Abstract
The biochemical composition of the cell envelope of Renibacterium salmoninarum was investigated in a total of 13 strains isolated from different salmonid fish species at various geographical locations of the United States, Canada, and Europe. A marked similarity with the type strain R. salmoninarum ATCC 33209 was found both in the peptidoglycan and the cell wall polysaccharide. The primary structure of the peptidoglycan was found to be consistent with lysine in the third position of the peptide subunit, a glycyl-alanine interpeptide bridge between lysine and D-alanine of adjacent peptide subunits, and a D-alanine amide substituent at the alpha-carboxyl group of D-glutamic acid in position 2 of the peptide subunit. The cell wall polysaccharide contained galactose as the major sugar component which was accompanied by rhamnose, N-acetylglucosamine, and N-acetylfucosamine. The polysaccharide amounted to more than 60% of the dry weight of the cell walls. It was found to be covalently linked to the peptidoglycan and was released by hot formamide treatment. On gel filtration chromatography the extracted polysaccharide behaved like a homogeneous polymeric compound. The purified cell wall polysaccharide showed antigenic activity with antiserum obtained by immunization of rabbits with heat-inactivated trypsinized cells of R. salmoninarum. Immunoblotting experiments with nontrypsinized cell walls and antisera raised against R. salmoninarum cells revealed that antigenic proteins were attached to the cell walls.
Full text
PDF
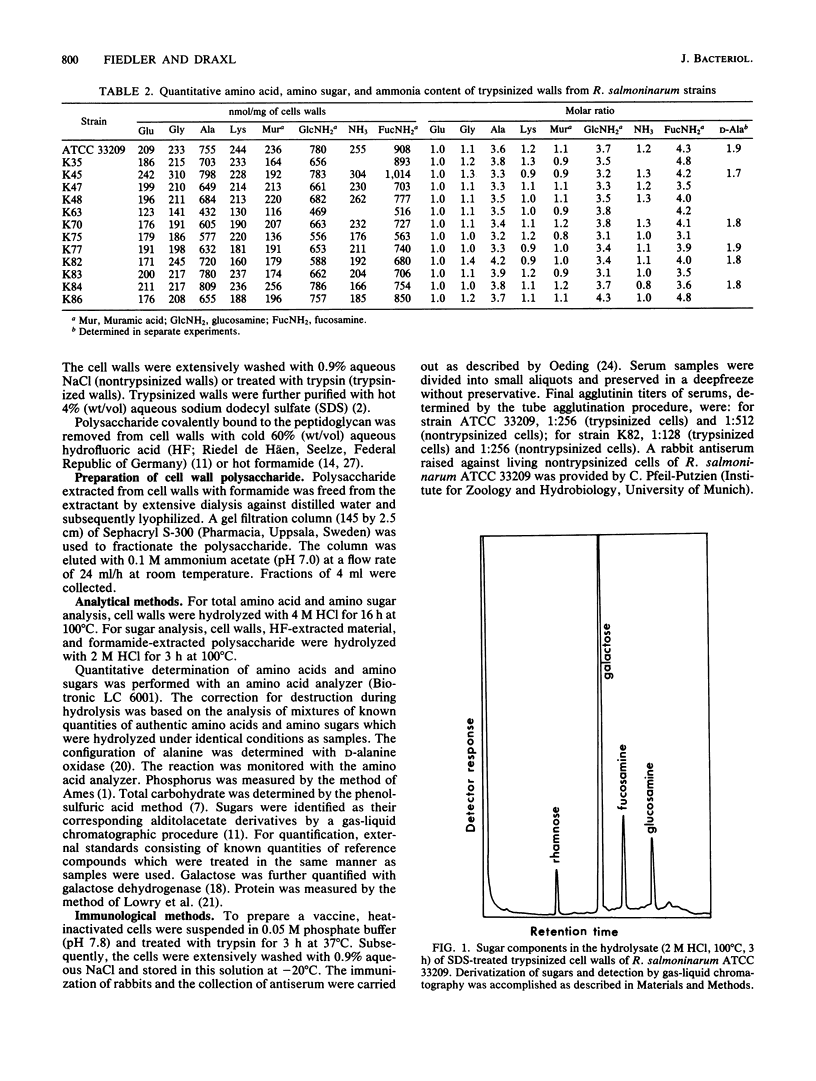
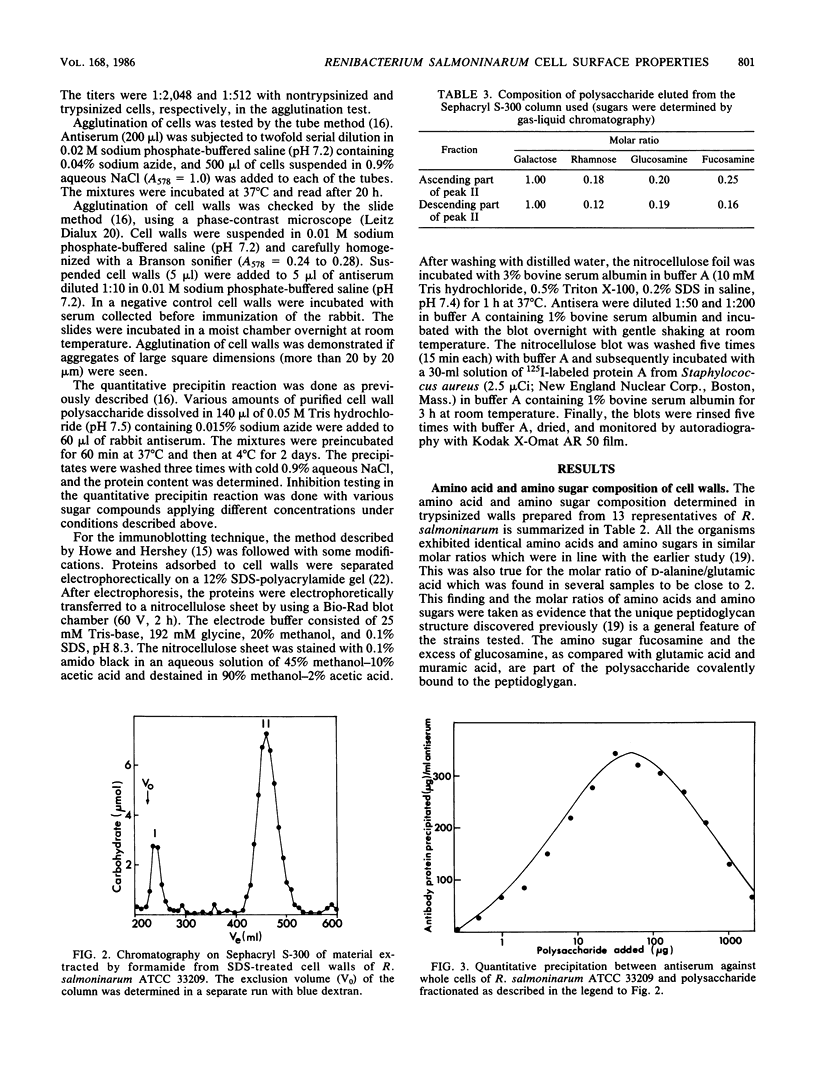

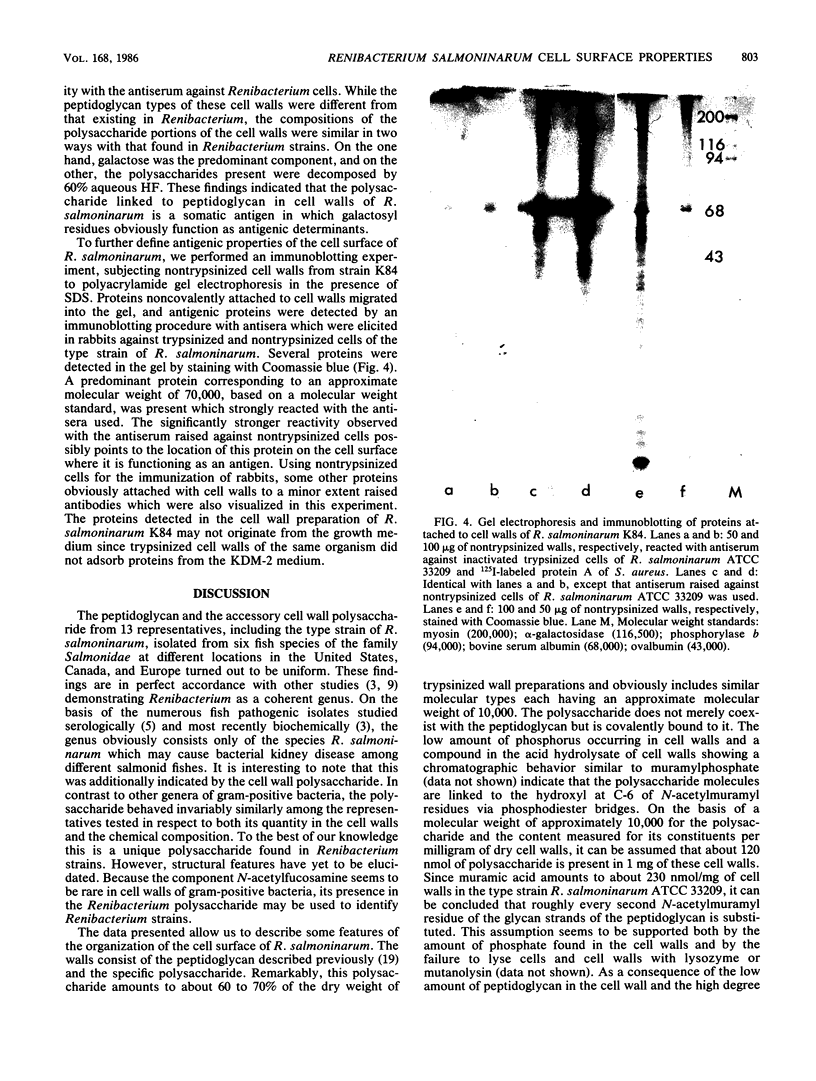

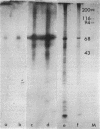
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Bullock G. L., Stuckey H. M., Chen P. K. Corynebacterial kidney disease of salmonids: growth and serological studies on the causative bacterium. Appl Microbiol. 1974 Nov;28(5):811–814. doi: 10.1128/am.28.5.811-814.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler F., Schleifer K., Kandler O. Amino acid sequence of the threonine-containing mureins of coryneform bacteria. J Bacteriol. 1973 Jan;113(1):8–17. doi: 10.1128/jb.113.1.8-17.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer J. L., Sanders J. E. Bacterial kidney disease of salmonid fish. Annu Rev Microbiol. 1981;35:273–298. doi: 10.1146/annurev.mi.35.100181.001421. [DOI] [PubMed] [Google Scholar]
- Howe J. G., Hershey J. W. A sensitive immunoblotting method for measuring protein synthesis initiation factor levels in lysates of Escherichia coli. J Biol Chem. 1981 Dec 25;256(24):12836–12839. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larson D. M., Snetsinger D. C., Waibel P. E. Procedure for determination of D-amino acids. Anal Biochem. 1971 Feb;39(2):395–401. doi: 10.1016/0003-2697(71)90429-5. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Nakel M., Ghuysen J. M., Kandler O. Wall peptidoglycan in Aerococcus viridans strains 201 Evans and ATCC 11563 and in Gaffkya homari strain ATCC 10400. Biochemistry. 1971 May 25;10(11):2170–2175. doi: 10.1021/bi00787a033. [DOI] [PubMed] [Google Scholar]
- ORDAL E. J., EARP B. J. Cultivation and transmission of etiological agent of kidney disease in salmonid fishes. Proc Soc Exp Biol Med. 1956 May;92(1):85–88. doi: 10.3181/00379727-92-22392. [DOI] [PubMed] [Google Scholar]
- Op den Camp H. J., Veerkamp J. H., Oosterhof A., Van Halbeek H. Structure of the lipoteichoic acids from Bifidobacterium bifidum spp. pennsylvanicum. Biochim Biophys Acta. 1984 Sep 12;795(2):301–313. doi: 10.1016/0005-2760(84)90080-8. [DOI] [PubMed] [Google Scholar]
- PERKINS H. R. THE ACTION OF HOT FORMAMIDE ON BACTERIAL CELL WALLS. Biochem J. 1965 Jun;95:876–882. doi: 10.1042/bj0950876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Zur chemischen Zusammensetzung der Zellwand der Streptokokken. I. Die Aminosäuresequenz des Mureins von Str. thermophilus und Str. faecalis. Arch Mikrobiol. 1967 Jul 6;57(4):335–364. [PubMed] [Google Scholar]
- Stackebrandt E., Fiedler F., Kandler O. Peptidoglycantyp und Zusammensetzung der Zellwandpolysaccharide von Cellulomonas cartalyticum und einigen coryneformen organismen. Arch Microbiol. 1978 Apr 27;117(1):115–118. doi: 10.1007/BF00689360. [DOI] [PubMed] [Google Scholar]