Abstract
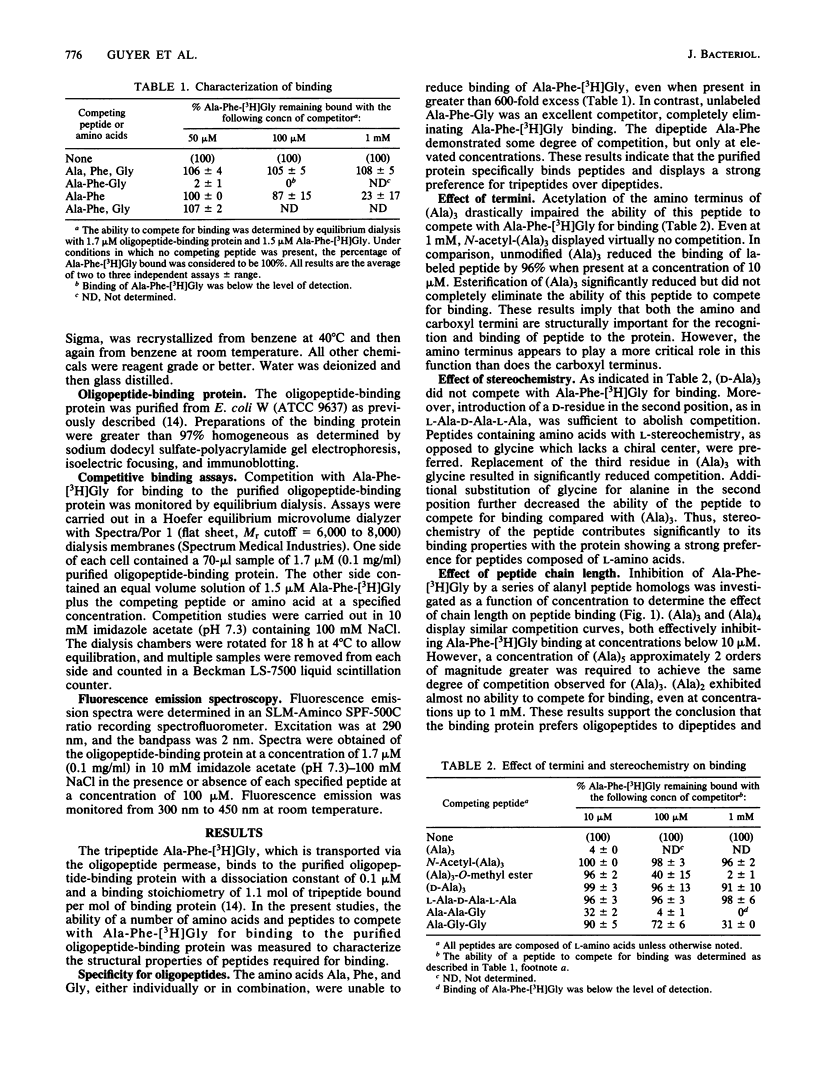
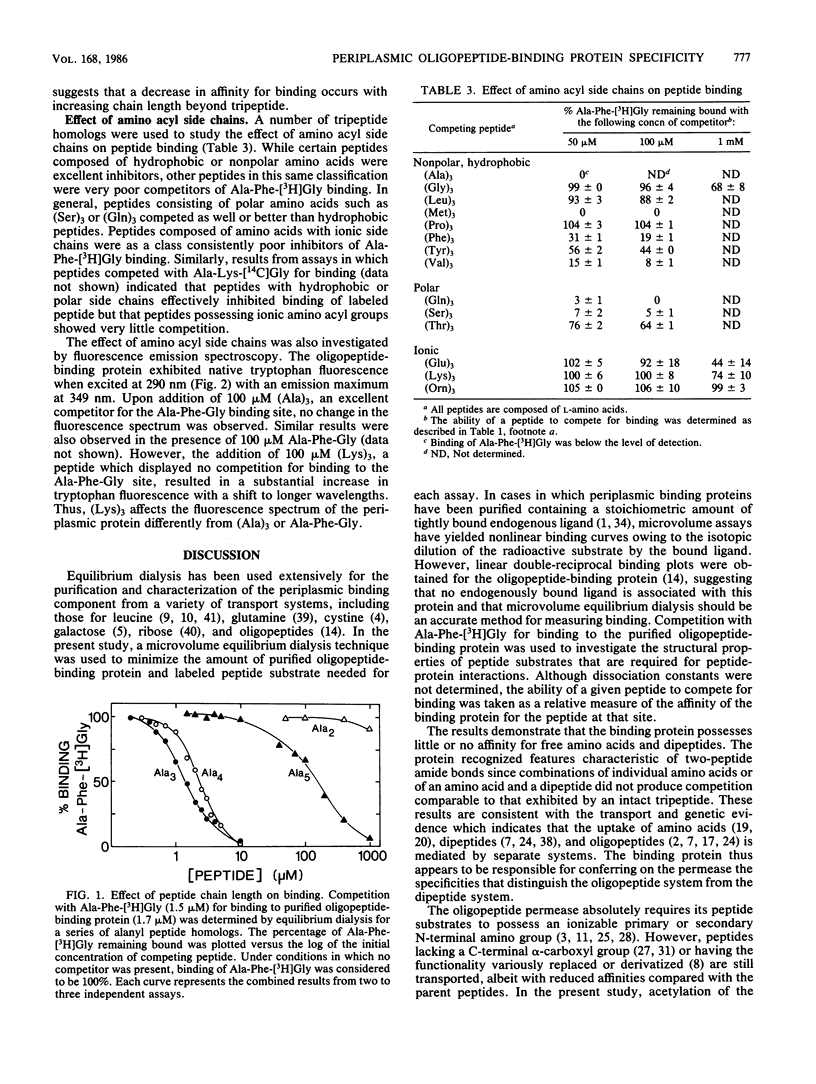
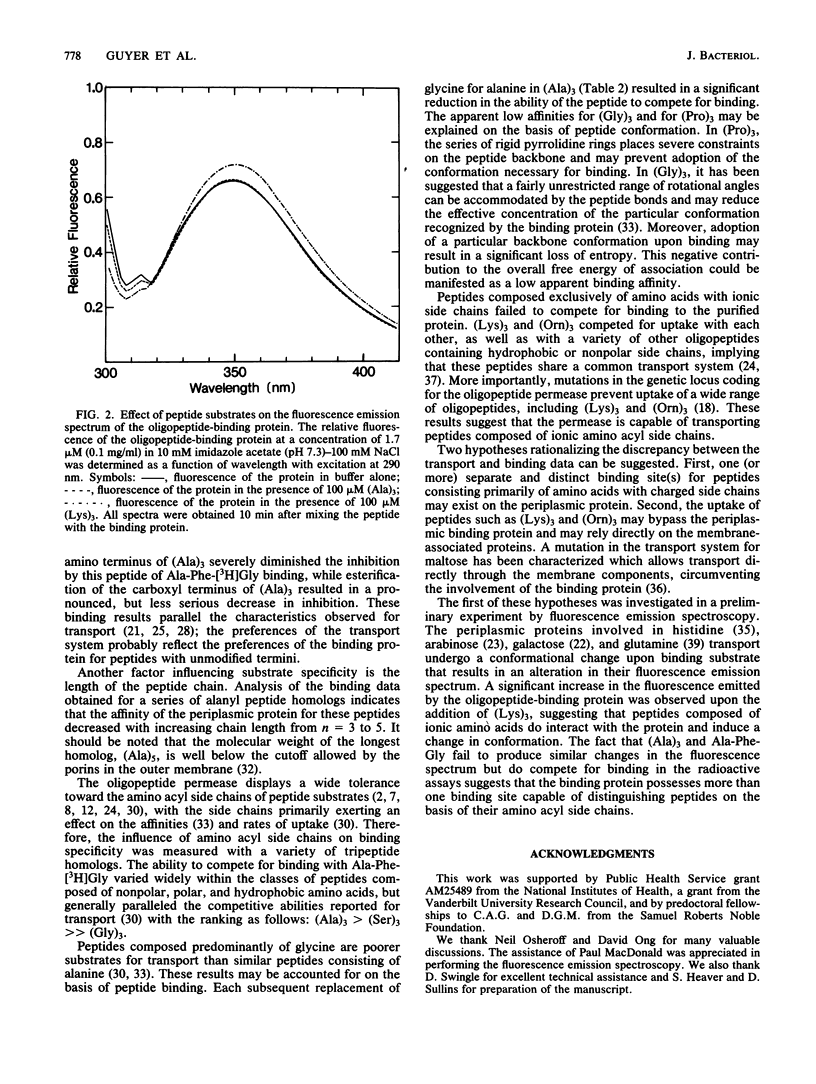
The structural properties required for the binding of peptide substrates to the Escherichia coli periplasmic protein involved in oligopeptide transport were surveyed by measuring the ability of different peptides to compete for binding in an equilibrium dialysis assay with the tripeptide Ala-Phe-[3H]Gly. The protein specifically bound oligopeptides and failed to bind amino acids or dipeptides. Acetylation of the peptide amino terminus of (Ala)3 severely impaired binding, whereas esterification of the carboxyl terminus significantly reduced but did not completely eliminate binding. Peptides composed of L-amino acids competed more effectively than did peptides containing D-residues or glycine. Experiments with a series of alanyl peptide homologs demonstrated a decrease in competitive ability with increasing chain length beyond tripeptide. Competition studies with tripeptide homologs indicated that a wide variety of amino acyl side chains were tolerated by the periplasmic protein, but side-chain composition did affect binding. Fluorescence emission data suggested that this periplasmic protein possesses more than one substrate-binding site capable of distinguishing peptides on the basis of amino acyl side chains.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amanuma H., Itoh J., Anraku Y. Transport of sugars and amino acids in bacteria. XVII. On the existence and nature of substrate amino acids bound to purified branched chain amino acid-binding proteins of Escherichia coli. J Biochem. 1976 Jun;79(6):1167–1182. doi: 10.1093/oxfordjournals.jbchem.a131172. [DOI] [PubMed] [Google Scholar]
- Barak Z., Gilvarg C. Triornithine-resistant strains of Escherichia coli. Isolation, definition, and genetic studies. J Biol Chem. 1974 Jan 10;249(1):143–148. [PubMed] [Google Scholar]
- Becker J. M., Naider F. Stereospecificity of tripeptide utilization in a methionine auxotroph of Escherichia coli K-12. J Bacteriol. 1974 Oct;120(1):191–196. doi: 10.1128/jb.120.1.191-196.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A., Heppel L. A. A binding protein involved in the transport of cystine and diaminopimelic acid in Escherichia coli. J Biol Chem. 1972 Dec 10;247(23):7684–7694. [PubMed] [Google Scholar]
- Boos W., Gordon A. S., Hall R. E., Price H. D. Transport properties of the galactose-binding protein of Escherichia coli. Substrate-induced conformational change. J Biol Chem. 1972 Feb 10;247(3):917–924. [PubMed] [Google Scholar]
- Cowell J. L. Energetics of glycylglycine transport in Escherichia coli. J Bacteriol. 1974 Oct;120(1):139–146. doi: 10.1128/jb.120.1.139-146.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice M., Guardiola J., Lamberti A., Iaccarino M. Escherichia coli K-12 mutants altered in the transport systems for oligo- and dipeptides. J Bacteriol. 1973 Nov;116(2):751–756. doi: 10.1128/jb.116.2.751-756.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickel T. E., Gilvarg C. Transport of impermeant substances in E. coli by way of oligopeptide permease. Nat New Biol. 1973 Feb 7;241(110):161–163. doi: 10.1038/newbio241161a0. [DOI] [PubMed] [Google Scholar]
- Furlong C. E., Morris R. G., Kandrach M., Rosen B. P. A multichamber equilibrium dialysis apparatus. Anal Biochem. 1972 Jun;47(2):514–526. doi: 10.1016/0003-2697(72)90146-7. [DOI] [PubMed] [Google Scholar]
- Furlong C. E., Weiner J. H. Purification of a leucine-specific binding protein from Escherichia coli. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1076–1083. doi: 10.1016/0006-291x(70)90349-9. [DOI] [PubMed] [Google Scholar]
- GILVARG C., KATCHALSKI E. PEPTIDE UTILIZATION IN ESCHERICHIA COLI. J Biol Chem. 1965 Jul;240:3093–3098. [PubMed] [Google Scholar]
- Gilvarg C., Levin Y. Response of Escherichia coli to ornithyl peptides. J Biol Chem. 1972 Jan 25;247(2):543–549. [PubMed] [Google Scholar]
- Gutnick D., Calvo J. M., Klopotowski T., Ames B. N. Compounds which serve as the sole source of carbon or nitrogen for Salmonella typhimurium LT-2. J Bacteriol. 1969 Oct;100(1):215–219. doi: 10.1128/jb.100.1.215-219.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer C. A., Morgan D. G., Osheroff N., Staros J. V. Purification and characterization of a periplasmic oligopeptide binding protein from Escherichia coli. J Biol Chem. 1985 Sep 5;260(19):10812–10818. [PubMed] [Google Scholar]
- Higgins C. F., Hardie M. M., Jamieson D., Powell L. M. Genetic map of the opp (Oligopeptide permease) locus of Salmonella typhimurium. J Bacteriol. 1983 Feb;153(2):830–836. doi: 10.1128/jb.153.2.830-836.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Hardie M. M. Periplasmic protein associated with the oligopeptide permeases of Salmonella typhimurium and Escherichia coli. J Bacteriol. 1983 Sep;155(3):1434–1438. doi: 10.1128/jb.155.3.1434-1438.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiles I. D., Higgins C. F. Peptide uptake by Salmonella typhimurium. The periplasmic oligopeptide-binding protein. Eur J Biochem. 1986 Aug 1;158(3):561–567. doi: 10.1111/j.1432-1033.1986.tb09791.x. [DOI] [PubMed] [Google Scholar]
- Hogarth B. G., Higgins C. F. Genetic organization of the oligopeptide permease (opp) locus of Salmonella typhimurium and Escherichia coli. J Bacteriol. 1983 Mar;153(3):1548–1551. doi: 10.1128/jb.153.3.1548-1551.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESSEL D., LUBIN M. Transport of proline in Escherichia coli. Biochim Biophys Acta. 1962 Feb 12;57:32–43. doi: 10.1016/0006-3002(62)91074-0. [DOI] [PubMed] [Google Scholar]
- LEVINE E. M., SIMMONDS S. Further studies on metabolite uptake by serine-glycine auxotrophs of Escherichia coli. J Biol Chem. 1962 Dec;237:3718–3724. [PubMed] [Google Scholar]
- Miller D. M., 3rd, Olson J. S., Quiocho F. A. The mechanism of sugar binding to the periplasmic receptor for galactose chemotaxis and transport in Escherichia coli. J Biol Chem. 1980 Mar 25;255(6):2465–2471. [PubMed] [Google Scholar]
- Parsons R. G., Hogg R. W. Crystallization and characterization of the L-arabinose-binding protein of Escherichia coli B-r. J Biol Chem. 1974 Jun 10;249(11):3602–3607. [PubMed] [Google Scholar]
- Payne J. W., Bell G. Direct determination of the properties of peptide transport systems in Escherichia coli, using a fluorescent-labeling procedure. J Bacteriol. 1979 Jan;137(1):447–455. doi: 10.1128/jb.137.1.447-455.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne J. W. Effects of N-methyl peptide bonds on peptide utilization by Escherichia coli. J Gen Microbiol. 1972 Jul;71(2):259–265. doi: 10.1099/00221287-71-2-259. [DOI] [PubMed] [Google Scholar]
- Payne J. W., Gilvarg C. Size restriction on peptide utilization in Escherichia coli. J Biol Chem. 1968 Dec 10;243(23):6291–6299. [PubMed] [Google Scholar]
- Payne J. W., Gilvarg C. The role of the terminal carboxyl group on peptide transport in Escherichia coli. J Biol Chem. 1968 Jan 25;243(2):335–340. [PubMed] [Google Scholar]
- Payne J. W. Oligopeptide transport in Escherichia coli. Specificity with respect to side chain and distinction from dipeptide transport. J Biol Chem. 1968 Jun 25;243(12):3395–3403. [PubMed] [Google Scholar]
- Payne J. W. Peptide transport in Escherichia coli: permease specificity towards terminal amino group substituents. J Gen Microbiol. 1974 Jan;80(1):269–276. doi: 10.1099/00221287-80-1-269. [DOI] [PubMed] [Google Scholar]
- Payne J. W. Peptides and micro-organisms. Adv Microb Physiol. 1976;13:55–113. doi: 10.1016/s0065-2911(08)60038-7. [DOI] [PubMed] [Google Scholar]
- Payne J. W. The requirement for the protonated -amino group for the transport of peptides in Escherichia coli. Biochem J. 1971 Jun;123(2):245–253. doi: 10.1042/bj1230245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D., Gilvarg C. Spectrophotometric determination of affinities of peptides for their transport systems in Escherichia coli. J Bacteriol. 1984 Dec;160(3):943–948. doi: 10.1128/jb.160.3.943-948.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richarme G., Kepes A. Release of glucose from purified galactose-binding protein of Escherichia coli upon addition of galactose. Eur J Biochem. 1974 Jun 1;45(1):127–133. doi: 10.1111/j.1432-1033.1974.tb03537.x. [DOI] [PubMed] [Google Scholar]
- Shuman H. A. The maltose-maltodextrin transport system of Escherichia coli. Ann Microbiol (Paris) 1982 Jan;133A(1):153–159. [PubMed] [Google Scholar]
- Smith R. L., Archer E. G., Dunn F. W. Uptake of [14C]-labeled tri-, tetra-, and pentapeptides of phenylalanine and glycine by Escherichia coli. J Biol Chem. 1970 Jun 10;245(11):2967–2971. [PubMed] [Google Scholar]
- Vonder Haar R. A., Umbarger H. E. Isoleucine and valine metabolism in Escherichia coli. XIX. Inhibition of isoleucine biosynthesis by glycyl-leucine. J Bacteriol. 1972 Oct;112(1):142–147. doi: 10.1128/jb.112.1.142-147.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis R. C., Furlong C. E. Purification and properties of a ribose-binding protein from Escherichia coli. J Biol Chem. 1974 Nov 10;249(21):6926–6929. [PubMed] [Google Scholar]


