Abstract
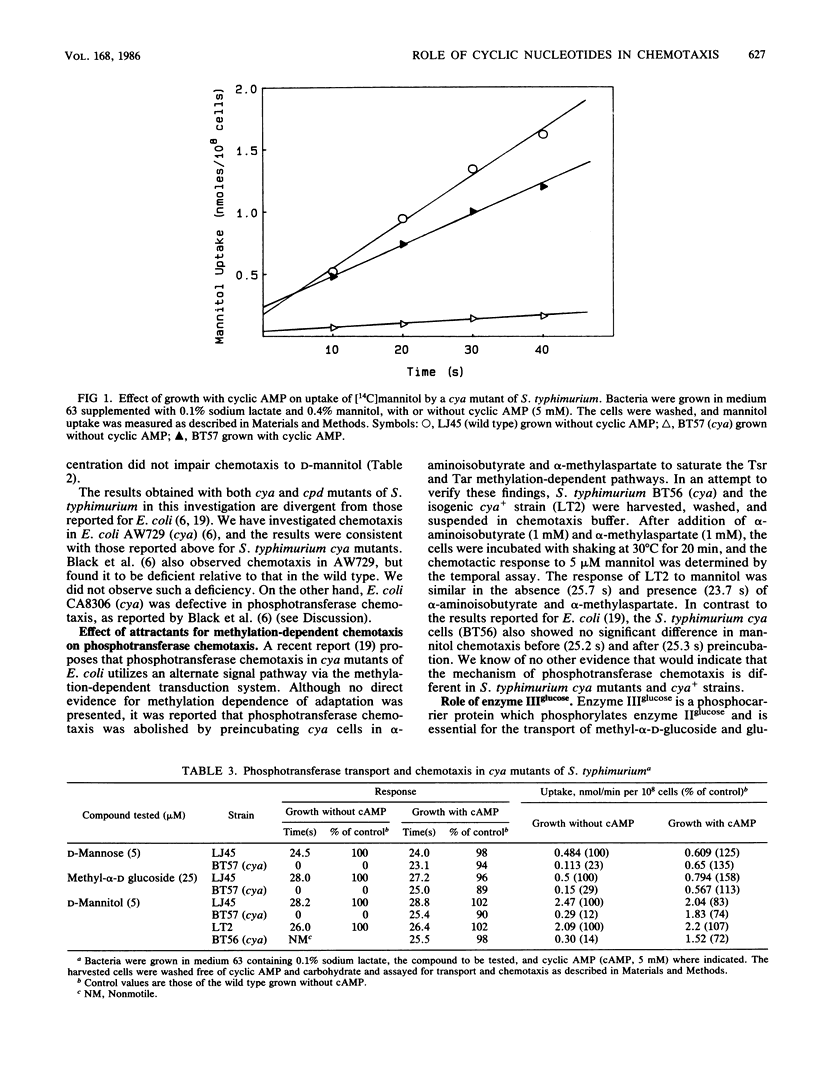
Defects in phosphotransferase chemotaxis in cya and cpd mutants previously cited as evidence of a cyclic GMP or cyclic AMP intermediate in signal transduction were not reproduced in a study of chemotaxis in Escherichia coli and Salmonella typhimurium. In cya mutants, which lack adenylate cyclase, the addition of cyclic AMP was required for synthesis of proteins that were necessary for phosphotransferase transport and chemotaxis. However, the induced cells retained normal phosphotransferase chemotaxis after cyclic AMP was removed. Phosphotransferase chemotaxis was normal in a cpd mutant of S. typhimurium that has elevated levels of cyclic GMP and cyclic AMP. S. typhimurium crr mutants are deficient in enzyme III glucose, which is a component of the glucose transport system, and a regulator of adenylate cyclase. After preincubation with cyclic AMP, the crr mutants were deficient in enzyme II glucose-mediated transport and chemotaxis, but other chemotactic responses were normal. It is concluded that cyclic GMP does not determine the frequency of tumbling and is probably not a component of the transduction pathway. The only known role of cyclic AMP is in the synthesis of some proteins that are subject to catabolite repression.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. Chemotaxis in bacteria. Annu Rev Biochem. 1975;44:341–356. doi: 10.1146/annurev.bi.44.070175.002013. [DOI] [PubMed] [Google Scholar]
- Adler J., Epstein W. Phosphotransferase-system enzymes as chemoreceptors for certain sugars in Escherichia coli chemotaxis. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2895–2899. doi: 10.1073/pnas.71.7.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper M. D., Ames B. N. Cyclic 3', 5'-adenosine monophosphate phosphodiesterase mutants of Salmonella typhimurium. J Bacteriol. 1975 Jun;122(3):1081–1090. doi: 10.1128/jb.122.3.1081-1090.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley G. S., Hansen D. E., Jacobson G. R., Knowles J. R. Stereochemical course of the reactions catalyzed by the bacterial phosphoenolpyruvate:glucose phosphotransferase system. Biochemistry. 1982 Oct 26;21(22):5552–5556. doi: 10.1021/bi00265a026. [DOI] [PubMed] [Google Scholar]
- Black R. A., Hobson A. C., Adler J. Adenylate cyclase is required for chemotaxis to phosphotransferase system sugars by Escherichia coli. J Bacteriol. 1983 Mar;153(3):1187–1195. doi: 10.1128/jb.153.3.1187-1195.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R. A., Hobson A. C., Adler J. Involvement of cyclic GMP in intracellular signaling in the chemotactic response of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3879–3883. doi: 10.1073/pnas.77.7.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botsford J. L. Cyclic AMP phosphodiesterase in Salmonella typhimurium: characteristics and physiological function. J Bacteriol. 1984 Nov;160(2):826–830. doi: 10.1128/jb.160.2.826-830.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dills S. S., Apperson A., Schmidt M. R., Saier M. H., Jr Carbohydrate transport in bacteria. Microbiol Rev. 1980 Sep;44(3):385–418. doi: 10.1128/mr.44.3.385-418.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erni B., Zanolari B. The mannose-permease of the bacterial phosphotransferase system. Gene cloning and purification of the enzyme IIMan/IIIMan complex of Escherichia coli. J Biol Chem. 1985 Dec 15;260(29):15495–15503. [PubMed] [Google Scholar]
- Feucht B. U., Saier M. H., Jr Fine control of adenylate cyclase by the phosphoenolpyruvate:sugar phosphotransferase systems in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1980 Feb;141(2):603–610. doi: 10.1128/jb.141.2.603-610.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier F. C., Hayward I., Novotny M. J., Leonard J. E., Saier M. H., Jr Identification of the phosphocarrier protein enzyme IIIgut: essential component of the glucitol phosphotransferase system in Salmonella typhimurium. J Bacteriol. 1985 Mar;161(3):1017–1022. doi: 10.1128/jb.161.3.1017-1022.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett B. J., Bar-Tana J. Polyprenyl p-hydroxybenzoate carboxylase in flagellation of Salmonella typhimurium. J Bacteriol. 1980 Aug;143(2):644–651. doi: 10.1128/jb.143.2.644-651.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler J., Auburger A. M., Mayer R., Pecher A. The phosphoenolpyruvate-dependent carbohydrate: phosphotransferase system enzymes II as chemoreceptors in chemotaxis of Escherichia coli K 12. Mol Gen Genet. 1981;183(1):163–170. doi: 10.1007/BF00270156. [DOI] [PubMed] [Google Scholar]
- Meadow N. D., Rosenberg J. M., Pinkert H. M., Roseman S. Sugar transport by the bacterial phosphotransferase system. Evidence that crr is the structural gene for the Salmonella typhimurium glucose-specific phosphocarrier protein IIIGlc. J Biol Chem. 1982 Dec 10;257(23):14538–14542. [PubMed] [Google Scholar]
- Melton T., Hartman P. E., Stratis J. P., Lee T. L., Davis A. T. Chemotaxis of Salmonella typhimurium to amino acids and some sugars. J Bacteriol. 1978 Feb;133(2):708–716. doi: 10.1128/jb.133.2.708-716.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S. O., Scholte B. J., Postma P. W. Phosphoenolpyruvate:sugar phosphotransferase system-mediated regulation of carbohydrate metabolism in Salmonella typhimurium. J Bacteriol. 1982 May;150(2):604–615. doi: 10.1128/jb.150.2.604-615.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S. O., Schuitema A. R., Benne R., van der Ploeg L. H., Plijter J. S., Aan F., Postma P. W. Molecular cloning, sequencing, and expression of the crr gene: the structural gene for IIIGlc of the bacterial PEP:glucose phosphotransferase system. EMBO J. 1984 Jul;3(7):1587–1593. doi: 10.1002/j.1460-2075.1984.tb02015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S. O., Wright J. K., Postma P. W. The mechanism of inducer exclusion. Direct interaction between purified III of the phosphoenolpyruvate:sugar phosphotransferase system and the lactose carrier of Escherichia coli. EMBO J. 1983;2(5):715–720. doi: 10.1002/j.1460-2075.1983.tb01490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwano M., Taylor B. L. Novel sensory adaptation mechanism in bacterial chemotaxis to oxygen and phosphotransferase substrates. Proc Natl Acad Sci U S A. 1982 Jan;79(1):11–15. doi: 10.1073/pnas.79.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny M. J., Frederickson W. L., Waygood E. B., Saier M. H., Jr Allosteric regulation of glycerol kinase by enzyme IIIglc of the phosphotransferase system in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1985 May;162(2):810–816. doi: 10.1128/jb.162.2.810-816.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osumi T., Saier M. H., Jr Regulation of lactose permease activity by the phosphoenolpyruvate:sugar phosphotransferase system: evidence for direct binding of the glucose-specific enzyme III to the lactose permease. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1457–1461. doi: 10.1073/pnas.79.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Adhya S. Cyclic adenosine 5'-monophosphate in Escherichia coli. Bacteriol Rev. 1976 Sep;40(3):527–551. doi: 10.1128/br.40.3.527-551.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Epstein W., Schuitema A. R., Nelson S. O. Interaction between IIIGlc of the phosphoenolpyruvate:sugar phosphotransferase system and glycerol kinase of Salmonella typhimurium. J Bacteriol. 1984 Apr;158(1):351–353. doi: 10.1128/jb.158.1.351-353.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W. Galactose transport in Salmonella typhimurium. J Bacteriol. 1977 Feb;129(2):630–639. doi: 10.1128/jb.129.2.630-639.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rephaeli A. W., Saier M. H., Jr Regulation of genes coding for enzyme constituents of the bacterial phosphotransferase system. J Bacteriol. 1980 Feb;141(2):658–663. doi: 10.1128/jb.141.2.658-663.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U. Coordinate regulation of adenylate cyclase and carbohydrate permeases by the phosphoenolpyruvate:sugar phosphotransferase system in Salmonella typhimurium. J Biol Chem. 1975 Sep 10;250(17):7078–7080. [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U., Hofstadter L. J. Regulation of carbohydrate uptake and adenylate cyclase activity mediated by the enzymes II of the phosphoenolpyruvate: sugar phosphotransferase system in Escherichia coli. J Biol Chem. 1976 Feb 10;251(3):883–892. [PubMed] [Google Scholar]
- Saier M. H., Jr, Roseman S. Sugar transport. 2nducer exclusion and regulation of the melibiose, maltose, glycerol, and lactose transport systems by the phosphoenolpyruvate:sugar phosphotransferase system. J Biol Chem. 1976 Nov 10;251(21):6606–6615. [PubMed] [Google Scholar]
- Saier M. H., Jr, Roseman S. Sugar transport. The crr mutation: its effect on repression of enzyme synthesis. J Biol Chem. 1976 Nov 10;251(21):6598–6605. [PubMed] [Google Scholar]
- Scholte B. J., Postma P. W. Mutation in the crp gene of Salmonella typhimurium which interferes with inducer exclusion. J Bacteriol. 1980 Feb;141(2):751–757. doi: 10.1128/jb.141.2.751-757.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte B. J., Schuitema A. R., Postma P. W. Characterization of factor IIIGLc in catabolite repression-resistant (crr) mutants of Salmonella typhimurium. J Bacteriol. 1982 Feb;149(2):576–586. doi: 10.1128/jb.149.2.576-586.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M., Takebe Y., Kaziro Y. A possible involvement of cya gene in the synthesis of cyclic guanosine 3':5'-monophosphate in E. coli. Cell. 1977 Oct;12(2):521–528. doi: 10.1016/0092-8674(77)90128-3. [DOI] [PubMed] [Google Scholar]
- Simoni R. D., Roseman S., Saier M. H., Jr Sugar transport. Properties of mutant bacteria defective in proteins of the phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1976 Nov 10;251(21):6584–6597. [PubMed] [Google Scholar]
- Tao M., Huberman A. Some properties of Escherichia coli adenyl cyclase. Arch Biochem Biophys. 1970 Nov;141(1):236–240. doi: 10.1016/0003-9861(70)90127-x. [DOI] [PubMed] [Google Scholar]
- Taylor B. L. Role of proton motive force in sensory transduction in bacteria. Annu Rev Microbiol. 1983;37:551–573. doi: 10.1146/annurev.mi.37.100183.003003. [DOI] [PubMed] [Google Scholar]
- Tsang N., Macnab R., Koshland D. E., Jr Common mechanism for repellents and attractants in bacterial chemotaxis. Science. 1973 Jul 6;181(4094):60–63. doi: 10.1126/science.181.4094.60. [DOI] [PubMed] [Google Scholar]
- Waygood E. B., Mattoo R. L., Peri K. G. Phosphoproteins and the phosphoenolpyruvate: sugar phosphotransferase system in Salmonella typhimurium and Escherichia coli: evidence for IIImannose, IIIfructose, IIIglucitol, and the phosphorylation of enzyme IImannitol and enzyme IIN-acetylglucosamine. J Cell Biochem. 1984;25(3):139–159. doi: 10.1002/jcb.240250304. [DOI] [PubMed] [Google Scholar]
- Yokota T., Gots J. S. Requirement of adenosine 3', 5'-cyclic phosphate for flagella formation in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1970 Aug;103(2):513–516. doi: 10.1128/jb.103.2.513-516.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


