Abstract
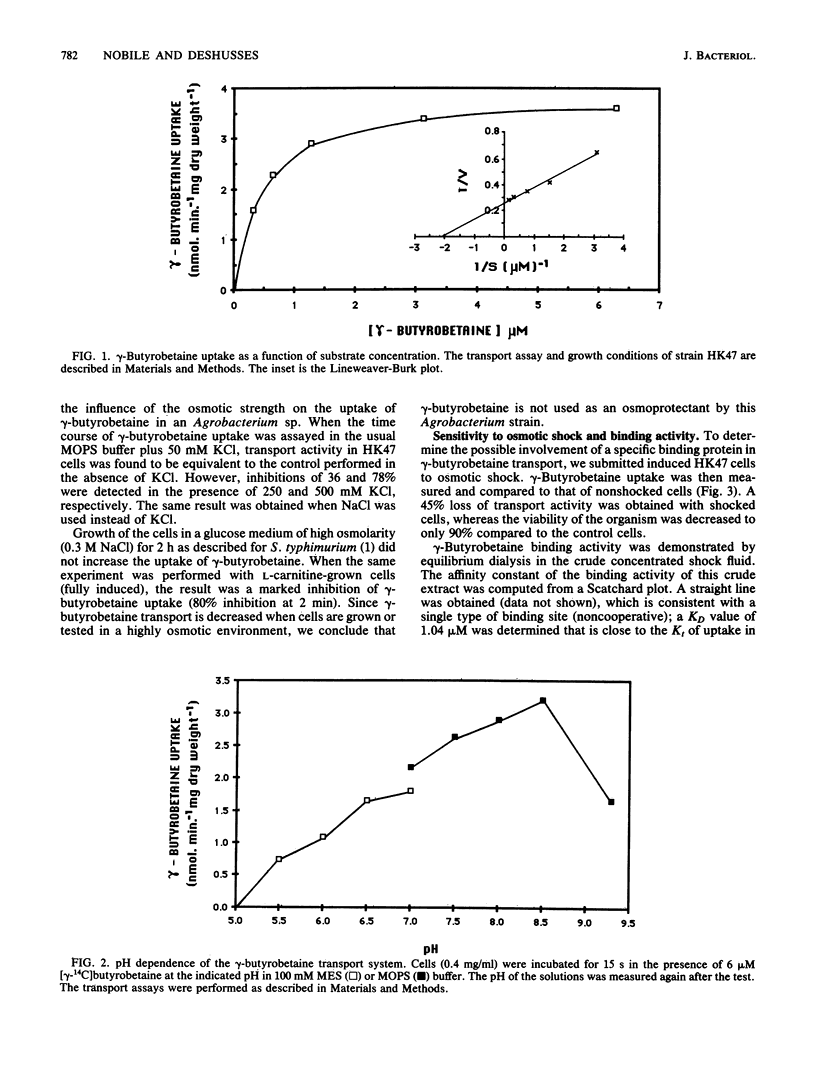
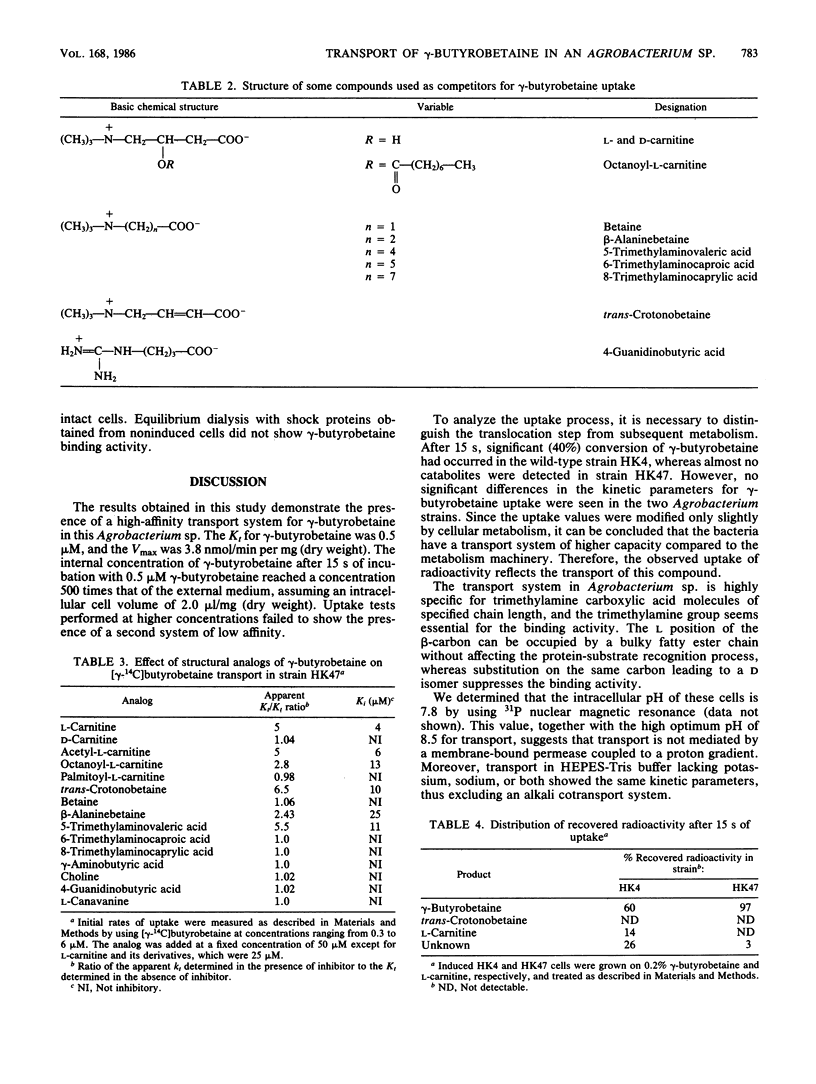
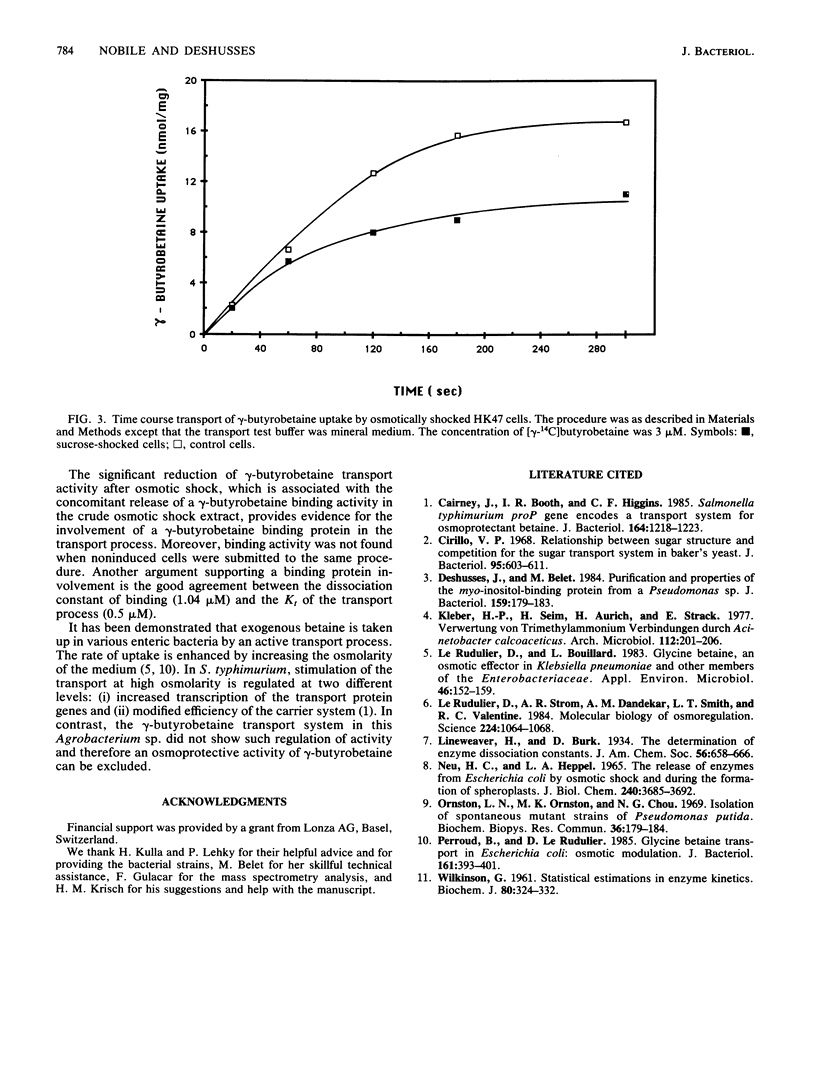
An Agrobacterium sp. isolated from soil by selective growth on gamma-butyrobetaine (gamma-trimethylaminobutyrate) as the sole source of both carbon and nitrogen has been shown to possess an inducible transport system for this growth substrate. This transport system has a Kt of 0.5 microM and a maximal velocity of 3.8 nmol/min per mg (dry weight). The influx of gamma-butyrobetaine is optimal at pH 8.5 and operates against a concentration gradient. The transport system shows a high specificity for trimethylamine carboxylic acid molecules of defined chain length. gamma-Butyrobetaine uptake was significantly reduced in osmotically shocked cells and a gamma-butyrobetaine binding activity was detected in the crude shock fluid. This suggests a transport mechanism involving a periplasmic gamma-butyrobetaine binding protein.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cairney J., Booth I. R., Higgins C. F. Salmonella typhimurium proP gene encodes a transport system for the osmoprotectant betaine. J Bacteriol. 1985 Dec;164(3):1218–1223. doi: 10.1128/jb.164.3.1218-1223.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo V. P. Relationship between sugar structure and competition for the sugar transport system in Bakers' yeast. J Bacteriol. 1968 Feb;95(2):603–611. doi: 10.1128/jb.95.2.603-611.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshusses J., Belet M. Purification and properties of the myo-inositol-binding protein from a Pseudomonas sp. J Bacteriol. 1984 Jul;159(1):179–183. doi: 10.1128/jb.159.1.179-183.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleber H-P, Seim H., Aurich H., Strack E. Verwertung von Trimethylammoniumverbindungen durch Acinetobacter calcoaceticus. Arch Microbiol. 1977 Mar 1;112(2):201–206. doi: 10.1007/BF00429336. [DOI] [PubMed] [Google Scholar]
- Le Rudulier D., Bouillard L. Glycine betaine, an osmotic effector in Klebsiella pneumoniae and other members of the Enterobacteriaceae. Appl Environ Microbiol. 1983 Jul;46(1):152–159. doi: 10.1128/aem.46.1.152-159.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Rudulier D., Strom A. R., Dandekar A. M., Smith L. T., Valentine R. C. Molecular biology of osmoregulation. Science. 1984 Jun 8;224(4653):1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Ornston L. N., Ornston M. K., Chou G. Isolation of spontaneous mutant strains of Pseudomonas putida. Biochem Biophys Res Commun. 1969 Jul 7;36(1):179–184. doi: 10.1016/0006-291x(69)90666-4. [DOI] [PubMed] [Google Scholar]
- Perroud B., Le Rudulier D. Glycine betaine transport in Escherichia coli: osmotic modulation. J Bacteriol. 1985 Jan;161(1):393–401. doi: 10.1128/jb.161.1.393-401.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]


