Abstract
Induction of the immediate early gene protein product Fos has been used extensively to assess neural activation in the striatum after repeated amphetamine administration to rats in their home cages. However, this technique has not been used to examine striatal activation after repeated administration outside the home cage, an environment where repeated drug administration produces more robust psychomotor sensitization. We determined the dose-response relationship for amphetamine-induced psychomotor activity and Fos expression in nucleus accumbens and caudate-putamen one week after repeated administration of amphetamine or saline in locomotor activity chambers. Repeated administration of amphetamine enhanced amphetamine-induced locomotor activity and stereotypy and Fos expression in nucleus accumbens, but not in caudate-putamen. In comparison, levels of Fos expression induced by 1 mg/kg amphetamine were not altered in nucleus accumbens or caudate-putamen by repeated amphetamine administration in the home cage. Double-labeling of Fos protein and enkephalin mRNA indicates that Fos is expressed in approximately equal numbers of enkephalin-negative and enkephalin-positive neurons in nucleus accumbens and caudate-putamen following injections outside the home cage. Furthermore, repeated amphetamine administration increased drug-induced Fos expression in enkephalin-positive, but not enkephalin-negative, neurons in nucleus accumbens. We conclude that repeated amphetamine administration outside the home cage recruits the activation of enkephalin-containing nucleus accumbens neurons during sensitized amphetamine-induced psychomotor activity.
Keywords: sensitization, locomotor activity, stereotypy, striatum, environmental modulation, enkephalin, in situ hybridization
Repeated psychostimulant drug administration produces a progressive augmentation, or sensitization, of psychomotor activating effects including locomotor activity and stereotyped behaviors (Kalivas and Stewart, 1991; Randrup and Munkvad, 1975; Robinson and Becker, 1986). Since these drug-induced behaviors are mediated in part by neuronal activity in nucleus accumbens and caudate-putamen (Kelly et al., 1975; Robinson and Becker, 1986; Vanderschuren and Kalivas, 2000; Wise and Bozarth, 1985), Fos immunohistochemistry and c-fos in situ hybridization have been used to assess sensitization-related changes of drug-induced neuronal activation in these regions (Cole et al., 1995; Jaber et al., 1995; Persico et al., 1993; Persico et al., 1995; Simpson et al., 1995; Turgeon et al., 1997). The majority of these studies have found that repeated administration of amphetamine or cocaine to rats attenuates subsequent drug-induced Fos expression in nucleus accumbens and caudate-putamen (Cole et al., 1995; Jaber et al., 1995; Persico et al., 1993; Persico et al., 1995; Simpson et al., 1995; Turgeon et al., 1997).
However, recent studies indicate that the environment in which the drug is administered powerfully modulates the effects of amphetamine or cocaine administration on both psychomotor activity and Fos expression in the striatum. First, amphetamine-induced psychomotor activity and sensitization is greater when the drug is administered in a novel environment (outside the rat’s home cage) than in the home cage (Badiani et al., 1995a; Badiani et al., 1995b; Badiani et al., 1997; Badiani et al., 1998; Crombag et al., 1996; Crombag et al., 1999; Fraioli et al., 1999; Uslaner et al., 2001a; Uslaner et al., 2001b). Second, Fos expression induced by a single (acute) injection of amphetamine or cocaine is greater when the drug is administered in a novel environment (Badiani et al., 1998; Uslaner et al., 2001a; Uslaner et al., 2001b). Furthermore, amphetamine or cocaine induces Fos in both D1 receptor/dynorphin and D2 receptor/enkephalin-containing medium spiny neurons in the striatum when the drug is administered in a novel environment outside the home cage, while Fos is induced in only D1 receptor/dynorphin-containing neurons when the drug is administered in the home cage (Badiani et al., 1999; Uslaner et al., 2001b).
The drug administration environment modulates the effects of repeated psychostimulant administration on subsequent drug-induced Fos expression. Repeated cocaine administration outside the home cage enhances cocaine-induced Fos expression in the nucleus accumbens (Crombag et al., 2002; Hope et al., 2006; Todtenkopf et al., 2002). In the only related amphetamine study, repeated amphetamine administration did not alter amphetamine-induced c-fos mRNA levels in nucleus accumbens of unilateral 6-hydroxydopamine-lesioned rats (Ostrander et al., 2003). However, this study used only a single challenge dose of amphetamine, while we found that enhanced Fos protein expression following repeated cocaine administration is critically dependent on challenge dose (Crombag et al., 2002). Additionally, the thresholds for detecting Fos protein and c-fos mRNA in situ are different and 6-hydroxydopamine lesions may have altered neural responsiveness in the intact side. Therefore in the present study, we examined the effects of different challenge doses of amphetamine on Fos expression in nucleus accumbens and caudate-putamen of intact rats one week after repeated drug administration outside the home cage and assessed which types of striatal medium spiny neurons express Fos.
Experimental procedures
Subjects
Male Sprague-Dawley rats (Charles River, Raleigh, NC) weighing 200–250g were used in all our experiments. They were housed individually in standard plastic cages in a temperature and humidity controlled room and maintained on a 12:12 hr reverse light/dark cycle (lights on at 8:00 PM) with free access to food and water. They were acclimatized to these housing conditions for a minimum of 7 days prior to drug treatments.
Drug treatments and behavioral assays
Experiment 1: Repeated amphetamine administration in locomotor activity chambers
Amphetamine or saline was administered in locomotor activity chambers once daily for five days. Each day during repeated drug administration, the rats were removed from their home cages and placed in locomotor activity chambers for 30 minutes to habituate. At the end of the habituation period, the rats received a single intraperitoneal (IP) injection of amphetamine (2 mg/kg; n=32) or saline vehicle (1ml/kg; n=31) and were immediately placed back into the activity chambers for 120 additional minutes. The rats were brought back to their home cages after each daily session. The locomotor activity chamber consisted of a 43 × 43 cm plexiglass activity monitor in a light- and sound-attenuating chamber. The monitor used 2 ×16 infrared photobeams to quantify locomotor activity. The distance traveled before and after the injection was calculated using activity monitor software from Med Associates (Georgia, VT).
Following the repeated drug administration phase, the rats were kept in their home cages for 1 week (withdrawal phase). Finally, on the test day the rats were removed from their home cages, placed in the same locomotor activity chambers for 30 minutes to habituate, and then injected with 0, 0.5, 1, or 2 mg/kg amphetamine (IP; 8 rats for each dose). Locomotor activity was monitored for 120 additional minutes and during this time-period the rats’ behavior was also monitored with cameras mounted in the locomotor activity chambers. Stereotyped behaviors were assessed every 4 minutes for 30 sec each time using a 9-point rating scale adapted from Ellinwood and Balster (1974): 1-asleep; 2-inactive; 3-normal in place activity; 4-normal, alert, rearing, normal level of locomotor activity; 5-rearing, high level of locomotor activity; 6-slow patterned behaviors, no rearing, normal level of locomotor activity; 7-faster patterned behaviors, no rearing, high level of locomotor activity; 8-highly repetitive patterned behaviors in a restricted area; 9-backing up, abnormally maintained posture.
Two hours after the challenge injection, each rat was deeply anesthetized with a mixture of ketamine+xylazine (100+10 mg/kg, IP) and perfused transcardially with 100 ml of ice-cold 0.1M phosphate-buffered saline (PBS) followed by 300 ml of freshly prepared 4% paraformaldehyde in 0.1M sodium phosphate (pH=7.4). The brain was removed and post-fixed in the same fixative for 2 hours before being placed in 20% sucrose, 0.1M sodium phosphate buffer (pH=7.4) at 4°C for 48 hours. Finally, the brain was frozen in powdered dry ice and stored at −80°C until sectioning for Fos immunohistochemistry.
Experiment 2: Repeated amphetamine administration for double-labeling histochemistry
To determine which neuronal types express Fos, we repeatedly administered amphetamine or saline to rats using the same procedure described in Experiment 1, with the difference that only saline and 1 mg/kg amphetamine were administered on test day. Two hours after challenge injections, each rat was deeply anesthetized with a mixture of ketamine+xylazine (100+10 mg/kg, i.p.) and the brain was processed as described above. These brains were used for double-labeling with Fos immunohistochemistry and enkephalin in situ hybridization as described below.
Experiment 3: Repeated amphetamine administration in the home cage
Amphetamine or saline was repeatedly administered to 28 rats using the same treatment regimen described above except that amphetamine or saline injections were administered to rats in their home cages. Also, a single challenge dose of 1 mg/kg amphetamine was administered: this dose was found to produce robust expression of psychomotor sensitization and enhanced Fos expression when the drug was administered in locomotor activity chambers. Thus, five once daily IP injections of either amphetamine (2 mg/kg; n=14) or saline vehicle (1ml/kg; n=14) were administered to rats in their home cage. One week after the last administration, each of the repeated saline or amphetamine groups was further divided into 2 groups of 7 rats that received an IP challenge injection of 1 mg/kg of amphetamine or saline vehicle (1 ml/kg) in the home cage. Locomotor activity and stereotypy were not assessed for this experiment. Two hours following the challenge injection, each rat was deeply anesthetized with a mixture of ketamine+xylazine (100+10 mg/kg, i.p.) and the brain was processed for Fos immunohistochemistry as described above.
Fos immunohistochemistry
Twenty micrometer coronal sections were cut in a cryostat (Reichert-Jung 28000E, Leica Inc., Deerfield, IL) and placed into cryopreservant solution containing 0.1M sodium phosphate buffer (pH=7.4), 20% glycerol and 2% DMSO. Sections were kept frozen at −80°C until further processing. Sections were thawed, washed three times in phosphate-buffered saline (PBS) and placed in blocking buffer: PBS with 3% normal goat serum (NGS) and 0.3% Triton X-100 for 2 hours at 22°C. Sections were then incubated overnight at 4°C with the anti-Fos primary antibody (Ab-5; Calbiochem, EMD Biosciences, La Jolla, CA) diluted 1:10,000 in PBS containing 0.3% Triton X-100 and 2% NGS. Sections were washed again with PBS and incubated for 2 hr in biotinylated anti-rabbit secondary antibody (1:600), washed in PBS, and then incubated for 2 hours in avidin-biotin-peroxidase complex (ABC Elite kit, PK-6100; Vector Laboratories, Burlingame, CA). Finally, sections were washed and developed in 3, 3′-diaminobenzidine (DAB) for approximately 4 min, transferred into PBS and mounted onto chrom-alum/gelatin-coated slides. Once dry, the slides were dehydrated through a graded series of alcohol (70, 95, 95, 100, 100% ethanol) and cleared with Citrasolv (Fisher Scientific, Fairlawn, NJ) before coverslipping with Permount (Sigma, St. Louis, MO).
Bright-field images of brain sections were captured and digitized using a CCD camera (Coolsnap Photometrics, Roper Scientific Inc., Trenton, NJ) attached to a Zeiss Axioskop 2 light microscope. The size of the image captured from each section was 1.76 × 1.36 mm with a total area of 2.39 mm2. For analysis of the whole caudate-putamen and nucleus accumbens, Fos-immunoreactive nuclei were counted from the entire captured image. For analysis of nucleus accumbens core and shell subregions, Fos-immunoreactive nuclei were counted from smaller areas (0.62 × 0.41 mm with a total area of 0.25 mm2) of the same captured images. Fos-immunoreactive nuclei were counted using IPLab software for Macintosh (Scanalytics, Inc., Fairfax, VA). Image capture and quantification of Fos-positive nuclei were conducted by an observer blind to the experimental conditions. The pixel density threshold for detecting Fos-immunoreactive nuclei was chosen to match that of experimenter-determined counts from monitor images and microscope fields of view. Results were not significantly different when we compared the automated method of quantifying Fos-immunoreactive nuclei using IPLab with experimenter-determined counts by two different observers.
Double-labeling with Fos immunohistochemistry and enkephalin in situ hybridization
Double-labeling experiments were used to determine which types of medium spiny neurons express Fos following repeated amphetamine administration outside the home cage. Sections were obtained from rats in Experiment 2. The procedure for in situ hybridization is a modification of that used in Backman et al (2001) for free-floating sections. Sections frozen in cryopreservant solution were thawed and washed three times in PBS. Sections were then pretreated with 0.5% Triton X-100 for 10 minutes, given two washes in PBS, 0.2N HCl for 10 minutes, two washes in PBS, and 0.25% acetic anhydride in 0.5M triethanolamine, pH=8.0 for 10 minutes. Sections were then washed twice in PBS and dehydrated with 50%, 70%, 95%, and 100% ethanol before three final washes in PBS. Sections were incubated for 16 hours at 55°C with 4×107 cpm/ml of S35-labeled enkephalin riboprobe in hybridization solution containing 50% formamide, 0.62M NaCl, 5X Denhardt’s solution, 20mM PIPES buffer (pH=6.8), 10mM EDTA, 10% dextran sulfate, 250 μg/ml salmon sperm DNA, 250 μg/ml yeast transfer RNA, 0.2% SDS, and 50mM dithiothreitol. The riboprobe was transcribed with SP6 polymerase and S35-labeled UTP from a plasmid encoding the rat enkephalin gene (obtained from Dr. Steve Sabol, NIH, Bethesda, MD).
The next day, sections were rinsed for 30 minutes at 22°C with 2XSSC and 10mM β-mercaptoethanol before incubating them for 1 hour at 37°C in RNAse solution containing 5μg/ml RNAse A, 0.5M NaCl, 10mM Tris-Cl, pH=7.6, 10mM EDTA. Sections were then rinsed for 1 hour at 55°C in 0.5XSSC, 50% formamide, 10mM β-mercaptoethanol followed by 1 hour at 60°C in 0.1XSSC and 10mM β-mercaptoethanol. Sections were then washed three times in PBS before processing with Fos immunohistochemistry as described above.
After development of Fos-immunoreactivity, sections were mounted onto glass slides, air-dried, and exposed for 3 days to Hyperfilm MP (Amersham Biosciences, Piscataway, NJ) to confirm successful labeling. Slides were then dipped in NTB-2 photographic emulsion (Kodak Molecular Imaging Systems, New Haven, CT) with 0.2 M ammonium sulfate added, air-dried, and exposed for 3–5 weeks at 4°C with dessicant. Slides were developed for 2 minutes in Kodak D19 developer, rinsed in water, and fixed for 5 minutes in Kodak rapid fixer without hardener. Slides were counterstained with cresyl violet and dehydrated through a graded series of alcohol (70, 95, 95, 100, 100% ethanol) and cleared with Citrasolv (Fisher Scientific) before coverslipping with Permount (Sigma).
Bright-field images of each brain section were captured and digitized using the same microscope, camera, and software but using a 40X objective. Images from four areas of the nucleus accumbens, including core and shell subregions, and four areas of caudate-putamen were captured from coronal sections 2 mm anterior to Bregma. The size of each image was 0.4 × 0.34 mm with an area of 0.14 mm2. Two images were captured from each of these areas before and after ferricyanide treatment. The first images had both Fos-labeled nuclei and silver grains from in situ hybridization. High levels of silver grains from in situ hybridization labeling obscured some of the underlying Fos-IR in the sections. Thus silver grains were removed by oxidizing with ferricyanide using a modification of a recommended technique from Kodak Applied Sciences. The slides were soaked in Citrasolv to remove coverslips and rehydrated through 100, 95, 70% ethanol, and water. The slides were incubated for 2 minutes in 7.5% potassium ferricyanide and rinsed three times in water. The slides were then dehydrated and coverslipped as before and a second image of the same area was captured again. The number of Fos-labeled, enkephalin-labeled, and double-labeled neurons were hand-counted by an observer blind to the experimental conditions. Afterwards, the numbers obtained from the four images of nucleus accumbens and the four images of caudate-putamen were added together to produce total values for each brain region from each rat.
Data analysis
The behavior and histochemical data were analyzed using two-way analyses of variance (ANOVA) with Challenge (Amphetamine versus Saline) and Repeated administration (Amphetamine versus Saline) as the two main factors. Fisher’s PLSD post hoc tests were used to make specific group comparisons. Effects were considered significant when p < 0.05.
Results
Experiment 1: Repeated amphetamine administration outside the home cage enhanced psychomotor activation
Amphetamine-induced locomotor activity was significantly enhanced one week after repeated amphetamine administration in the locomotor activity chambers (Figure 1A; two-way ANOVA, effect of Challenge dose, F(3,55)=27.9, p<0.0001 and effect of Repeated administration, F(1,55)=20.2, p<0.0001). Repeated amphetamine administration significantly enhanced amphetamine-induced locomotor activity for 0.5, 1, and 2 mg/kg amphetamine challenge doses. Locomotor activity following saline challenge injections was not significantly different following repeated amphetamine versus repeated saline administration, which indicated that conditioned locomotion due to environmental exposure alone was not induced. Habituation prior to each drug injection has previously been shown to prevent this form of conditioned locomotion after repeated amphetamine administration (Crombag et al., 2001). Test injections of 1 and 2 mg/kg, but not 0.5 mg/kg, amphetamine significantly increased locomotor activity following repeated saline administration, while all doses of amphetamine significantly increased locomotor activity following repeated amphetamine administration.
Figure 1.
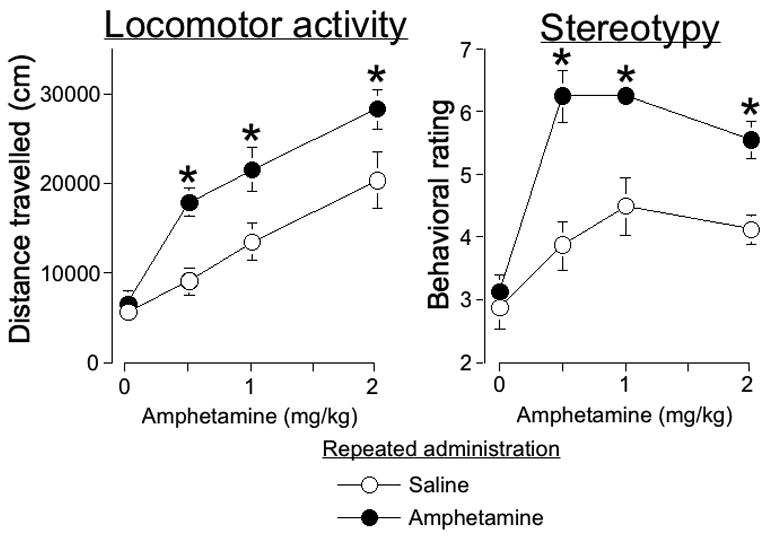
(A) Locomotor activity and stereotyped behavior are sensitized after repeated amphetamine administration in the locomotor activity chamber. Values for locomotor activity represent mean±SEM (n=7–8 per group) of distance traveled during 2 hours following challenge injections of different doses of amphetamine after repeated administration of amphetamine or saline. (B) Values for stereotyped behavior represent mean±SEM (n=7–8 per group) assessed during the 30-second interval 20 minutes following challenge injections. * indicates significantly more amphetamine-induced locomotor activity or stereotyped behavior for each challenge dose after repeated amphetamine versus repeated saline administration.
Following challenge injections, stereotypy was assessed every 4 minutes for 30 sec each time. Since amphetamine-induced stereotypy was most pronounced 20 minutes following challenge injections, we present data from only this time point in Figure 1. Amphetamine-induced stereotyped behavior was significantly enhanced one week after repeated amphetamine administration in the locomotor activity chambers (Figure 1B; two-way ANOVA, Challenge dose, F(3,55)=20.02, p<0.0001, Repeated administration, F(1,55)=36.5, p<0.0001, Challenge dose X Repeated administration, F(3,55)=3.5, p<0.05). Repeated amphetamine administration significantly enhanced amphetamine-induced stereotypy for 0.5, 1, and 2 mg/kg amphetamine challenge doses. All test doses of amphetamine injections significantly increased stereotypy following repeated saline administration and repeated amphetamine administration.
Repeated amphetamine administration outside the home cage enhanced amphetamine-induced Fos expression
Amphetamine challenge injections induced Fos expression in nucleus accumbens following repeated amphetamine administration outside the home cage (example image in Figure 2C). Similar to our previous studies (Crombag et al., 2002; Hope et al., 2006), we observed strongly labeled brown oval-shaped Fos-immunoreactive nuclei with negligible neuropil staining. Amphetamine-induced Fos expression was enhanced in the rostral nucleus accumbens after repeated amphetamine administration (Figure 3, top row; two-way ANOVA, effect of Challenge dose, F(3,51)=7.4, p<0.0005, Repeated administration, F(1,51)=8.3, p<0.01, Challenge dose X Repeated administration, F(3,51)=3.0, p<0.05). This effect, however, was dose-dependent as repeated amphetamine administration significantly enhanced drug-induced Fos expression at 1 and 2, but not 0.5 mg/kg amphetamine (post-hoc analysis).
Figure 2.
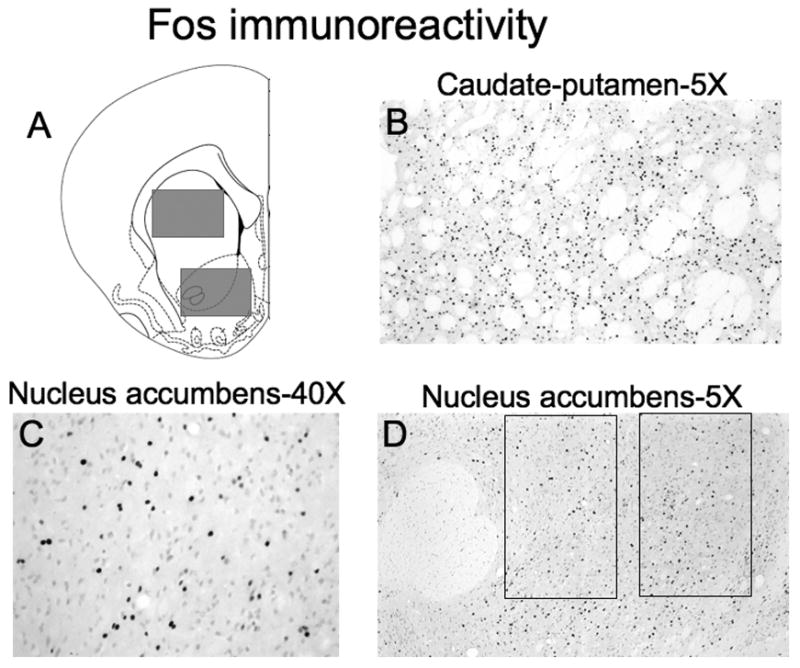
(A) Schematic representation of brain regions captured for image analysis. Images were captured from coronal sections approximately 2 and 1 mm anterior to Bregma for nucleus accumbens and approximately 2, 1, and 0 mm anterior to Bregma for caudate-putamen. Fos-IR nuclei were quantified from images of the regions indicated with gray rectangles. The size of each image was 1.76 × 1.36 mm with a total area of 2.39 mm2. Drawings of coronal section and coordinates were obtained from Paxinos and Watson (1998). Photomicrographs (with 5X objective) of amphetamine-induced Fos-IR nuclei in (B) caudate-putamen and (D) nucleus accumbens following repeated amphetamine administration. The large round area of white matter in the left-hand side of the nucleus accumbens image is the anterior commissure. The two rectangles in the nucleus accumbens image indicate core and shell subregions quantified and shown in Figure 4; the size of each rectangle was 0.62 × 0.41 mm with a total area of 0.25 mm2. (C) Higher magnification photomicrograph (with 40X objective) of nucleus accumbens.
Figure 3.
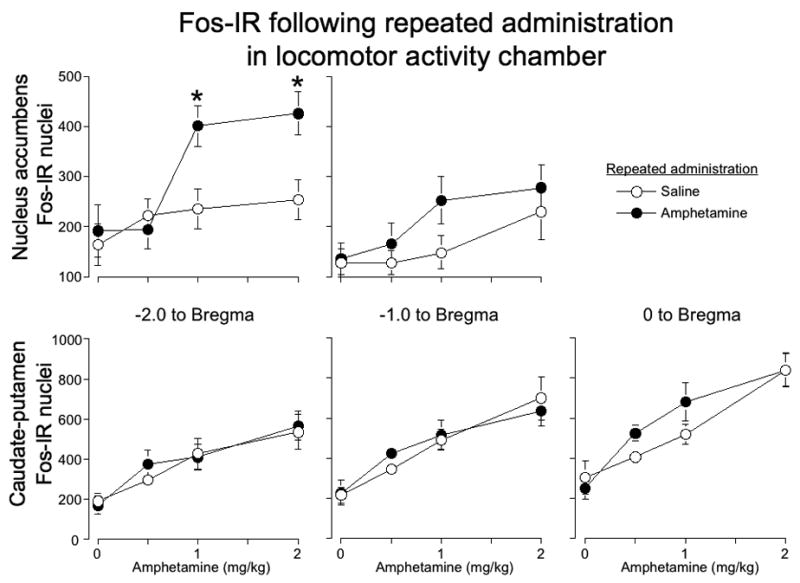
Amphetamine-induced Fos expression after repeated drug administration in the locomotor activity chamber. Graphs indicate the number of Fos-IR nuclei in nucleus accumbens (top row) and caudate-putamen (bottom row) at 2, 1, and 0 mm anterior to Bregma after repeated administration of amphetamine or saline. Values represent mean±SEM (n=6–8 per group). * indicates significantly more amphetamine-induced Fos-IR for each challenge dose after repeated administration of amphetamine versus saline.
We also analyzed the challenge effect separately for the repeated amphetamine and repeated saline groups. Challenge doses of 1 and 2, but not 0.5 mg/kg, amphetamine increased Fos expression following repeated amphetamine administration. None of the challenge doses increased Fos expression following repeated saline administration. Amphetamine challenge injections had a significant overall effect on Fos expression in the caudal nucleus accumbens as well (Figure 3, top row; effect of Challenge dose, F(3,51)=3.9, p<0.05), but there was merely a trend towards enhanced drug-induced Fos expression after repeated amphetamine administration (effect of Repeated administration, F(1,51)=3.3, p=0.07). We also analyzed the challenge effect in the caudal accumbens separately for repeated amphetamine and repeated saline groups. Challenge doses of 1 and 2, but not 0.5 mg/kg, amphetamine increased Fos expression following repeated amphetamine administration. None of the challenge doses increased Fos expression following repeated saline administration.
Amphetamine challenge injections induced Fos expression in caudate-putamen following repeated saline or repeated amphetamine administration outside the home cage (example image in Figure 2B). Amphetamine challenge injections significantly increased Fos expression in all three coronal planes of caudate-putamen (Figure 3 bottom row; effect of Challenge dose for rostral F(3, 52)=10.6; p<0.0001, for medial F(3, 55)=16.7; p<0.0001, for caudal F(3, 52)=21.4; p<0.0001). However, drug-induced Fos expression was not enhanced after repeated amphetamine administration. We analyzed the challenge effect separately for the repeated amphetamine and repeated saline groups. For all three coronal planes, 0.5, 1, and 2 mg/kg amphetamine increased Fos expression following repeated amphetamine, while only 1 and 2 mg/kg amphetamine increased Fos expression following repeated saline administration.
We further examined Fos-immunoreactive nuclei in core and shell subregions of nucleus accumbens (indicated by rectangles in Figure 2D). We assayed Fos-immunoreactive nuclei in only the rostral (−2.0 mm to Bregma) nucleus accumbens since amphetamine-induced Fos expression was significantly enhanced in only this rostral plane. In the core subregion, amphetamine-induced Fos expression was enhanced after repeated amphetamine administration (Figure 4A; two-way ANOVA, effect of Challenge dose, F(3,50)=5.1, p<0.005, Repeated administration, F(1,50)=5.0, p<0.05, the interaction was not significant). This effect, however, was dose-dependent as repeated amphetamine administration significantly enhanced drug-induced Fos expression at 1 and 2 mg/kg, but not 0.5 mg/kg amphetamine. We analyzed the challenge effect separately for the repeated amphetamine and repeated saline groups. Challenge doses of 1 and 2, but not 0.5 mg/kg, amphetamine increased Fos expression following repeated amphetamine administration. None of the challenge doses increased Fos expression following repeated saline administration.
Figure 4.
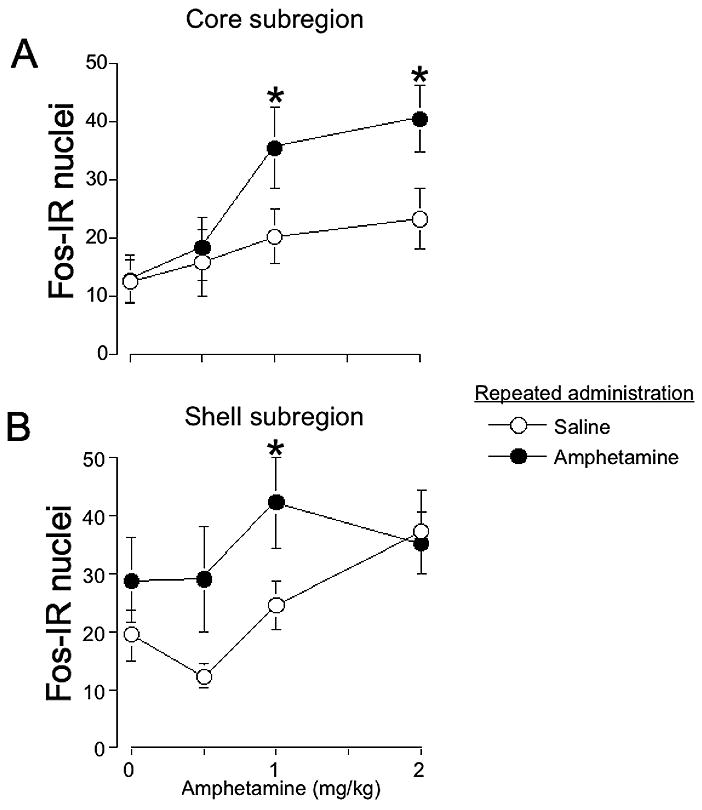
Amphetamine-induced Fos expression is enhanced in core and shell subregions after repeated amphetamine administration in the locomotor activity chamber. Graphs indicate the number of Fos-IR nuclei in core (A) and shell (B) 2 mm anterior to Bregma. Values represent mean±SEM (n=6–8 per group). * indicates significantly more amphetamine-induced Fos-IR for each challenge dose after repeated administration of amphetamine versus saline.
In the shell subregion, amphetamine-induced Fos expression was also enhanced after repeated drug administration (Figure 4B; two-way ANOVA, effect of Challenge dose, F(3,50)=2.8, p<0.05, Repeated administration, F(1,50)=5.6, p<0.05, the interaction was not significant). This effect, however, was dose-dependent as repeated amphetamine administration significantly enhanced drug-induced Fos expression at 1 mg/kg, but not 0.5 or 2 mg/kg amphetamine. We analyzed the challenge effect separately for the repeated amphetamine and repeated saline groups. None of the challenge doses of amphetamine increased Fos expression following repeated amphetamine administration. Only the challenge dose of 2 mg/kg amphetamine increased Fos expression following repeated saline administration. Overall, regulation of Fos expression in the core subregion appears similar to that observed for the whole rostral nucleus accumbens (Figure 3, nucleus accumbens at −2.0 mm to Bregma).
Experiment 2: Double-labeling with Fos histochemistry and enkephalin in situ hybridization
We used in situ hybridization for enkephalin mRNA along with Fos immunohistochemistry to identify which neurons in rostral nucleus accumbens and caudate-putamen expressed Fos after repeated saline and amphetamine administration in the locomotor activity chambers. Before ferricyanide treatment, enkephalin containing neurons were strongly labeled by tightly grouped clusters of black-colored silver grains, while Fos-immunoreactive nuclei were indicated by small brown ovals as shown before (Figure 5B,D). Silver grains were removed by treatment with ferricyanide to reveal underlying Fos-immunoreactive nuclei that appeared similar, although darker brown and slightly grayer, to that observed following single-labeling for Fos protein (Figure 5C,E). Double-labeling was performed in two batches. We compensated for differences in Fos staining between these batches by normalizing with the average numbers of Fos-labeled neurons in the repeated amphetamine-amphetamine challenge groups from each batch. Although we identified a number of significant differences between groups, the relatively low number of saline-challenged rats (n=4) made it difficult to obtain significant effects for certain comparisons, particularly interactions between challenge injections and repeated administration.
Figure 5.
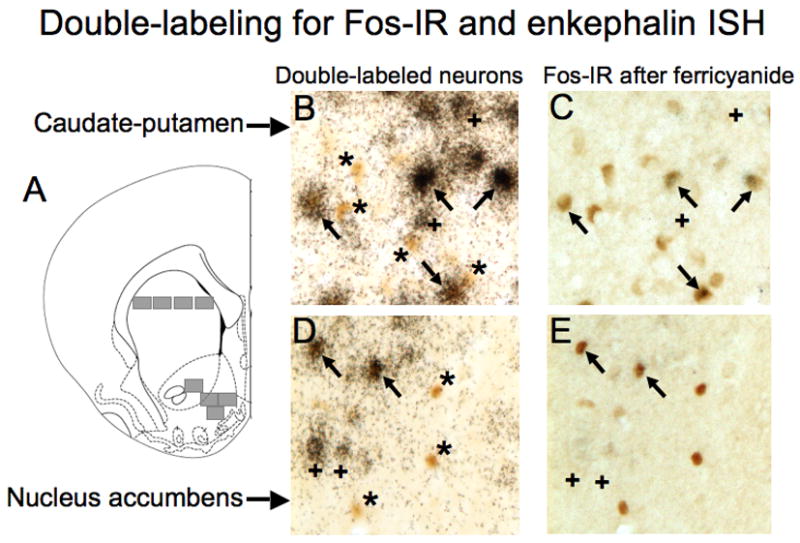
Representative images of Fos-immunoreactive nuclei expressed in enkephalin-positive and enkephalin-negative neurons in the caudate-putamen (B,C) and nucleus accumbens (D,E) after repeated amphetamine administration in the locomotor activity chamber. (A) Schematic representation of brain regions captured for image analysis. Images were captured from areas indicated with gray rectangles from coronal sections approximately 2 mm anterior to Bregma. The size of each image was 0.4 × 0.34 mm with a total area of 0.14 mm2. Drawings of coronal section and coordinates were obtained from Paxinos and Watson (1998). (B,D) Photomicrographs (400X) of sections double-labeled for Fos-immunoreactive nuclei (brown, oval objects) and enkephalin mRNA (clusters of black silver grains). (C,E) Photomicrographs (400X) of same sections after reduction of silver grains with ferricyanide. Arrows indicate enkephalin-positive Fos-immunoreactive neurons. Asterisks indicate enkephalin-negative Fos-immunoreactive neurons. Cross signs indicate enkephalin-positive Fos negative neurons.
In the nucleus accumbens, Fos was expressed in approximately equal numbers of enkephalin-positive and enkephalin-negative neurons: 32–63% of Fos-expressing neurons were enkephalin-positive. Amphetamine challenge injections significantly increased Fos expression in enkephalin-negative neurons (Figure 6A; F(1,22)=4.4, p<0.05). However, there was no indication that drug-induced Fos expression was further enhanced by repeated amphetamine administration. Meanwhile, amphetamine significantly decreased Fos expression in enkephalin-positive neurons of rats that received repeated saline administration (Figure 6B; F(1,10)=6.3; p<0.05). This acute amphetamine-induced decrease was not present in rats that received repeated amphetamine administration, largely because levels of Fos expression in amphetamine-challenged rats were significantly higher following repeated drug administration than following repeated saline administration (F(1,14)=5.5, p<0.05).
Figure 6.
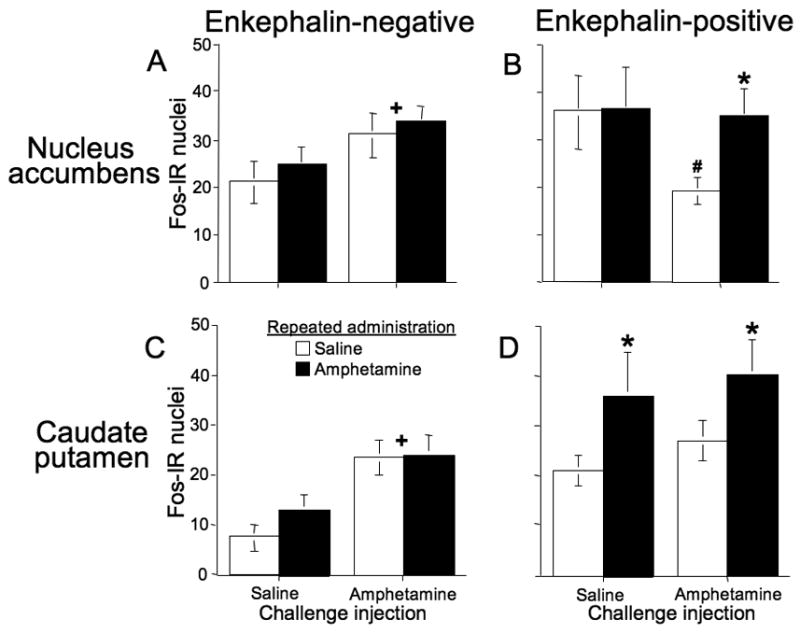
Levels of Fos expression in enkephalin-positive and enkephalin-negative neurons in the nucleus accumbens and caudate-putamen after repeated amphetamine administration in the locomotor activity chamber. For nucleus accumbens (A,B), asterisk indicates significantly more amphetamine-induced Fos-IR in enkephalin-positive neurons after repeated administration of amphetamine versus saline. Pound sign indicates that amphetamine challenge injections produced a significant decrease of Fos-IR in enkephalin-positive neurons after repeated saline administration. Cross sign indicates that amphetamine challenge injections significantly increased Fos-IR in enkephalin-negative neurons. For caudate-putamen (C,D), asterisks indicate significantly more Fos-IR after repeated administration of amphetamine versus saline. Cross signs indicate that amphetamine challenge injections significantly increased overall Fos-IR. Values represent mean±SEM (n=8 per group for amphetamine challenge injections; n=4 per group for saline challenge injections).
In the caudate-putamen, Fos was expressed more often in enkephalin-positive neurons than in enkephalin-negative neurons: 53–75% of Fos-expressing neurons were enkephalin-positive. Amphetamine challenge injections significantly increased Fos expression in enkephalin-negative neurons (Figure 6C; F(1,18)=11.4, p<0.005). However, there was no indication that drug-induced Fos expression was further enhanced by repeated amphetamine administration. Amphetamine challenge injections did not significantly affect Fos expression in enkephalin-positive neurons. However, repeated drug administration significantly enhanced overall Fos expression in these neurons (Figure 6D; F(1,18)=4.4, p<0.05).
Experiment 3: Repeated amphetamine administration in the home cage
When administered to rats in their home cages, challenge injections with 1 mg/kg amphetamine increased Fos expression in nucleus accumbens (Figure 7, top row; effect of Challenge for rostral F(1, 26)=29.2; p<0.0001, for caudal F(1, 26)=15.4; p<0.001). However, drug-induced Fos expression was not enhanced after repeated amphetamine administration in the home cage. Challenge injections of saline in the home cage induced much lower levels of Fos expression than that observed following saline injections in the locomotor chamber.
Figure 7.
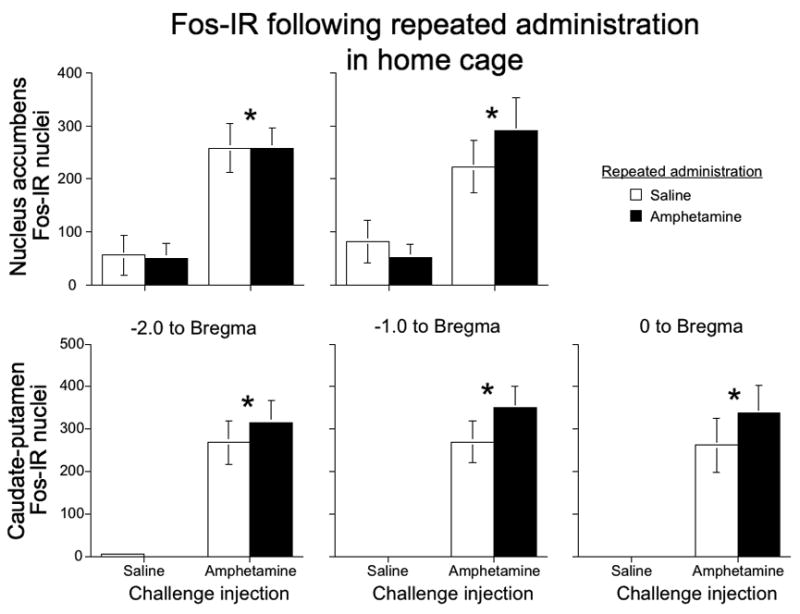
Amphetamine-induced Fos expression after repeated drug administration in the home cage. Graphs indicate the number of Fos-IR nuclei in nucleus accumbens (top row) and caudate-putamen (bottom row) at 2, 1, and 0 mm anterior to Bregma following challenge injections of 1 mg/kg amphetamine or saline after repeated administration of amphetamine or saline. Values represent mean±SEM (n=6–8 per group). * indicates significantly more Fos-IR induced by challenge injections of amphetamine; no significant differences between repeated administration of amphetamine and saline.
Similarly, challenge injections with 1 mg/kg amphetamine increased Fos expression in caudate-putamen (Figure 7, bottom row; effect of Challenge for rostral F(1, 27)=56.5; p<0.0001, for medial F(1, 27)=52.7; p<0.0001, and for caudal F(1, 27)=39.9; p<0.0001) sections, but again, drug-induced Fos expression was not enhanced after repeated amphetamine administration in the home cage. For both brain regions, challenge injections of saline in the home cage induced much lower levels of Fos expression than levels observed following saline injections in the locomotor chamber.
Discussion
Repeated amphetamine administration to rats outside their home cage (in the locomotor activity chambers) enhanced drug-induced Fos expression in nucleus accumbens, but not in caudate-putamen. Supporting the findings of Ostrander et al (2003), repeated amphetamine administration in our study did not enhance drug-induced Fos expression in the nucleus accumbens following the same 0.5 mg/kg amphetamine challenge dose used in their study. It is possible that neural activity is enhanced at lower challenge doses of amphetamine, but this enhancement can only be detected when the amphetamine dose is enough to induce high levels of c-fos mRNA and Fos protein. If Ostrander and colleagues had used 1 or 2 mg/kg amphetamine to induce c-fos mRNA, it is possible they also would have observed enhanced induction following repeated amphetamine administration. In contrast, when using 1 mg/kg amphetamine for test injections, repeated amphetamine administration in the home cage did not alter drug-induced Fos expression in nucleus accumbens or caudate-putamen. It is still possible that repeated amphetamine administration in the home cage could alter drug-induced Fos expression if different doses of amphetamine are used for test injections. Previous studies have observed attenuated drug-induced c-fos mRNA or Fos protein expression in both the nucleus accumbens and caudate-putamen 1–2 days following repeated amphetamine administration in the home cage (Cole et al., 1995; Jaber et al., 1995; Persico et al., 1993; Persico et al., 1995; Simpson et al., 1995; Turgeon et al., 1997). However, this attenuation of immediate early gene expression typically does not persist beyond 2 days (Persico et al., 1995) which is consistent with our finding of unaltered drug-induced expression 7 days after repeated drug administration in the home cage.
We previously found that the drug administration environment has similar effects on Fos expression following sensitization to cocaine. Repeated cocaine administration to rats outside their home cage enhanced drug-induced Fos expression in nucleus accumbens (Crombag et al., 2002; Todtenkopf et al., 2002), but not in caudate-putamen (Crombag et al., 2002; Todtenkopf et al., 2002; Uslaner et al., 2003a). In contrast, repeated cocaine administration in the home cage tends to attenuate drug-induced Fos expression in both nucleus accumbens and caudate-putamen (Ennulat et al., 1994; Hope et al., 1992; Jaber et al., 1995; Moratalla et al., 1996; Nye et al., 1995; Persico et al., 1993; Rosen et al., 1994; Steiner and Gerfen, 1993). Thus the effect of drug administration environment on drug-induced Fos expression generalizes to other psychostimulant drugs.
Amphetamine test injections in the home cage significantly increased levels of nucleus accumbens Fos expression following repeated saline administration, but did not significantly increase Fos expression when the drug was administered in the locomotor activity chamber. The lack of induction following repeated saline administration was due to higher levels of Fos expression following saline challenge injections in the locomotor activity chamber, which minimized the effect of acute amphetamine test injections. The higher basal levels of Fos expression following saline test injections are likely due to higher levels of novelty-associated arousal that increase excitatory input to the striatum in general (Ferguson and Robinson, 2004).
In our study, acute amphetamine administration induced Fos expression in both enkephalin-positive and enkephalin-negative medium spiny neurons in nucleus accumbens. Medium spiny neurons are GABAergic striatal projection neurons (Kita and Kitai, 1988) and constitute 90–95% of rat striatal neurons (Graveland and DiFiglia, 1985). In the caudate-putamen (reviewed in Gerfen, 1992), half of these medium spiny neurons produce dynorphin and substance P peptides, express primarily D1-type dopamine receptor mRNA, and project to midbrain dopaminergic neurons, while the other half of these neurons produce enkephalin, express primarily D2 dopamine receptor mRNA, and project to the pallidum. The nucleus accumbens is similarly organized (Fauchey et al., 2000; Kalivas et al., 1993; Le Moine and Bloch, 1995; Le Moine et al., 1991; Lu et al., 1998); but see (Meador-Woodruff et al., 1991) with the exception that D1/dynorphin/substance P neurons project equally to both the ventral pallidum and ventral tegmental area (Kalivas et al., 1993; Lu et al., 1997; Lu et al., 1998; Robertson and Jian, 1995).
Previous studies found that acute administration of amphetamine or cocaine to rats in their home cage induces c-fos or Fos primarily in D1-type medium spiny neurons (Badiani et al., 1999; Berretta et al., 1992; Berretta et al., 1993; Moratalla et al., 1996; Ruskin and Marshall, 1994; Uslaner et al., 2001b) while acute drug administration outside the home cage induces c-fos or Fos expression in both D1-type and D2-type medium spiny neurons in caudate-putamen (Badiani et al., 1999; Ferguson et al., 2003; Jaber et al., 1995; Uslaner et al., 2001b; Uslaner et al., 2003a). C-fos and Fos are also induced in both D1 and D2-type medium spiny neurons in rat caudate-putamen following repeated cocaine administration outside the home cage (Hope et al., 2006; Uslaner et al., 2003a).
Our present study extends these latter findings to Fos expression in both enkephalin-positive and enkephalin-negative neurons, presumably D2 and D1-type neurons, in nucleus accumbens following repeated amphetamine administration outside the home cage. To our knowledge, only Jaber et al 1995 previously used challenge injections of amphetamine outside the home cage after repeated drug administration to determine which cell types had altered drug-induced c-fos or Fos expression. Similar to our findings, Jaber et al. found approximately 50% of Fos-labeled neurons in caudate-putamen were enkephalin-positive. The slightly higher percentage (52–75%) of Fos induced in enkephalin-positive neurons in our study could be due to the fact that all drug injections were administered outside the home cage, rather than just the final test injection as in the Jaber et al. study. It is also noticeable that the percentage of amphetamine-activated enkephalin- or D2-positive neurons is consistently lower when c-fos in situ hybridization is employed rather than Fos immunohistochemistry (Ferguson and Robinson, 2004; Uslaner et al., 2001b; Uslaner et al., 2003b). First, c-fos in situ hybridization may require higher levels of neural activity than Fos immunohistochemistry for detection. If neural activation levels are slightly lower in enkephalin-positive neurons than in enkephalin-negative neurons, then c-fos would be detected less often than Fos in enkephalin-positive neurons and produce an overall appearance of a lower percentage of activated enkephalin-positive neurons. Second, Badiani, Robinson, and colleagues examined c-fos expression after only single administrations of amphetamine. Jaber et al. (1995) and our study have shown that the proportion of Fos-expressing neurons that are enkephalin-positive is higher following repeated drug administration than following a single administration of drug. Overall, it appears to be a general finding that amphetamine and cocaine induce Fos in both D1-type and D2-type neurons when the drug is injected outside the home cage, regardless of prior repeated drug administration.
The more important finding, however, is that repeated amphetamine administration increased Fos expression in enkephalin-positive neurons, but not in enkephalin-negative neurons, of the nucleus accumbens and caudate-putamen. The increase in nucleus accumbens Fos expression is partly due to amphetamine challenge injections producing a decrease of Fos expression in enkephalin-positive neurons following repeated saline administration. Decreased Fos expression in D2-type neurons is not entirely unexpected, since amphetamine-induced dopamine activates D2 receptors on these neurons, and D2 receptor agonists, such as quinpirole, have been shown to decrease levels of activity-dependent gene products, such as zif/268 (Gerfen et al., 1995; Keefe and Gerfen, 1995) and enkephalin (Angulo, 1992; Nisenbaum et al., 1994). Normally it is difficult to observe psychostimulant-induced decreases in c-fos or Fos expression because basal levels are already low when vehicle is administered to rats in their home cages. In the case of our study, the high basal levels of Fos expression when rats are injected outside the home cage (discussed above) allowed us to observe decreased Fos expression in the nucleus accumbens. However, the decrease in amphetamine-induced Fos expression in enkephalin-positive neurons is countered by increased expression in enkephalin-negative neurons; this gives the overall effect of no drug-induced Fos expression following repeated saline administration that was observed in our single-labeling experiments. Following repeated amphetamine administration, the lack of a drug-induced decrease of Fos expression in enkephalin-positive neurons allows increased Fos expression in enkephalin-negative neurons, leading to the overall effect observed in our single-labeling experiment of a drug-induced increase in Fos expression. The lack of congruence between double-labeling and single-labeling results in the caudate-putamen is more difficult to explain. It is possible that Fos expression in areas sampled in our single-labeling experiment, but not sampled in the much smaller areas sampled in our double-labeling experiments, countered the enhanced Fos expression we observed in enkephalin-positive neurons.
Amphetamine or cocaine-induced Fos expression is traditionally thought to be due to D1 receptor-dependent activation of the cAMP signal transduction pathway (Berretta et al., 1992; Robertson et al., 1991; Robertson et al., 1989; Stoof and Kebabian, 1981). Increased cAMP levels then activate PKA-dependent phosphorylation of the transcription factor CREB (Montminy and Bilezikjian, 1987) that induces c-fos transcription (Morgan and Curran, 1991; Sheng et al., 1990). However, amphetamine- and cocaine-induced Fos expression in D2-type neurons following drug administration outside the home cage cannot be mediated by activation of the cAMP pathway, since D2 receptors reduce cAMP signal transduction (Stoof and Kebabian, 1981). Amphetamine-induced Fos expression in D2-type neurons is more likely due to ERK/MAP kinase-dependent activation of CREB (Sgambato et al., 1998; Valjent et al., 2001; Vanhoutte et al., 1999) following strong glutamatergic activation of neural activity (Berretta et al., 1997; Berretta et al., 1999; Canales et al., 2002; Ferguson and Robinson, 2004; Fu and Beckstead, 1992; Gerfen et al., 2002; Liste et al., 1995; Parthasarathy and Graybiel, 1997; Sgambato et al., 1997; Sgambato et al., 1999). Indeed, we have previously shown that enhanced cocaine-induced activation of CREB following repeated cocaine administration in a novel environment is due to enhanced activation of ERK/MAP kinase, in the absence of altered PKA activation (Mattson et al., 2005). ERK/MAP kinase signaling may be similarly enhanced in D2-type neurons following amphetamine sensitization.
Amphetamine-induced locomotor activity and stereotyped behaviors are more strongly sensitized following repeated amphetamine administration in a novel environment than in the animal’s home cage (Badiani et al., 1995b; Badiani et al., 1997; Crombag et al., 1996; Crombag et al., 1999; Fraioli et al., 1999). At the same time, neuronal activity is increased in D2-type neurons by amphetamine injections in a novel environment, but not after amphetamine injections in the home cage. The addition of glutamatergic activation and neuronal activity in D2-type medium spiny neurons in nucleus accumbens may be an important factor underlying more robust sensitization following repeated drug administration in a novel environment. It should be noted, however, that psychomotor activity induced by the 0.5 mg/kg amphetamine challenge dose is sensitized while Fos expression at this challenge dose is not sensitized. This discrepancy is almost certainly due to differences in induction threshold, a common methodological issue encountered in most molecular and cellular assays. Fos is not sufficiently induced at 0.5 mg/kg amphetamine to show any differences in its expression. In any case, baclofen-muscimol inactivation of similar accumbens neurons following cocaine sensitization attenuated cocaine-induced locomotor activity, a fact which indicates that these neurons play an important role in mediating psychomotor behavior (Hope et al., 2006).
Acknowledgments
We thank Kristina Redmond for her technical assistance. This research was supported by the Intramural Research Program of the NIH, NIDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angulo JA. Involvement of dopamine D1 and D2 receptors in the regulation of proenkephalin mRNA abundance in the striatum and accumbens of the rat brain. J Neurochem. 1992;58:1104–9. doi: 10.1111/j.1471-4159.1992.tb09368.x. [DOI] [PubMed] [Google Scholar]
- Backman C, et al. Cellular mRNA expression of the transcription factor NGFI-B suggests a gene regulatory role in striatal opiate-peptide neurons. Brain Res. 2001;903:26–32. doi: 10.1016/s0006-8993(01)02332-0. [DOI] [PubMed] [Google Scholar]
- Badiani A, et al. The development of sensitization to the psychomotor stimulant effects of amphetamine is enhanced in a novel environment. Psychopharmacology (Berl) 1995a;117:443–52. doi: 10.1007/BF02246217. [DOI] [PubMed] [Google Scholar]
- Badiani A, et al. Influence of novel versus home environments on sensitization to the psychomotor stimulant effects of cocaine and amphetamine. Brain Res. 1995b;674:291–8. doi: 10.1016/0006-8993(95)00028-o. [DOI] [PubMed] [Google Scholar]
- Badiani A, et al. Enduring enhancement of amphetamine sensitization by drug-associated environmental stimuli. J Pharmacol Exp Ther. 1997;282:787–94. [PubMed] [Google Scholar]
- Badiani A, et al. Amphetamine-induced behavior, dopamine release, and c-fos mRNA expression: modulation by environmental novelty. J Neurosci. 1998;18:10579–93. doi: 10.1523/JNEUROSCI.18-24-10579.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A, et al. Environmental modulation of amphetamine-induced c-fos expression in D1 versus D2 striatal neurons. Behav Brain Res. 1999;103:203–9. doi: 10.1016/s0166-4328(99)00041-8. [DOI] [PubMed] [Google Scholar]
- Berretta S, et al. Local release of GABAergic inhibition in the motor cortex induces immediate-early gene expression in indirect pathway neurons of the striatum. J Neurosci. 1997;17:4752–63. doi: 10.1523/JNEUROSCI.17-12-04752.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta S, et al. Dopamine and glutamate agonists stimulate neuron-specific expression of Fos-like protein in the striatum. J Neurophysiol. 1992;68:767–77. doi: 10.1152/jn.1992.68.3.767. [DOI] [PubMed] [Google Scholar]
- Berretta S, et al. Neurochemically specialized projection neurons of the striatum respond differentially to psychomotor stimulants. Prog Brain Res. 1993;99:201–5. doi: 10.1016/s0079-6123(08)61347-3. [DOI] [PubMed] [Google Scholar]
- Berretta S, et al. Cortically driven Fos induction in the striatum is amplified by local dopamine D2-class receptor blockade. Eur J Neurosci. 1999;11:4309–19. doi: 10.1046/j.1460-9568.1999.00866.x. [DOI] [PubMed] [Google Scholar]
- Canales JJ, et al. Shifts in striatal responsivity evoked by chronic stimulation of dopamine and glutamate systems. Brain. 2002;125:2353–63. doi: 10.1093/brain/awf239. [DOI] [PubMed] [Google Scholar]
- Cole RL, et al. Neuronal adaptation to amphetamine and dopamine: molecular mechanisms of prodynorphin gene regulation in rat striatum. Neuron. 1995;14:813–23. doi: 10.1016/0896-6273(95)90225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, et al. The ability of environmental context to facilitate psychomotor sensitization to amphetamine can be dissociated from its effect on acute drug responsiveness and on conditioned responding. Neuropsychopharmacology. 2001;24:680–90. doi: 10.1016/S0893-133X(00)00238-4. [DOI] [PubMed] [Google Scholar]
- Crombag HS, et al. Signalled versus unsignalled intravenous amphetamine: large differences in the acute psychomotor response and sensitization. Brain Res. 1996;722:227–31. doi: 10.1016/0006-8993(96)00066-2. [DOI] [PubMed] [Google Scholar]
- Crombag HS, et al. Locomotor sensitization to cocaine is associated with increased Fos expression in the accumbens, but not in the caudate. Behav Brain Res. 2002;136:455–62. doi: 10.1016/s0166-4328(02)00196-1. [DOI] [PubMed] [Google Scholar]
- Crombag HS, et al. A comparison of two behavioral measures of psychomotor activation following intravenous amphetamine or cocaine: dose- and sensitization-dependent changes. Behav Pharmacol. 1999;10:205–13. doi: 10.1097/00008877-199903000-00009. [DOI] [PubMed] [Google Scholar]
- Ellinwood EH, Jr, Balster RL. Rating the behavioral effects of amphetamine. Eur J Pharmacol. 1974;28:35–41. doi: 10.1016/0014-2999(74)90109-5. [DOI] [PubMed] [Google Scholar]
- Ennulat DJ, et al. Persistent reduction of immediate early gene mRNA in rat forebrain following single or multiple doses of cocaine. Brain Res Mol Brain Res. 1994;26:106–12. doi: 10.1016/0169-328x(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Fauchey V, et al. Differential regulation of the dopamine D1, D2 and D3 receptor gene expression and changes in the phenotype of the striatal neurons in mice lacking the dopamine transporter. Eur J Neurosci. 2000;12:19–26. doi: 10.1046/j.1460-9568.2000.00876.x. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, et al. Amphetamine-evoked c-fos mRNA expression in the caudate-putamen: the effects of DA and NMDA receptor antagonists vary as a function of neuronal phenotype and environmental context. J Neurochem. 2003;86:33–44. doi: 10.1046/j.1471-4159.2003.01815.x. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Robinson TE. Amphetamine-evoked gene expression in striatopallidal neurons: regulation by corticostriatal afferents and the ERK/MAPK signaling cascade. J Neurochem. 2004;91:337–48. doi: 10.1111/j.1471-4159.2004.02712.x. [DOI] [PubMed] [Google Scholar]
- Fraioli S, et al. Susceptibility to amphetamine-induced locomotor sensitization is modulated by environmental stimuli. Neuropsychopharmacology. 1999;20:533–41. doi: 10.1016/S0893-133X(98)00079-7. [DOI] [PubMed] [Google Scholar]
- Fu L, Beckstead RM. Cortical stimulation induces fos expression in striatal neurons. Neuroscience. 1992;46:329–34. doi: 10.1016/0306-4522(92)90055-7. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, et al. D1 and D2 dopamine receptor function in the striatum: coactivation of D1- and D2-dopamine receptors on separate populations of neurons results in potentiated immediate early gene response in D1-containing neurons. J Neurosci. 1995;15:8167–76. doi: 10.1523/JNEUROSCI.15-12-08167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, et al. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2/MAP kinase. J Neurosci. 2002;22:5042–54. doi: 10.1523/JNEUROSCI.22-12-05042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveland GA, DiFiglia M. The frequency and distribution of medium-sized neurons with indented nuclei in the primate and rodent neostriatum. Brain Res. 1985;327:307–11. doi: 10.1016/0006-8993(85)91524-0. [DOI] [PubMed] [Google Scholar]
- Hope B, et al. Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc Natl Acad Sci U S A. 1992;89:5764–8. doi: 10.1073/pnas.89.13.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope BT, et al. Cocaine-induced locomotor activity and Fos expression in nucleus accumbens are sensitized for 6 months after repeated cocaine administration outside the home cage. Eur J Neurosci. 2006;24:867–75. doi: 10.1111/j.1460-9568.2006.04969.x. [DOI] [PubMed] [Google Scholar]
- Jaber M, et al. Acute and chronic amphetamine treatments differently regulate neuropeptide messenger RNA levels and Fos immunoreactivity in rat striatal neurons. Neuroscience. 1995;65:1041–50. doi: 10.1016/0306-4522(94)00537-f. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, et al. GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neuroscience. 1993;57:1047–60. doi: 10.1016/0306-4522(93)90048-k. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–44. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Keefe KA, Gerfen CR. D1-D2 dopamine receptor synergy in striatum: effects of intrastriatal infusions of dopamine agonists and antagonists on immediate early gene expression. Neuroscience. 1995;66:903–13. doi: 10.1016/0306-4522(95)00024-d. [DOI] [PubMed] [Google Scholar]
- Kelly PH, et al. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–22. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- Kita H, Kitai ST. Glutamate decarboxylase immunoreactive neurons in rat neostriatum: their morphological types and populations. Brain Res. 1988;447:346–52. doi: 10.1016/0006-8993(88)91138-9. [DOI] [PubMed] [Google Scholar]
- Le Moine C, Bloch B. D1 and D2 dopamine receptor gene expression in the rat striatum: sensitive cRNA probes demonstrate prominent segregation of D1 and D2 mRNAs in distinct neuronal populations of the dorsal and ventral striatum. J Comp Neurol. 1995;355:418–26. doi: 10.1002/cne.903550308. [DOI] [PubMed] [Google Scholar]
- Le Moine C, et al. Phenotypical characterization of the rat striatal neurons expressing the D1 dopamine receptor gene. Proc Natl Acad Sci U S A. 1991;88:4205–9. doi: 10.1073/pnas.88.10.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liste I, et al. Cortical stimulation induces Fos expression in striatal neurons via NMDA glutamate and dopamine receptors. Brain Res. 1995;700:1–12. doi: 10.1016/0006-8993(95)00958-s. [DOI] [PubMed] [Google Scholar]
- Lu XY, et al. Expression of D1 receptor mRNA in projections from the forebrain to the ventral tegmental area. Synapse. 1997;25:205–14. doi: 10.1002/(SICI)1098-2396(199702)25:2<205::AID-SYN11>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Lu XY, et al. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience. 1998;82:767–80. doi: 10.1016/s0306-4522(97)00327-8. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, et al. Cocaine-induced CREB phosphorylation in nucleus accumbens of cocaine-sensitized rats is enabled by enhanced activation of extracellular signal-related kinase, but not protein kinase A. J Neurochem. 2005;95:1481–94. doi: 10.1111/j.1471-4159.2005.03500.x. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, et al. Comparison of the distributions of D1 and D2 dopamine receptor mRNAs in rat brain. Neuropsychopharmacology. 1991;5:231–42. [PubMed] [Google Scholar]
- Montminy MR, Bilezikjian LM. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987;328:175–8. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Moratalla R, et al. Network-level changes in expression of inducible Fos-Jun proteins in the striatum during chronic cocaine treatment and withdrawal. Neuron. 1996;17:147–56. doi: 10.1016/s0896-6273(00)80288-3. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–51. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Nisenbaum LK, et al. Dopaminergic and muscarinic regulation of striatal enkephalin and substance P messenger RNAs following striatal dopamine denervation: effects of systemic and central administration of quinpirole and scopolamine. Neuroscience. 1994;63:435–49. doi: 10.1016/0306-4522(94)90541-x. [DOI] [PubMed] [Google Scholar]
- Nye HE, et al. Pharmacological studies of the regulation of chronic FOS-related antigen induction by cocaine in the striatum and nucleus accumbens. J Pharmacol Exp Ther. 1995;275:1671–80. [PubMed] [Google Scholar]
- Ostrander MM, et al. Environmental context and drug history modulate amphetamine-induced c-fos mRNA expression in the basal ganglia, central extended amygdala, and associated limbic forebrain. Neuroscience. 2003;120:551–71. doi: 10.1016/s0306-4522(03)00247-1. [DOI] [PubMed] [Google Scholar]
- Parthasarathy HB, Graybiel AM. Cortically driven immediate-early gene expression reflects modular influence of sensorimotor cortex on identified striatal neurons in the squirrel monkey. J Neurosci. 1997;17:2477–91. doi: 10.1523/JNEUROSCI.17-07-02477.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1998. [DOI] [PubMed] [Google Scholar]
- Persico AM, et al. Brain transcription factor expression: effects of acute and chronic amphetamine and injection stress. Brain Res Mol Brain Res. 1993;20:91–100. doi: 10.1016/0169-328x(93)90113-4. [DOI] [PubMed] [Google Scholar]
- Persico AM, et al. Brain transcription factor gene expression, neurotransmitter levels, and novelty response behaviors: alterations during rat amphetamine withdrawal and following chronic injection stress. Synapse. 1995;19:212–27. doi: 10.1002/syn.890190309. [DOI] [PubMed] [Google Scholar]
- Randrup A, Munkvad I. Stereotyped behavior. Pharmacol Ther [B] 1975;1:757–68. doi: 10.1016/0306-039x(75)90027-6. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Jian M. D1 and D2 dopamine receptors differentially increase Fos-like immunoreactivity in accumbal projections to the ventral pallidum and midbrain. Neuroscience. 1995;64:1019–34. doi: 10.1016/0306-4522(94)00426-6. [DOI] [PubMed] [Google Scholar]
- Robertson HA, et al. Expression of the immediate early gene c-fos in basal ganglia: induction by dopaminergic drugs. Can J Neurol Sci. 1991;18:380–3. doi: 10.1017/s0317167100032480. [DOI] [PubMed] [Google Scholar]
- Robertson HA, et al. D1-dopamine receptor agonists selectively activate striatal c-fos independent of rotational behaviour. Brain Res. 1989;503:346–9. doi: 10.1016/0006-8993(89)91689-2. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–98. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Rosen JB, et al. Differential induction of Fos protein and a Fos-related antigen following acute and repeated cocaine administration. Brain Res Mol Brain Res. 1994;25:168–72. doi: 10.1016/0169-328x(94)90295-x. [DOI] [PubMed] [Google Scholar]
- Ruskin DN, Marshall JF. Amphetamine- and cocaine-induced fos in the rat striatum depends on D2 dopamine receptor activation. Synapse. 1994;18:233–40. doi: 10.1002/syn.890180309. [DOI] [PubMed] [Google Scholar]
- Sgambato V, et al. Effect of electrical stimulation of the cerebral cortex on the expression of the Fos protein in the basal ganglia. Neuroscience. 1997;81:93–112. doi: 10.1016/s0306-4522(97)00179-6. [DOI] [PubMed] [Google Scholar]
- Sgambato V, et al. Effect of a functional impairment of corticostriatal transmission on cortically evoked expression of c-Fos and zif 268 in the rat basal ganglia. Neuroscience. 1999;93:1313–21. doi: 10.1016/s0306-4522(99)00267-5. [DOI] [PubMed] [Google Scholar]
- Sgambato V, et al. Extracellular signal-regulated kinase (ERK) controls immediate early gene induction on corticostriatal stimulation. J Neurosci. 1998;18:8814–25. doi: 10.1523/JNEUROSCI.18-21-08814.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, et al. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron. 1990;4:571–82. doi: 10.1016/0896-6273(90)90115-v. [DOI] [PubMed] [Google Scholar]
- Simpson JN, et al. Repeated amphetamine administration induces a prolonged augmentation of phosphorylated cyclase response element-binding protein and Fos-related antigen immunoreactivity in rat striatum. Neuroscience. 1995;69:441–57. doi: 10.1016/0306-4522(95)00274-m. [DOI] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Cocaine-induced c-fos messenger RNA is inversely related to dynorphin expression in striatum. J Neurosci. 1993;13:5066–81. doi: 10.1523/JNEUROSCI.13-12-05066.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoof JC, Kebabian JW. Opposing roles for D-1 and D-2 dopamine receptors in efflux of cyclic AMP from rat neostriatum. Nature. 1981;294:366–8. doi: 10.1038/294366a0. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, et al. Withdrawal duration differentially affects c-fos expression in the medial prefrontal cortex and discrete subregions of the nucleus accumbens in cocaine-sensitized rats. Neuroscience. 2002;114:1061–9. doi: 10.1016/s0306-4522(02)00272-5. [DOI] [PubMed] [Google Scholar]
- Turgeon SM, et al. Enhanced CREB phosphorylation and changes in c-Fos and FRA expression in striatum accompany amphetamine sensitization. f. Brain Res. 1997;749:120–6. doi: 10.1016/s0006-8993(96)01316-9. [DOI] [PubMed] [Google Scholar]
- Uslaner J, et al. Environmental context modulates the ability of cocaine and amphetamine to induce c-fos mRNA expression in the neocortex, caudate nucleus, and nucleus accumbens. Brain Res. 2001a;920:106–16. doi: 10.1016/s0006-8993(01)03040-2. [DOI] [PubMed] [Google Scholar]
- Uslaner J, et al. Amphetamine and cocaine induce different patterns of c-fos mRNA expression in the striatum and subthalamic nucleus depending on environmental context. Eur J Neurosci. 2001b;13:1977–83. doi: 10.1046/j.0953-816x.2001.01574.x. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, et al. Cocaine-induced psychomotor activity is associated with its ability to induce c-fos mRNA expression in the subthalamic nucleus: effects of dose and repeated treatment. Eur J Neurosci. 2003a;17:2180–6. doi: 10.1046/j.1460-9568.2003.02638.x. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, et al. Amphetamine-induced c-fos mRNA expression in the caudate-putamen and subthalamic nucleus: interactions between dose, environment, and neuronal phenotype. J Neurochem. 2003b;85:105–14. doi: 10.1046/j.1471-4159.2003.01646.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, et al. Mitogen-activated protein kinase/extracellular signal-regulated kinase induced gene regulation in brain: a molecular substrate for learning and memory? Mol Neurobiol. 2001;23:83–99. doi: 10.1385/MN:23:2-3:083. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P, et al. Glutamate induces phosphorylation of Elk-1 and CREB, along with c-fos activation, via an extracellular signal-regulated kinase-dependent pathway in brain slices. Mol Cell Biol. 1999;19:136–46. doi: 10.1128/mcb.19.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. Brain mechanisms of drug reward and euphoria. Psychiatr Med. 1985;3:445–60. [PubMed] [Google Scholar]


