Abstract
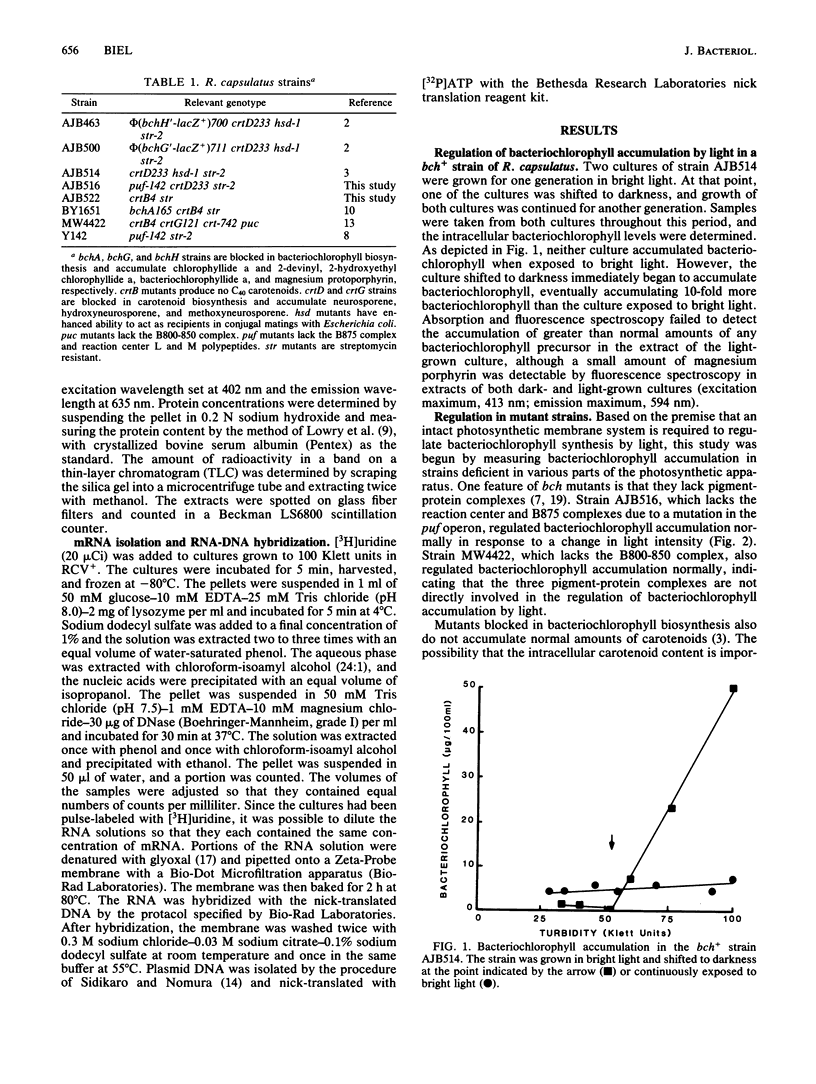
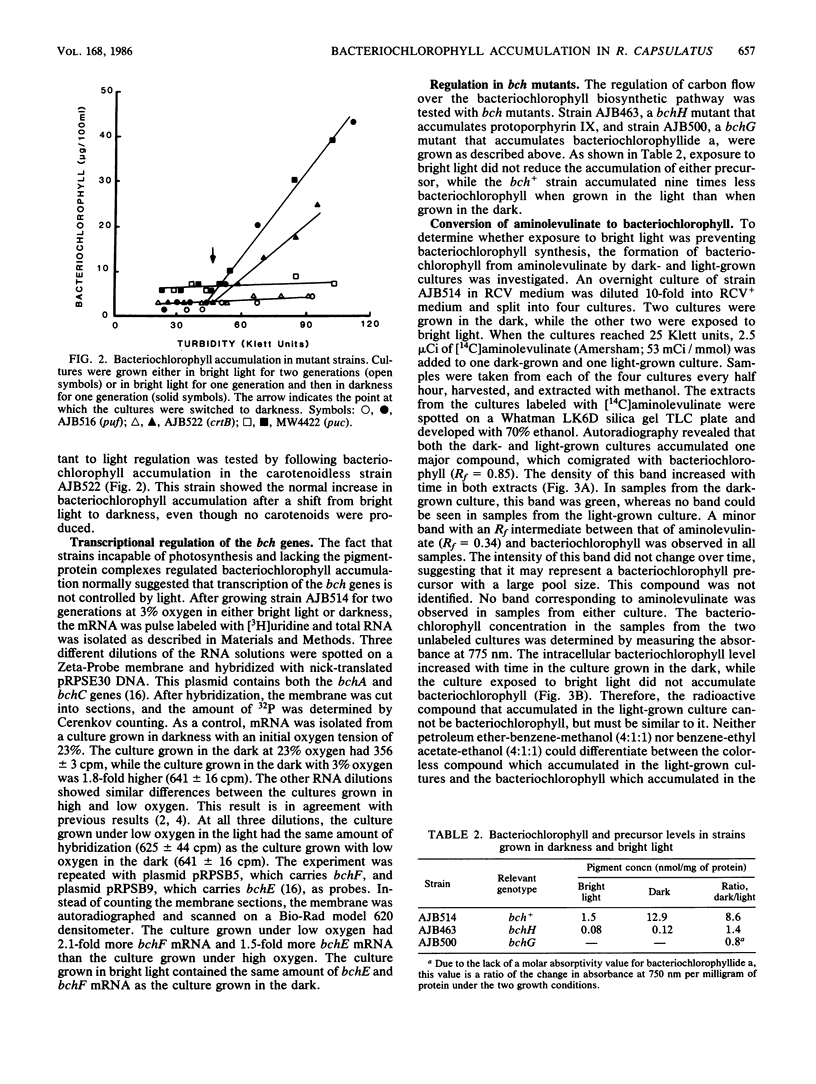
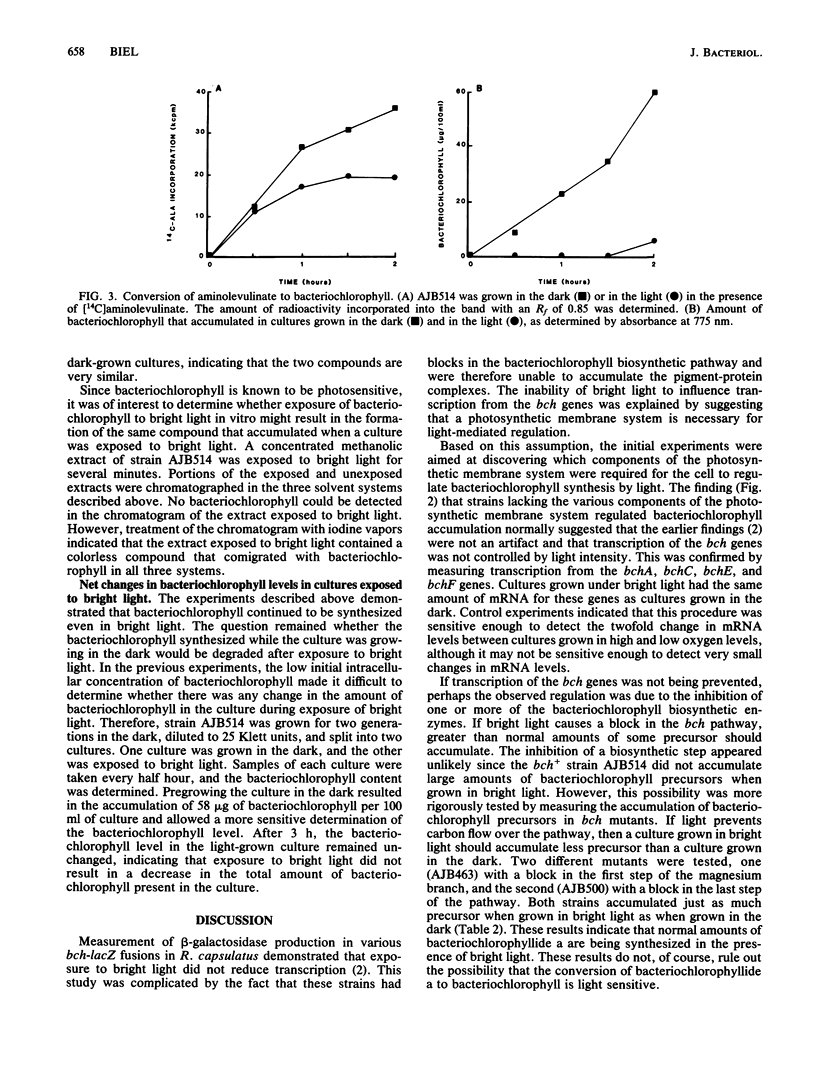
The accumulation of bacteriochlorophyll in Rhodobacter capsulatus grown either anaerobically or under low aeration is repressed by bright light. It has been proposed that an intact photosynthetic membrane system is required for light-mediated regulation. This was tested by measuring bacteriochlorophyll accumulation in various mutant strains grown under 3% oxygen. Mutants lacking either the reaction center and B875 complexes or the B800-850 complex exhibited normal regulation of bacteriochlorophyll accumulation by light, suggesting that neither photosynthesis nor the photosynthetic membrane system is involved in light-mediated regulation. Bright light did not reduce transcription from the bchA, bchC, bchE, or bchF gene. Neither a bch+ strain nor bchG or bchH mutants accumulated greater than normal amounts of any bacteriochlorophyll precursor when grown in bright light, indicating that carbon flow over the bacteriochlorophyll biosynthetic pathway was not being regulated by light intensity. When exposed to bright light, R. capsulatus converted aminolevulinate into a colorless compound with Rf values very similar to those of bacteriochlorophyll. These results suggest that in strains grown under low aeration, light intensity controls bacteriochlorophyll accumulation, but does not control bacteriochlorophyll synthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biel A. J., Marrs B. L. Oxygen does not directly regulate carotenoid biosynthesis in Rhodopseudomonas capsulata. J Bacteriol. 1985 Jun;162(3):1320–1321. doi: 10.1128/jb.162.3.1320-1321.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biel A. J., Marrs B. L. Transcriptional regulation of several genes for bacteriochlorophyll biosynthesis in Rhodopseudomonas capsulata in response to oxygen. J Bacteriol. 1983 Nov;156(2):686–694. doi: 10.1128/jb.156.2.686-694.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Clark W. G., Davidson E., Marrs B. L. Variation of levels of mRNA coding for antenna and reaction center polypeptides in Rhodopseudomonas capsulata in response to changes in oxygen concentration. J Bacteriol. 1984 Mar;157(3):945–948. doi: 10.1128/jb.157.3.945-948.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews G., Dierstein R., Schumacher A. Genetic transfer of the capacity to form bacteriochlorophyll-protein complexes in Rhodopseudomonas capsulata. FEBS Lett. 1976 Sep 15;68(1):132–136. doi: 10.1016/0014-5793(76)80421-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marrs B. Mobilization of the genes for photosynthesis from Rhodopseudomonas capsulata by a promiscuous plasmid. J Bacteriol. 1981 Jun;146(3):1003–1012. doi: 10.1128/jb.146.3.1003-1012.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher A., Drews G. Effects of light intensity on membrane differentiation in Rhodopseudomonas capsulata. Biochim Biophys Acta. 1979 Sep 11;547(3):417–428. doi: 10.1016/0005-2728(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Scolnik P. A., Zannoni D., Marrs B. L. Spectral and functional comparisons between the carotenoids of the two antenna complexes of Rhodopseudomonas capsulata. Biochim Biophys Acta. 1980 Dec 3;593(2):230–240. doi: 10.1016/0005-2728(80)90061-4. [DOI] [PubMed] [Google Scholar]
- Sidikaro J., Masayasu N. In vitro synthesis of the E3 immunity protein directed by Col E3 plasmid deoxyribonucleic acid. J Biol Chem. 1975 Feb 10;250(3):1123–1131. [PubMed] [Google Scholar]
- Takemoto J., Huang Kao M. Y. Effects of incident light levels on photosynthetic membrane polypeptide composition and assembly in Rhodopseudomonas sphaeroides. J Bacteriol. 1977 Feb;129(2):1102–1109. doi: 10.1128/jb.129.2.1102-1109.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. P., Cohen S. N., Clark W. G., Marrs B. L. Alignment of genetic and restriction maps of the photosynthesis region of the Rhodopseudomonas capsulata chromosome by a conjugation-mediated marker rescue technique. J Bacteriol. 1983 May;154(2):580–590. doi: 10.1128/jb.154.2.580-590.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver P. F., Wall J. D., Gest H. Characterization of Rhodopseudomonas capsulata. Arch Microbiol. 1975 Nov 7;105(3):207–216. doi: 10.1007/BF00447139. [DOI] [PubMed] [Google Scholar]
- Zsebo K. M., Hearst J. E. Genetic-physical mapping of a photosynthetic gene cluster from R. capsulata. Cell. 1984 Jul;37(3):937–947. doi: 10.1016/0092-8674(84)90428-8. [DOI] [PubMed] [Google Scholar]


