Abstract
Background
Over 180 different types of therapy have been used in the treatment and management of painful bladder syndrome/interstitial cystitis (PBS/IC), yet evidence from clinical trials remains inconclusive. This study aimed to evaluate the efficacy of pharmacological approaches to PBS/IC, quantify the effect size from randomized controlled trials, and begin to inform a clinical consensus of treatment efficacy for PBS/IC.
Methods
We identified randomized controlled trials for the pharmacological treatment of PBS/IC patients diagnosed on the basis of NIDDK or operational criteria. Study limitations include considerable patient heterogeneity as well as variability in the definition of symptoms and in outcome assessment.
Results
We included a total of 1470 adult patients from 21 randomized controlled trials. Only trials for pentosan polysulfate had sufficient numbers to allow a pooled analysis of effect. According to a random-effects model, the pooled estimate of the effect of pentosan polysulfate therapy suggested benefit, with a relative risk for patient-reported improvement in symptoms of 1.78 (95% confidence interval, 1.34 – 2.35). This result was not heterogeneous (p= 0.47) and was without evidence of publication bias (p= 0.18). Current evidence also suggests efficacy of DMSO and amitryptiline. Hydroxyzine, intravesical BCG and RTX failed to demonstrate efficacy, but evidence was inconclusive due to methodological limitations.
Conclusions
Pentosan polysulfate may be modestly beneficial for symptoms of PBS/IC. There is insufficient evidence for other pharmacological treatments. A consensus on standardized outcome measures is urgently needed.
INTRODUCTION
Painful bladder syndrome/interstitial cystitis (PBS/IC) is a poorly defined clinical condition characterized by three key symptoms: pelvic pain, urinary urgency, and frequency.1 These symptoms significantly overlap with those of other common conditions and are not associated with any known pathognomonic tissue, serum, or urine changes. PBS/IC is therefore primarily a diagnosis of exclusion.2
Further complicating diagnosis is a lack of a standard definition for the condition. In 1915, for example, Hunner described a form of bladder ulceration later designated as “classic IC”.3 In 1978, Messing and Stamey proposed that glomerulations apparent with bladder distension were diagnostic of IC in the absence of Hunner’s ulcerations (i.e., nonulcer IC).4 Most recently, in 1987, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) attempted to unify these contrasting approaches by developing a list of criteria to define IC.5 The NIDDK criteria, however, miss an estimated 60% of patients and, due to the criteria’s restrictiveness, are currently recommended for research use only.6 Moreover, Waxman7 and others8, 9 have called into question the specificity of the criteria by finding that 75% of healthy women show glomerulations even in the absence of symptoms. The term Painful Bladder Syndrome is now often used to describe the broader spectrum of patients who meet a more inclusive, symptom-based definition without the typical cystoscopic and histological features traditionally used to distinguish interstitial cystitis.2, 10
Prevalence estimates are highly variable, depending on what diagnostic criteria the epidemiologist is using. A number of surveys applying different methodologies have found IC incidence ranging from 1.6 per 100,000 women11 to 158 per 100,000 women.12 Self-reporting as part of the National Household Interview Survey found a rate of 450 per 100,000,13 and three studies that used O’Leary-Sant scores found a prevalence of approximately 300 per 100,000.14–16
Treatment and management approaches vary widely. As of 1997, 183 different types of dietary, interventional, pharmacologic, and behavioral therapies had been used.17 This diversity continues to be reflected within the broad range of pharmacologic agents currently applied to the condition.
Our aim was to synthesize and critically evaluate data from a wide range of current pharmacological approaches to PBS/IC, to quantify the effect size from randomized controlled trials, and begin to inform a clinical consensus of treatment efficacy for PBS/IC.
EVIDENCE ACQUISITION AND SYNTHESIS
Search strategy
A search strategy was developed for the purposes of the present review. The following databases were searched: PubMed (1966–2007), EMBASE (1988–2007), CINAHL (1982–2007), Healthstar (1975–2000), Current Contents (2000–2007), Web of Science (1980–2007), PsychInfo (1967–2007), Science Citation Indexes (1996–2007), and Cochrane Collaboration Reviews (1993–2007). The exploded Medical Subject Headings interstitial cystitis and painful bladder syndrome were combined with truncated keywords that described the type of publication, such as random, double–blind, random allocation, placebo, clinical trial, and comparative study and were limited to English–language studies in humans. Additional studies were identified through a manual search of the bibliographies of retrieved articles, recent reviews, monographs, and the Interstitial Cystitis Task Force—an NIH/NIDDK initiative on the epidemiology and definition of PBS/IC.1
Inclusion criteria
Articles on clinical trials were included if they met all of the following six inclusion criteria:18 a controlled clinical trial involving the pharmacologic treatment of PBS/IC; study population of adult patients; administration of a pharmacologic intervention to more than 10 patients; inclusion of a control group that received placebo therapy for PBS/IC; outcome measures of global status or individual PBS/IC symptoms (or both); and use of a randomized, double–blind, parallel–group or crossover design.
Data extraction
The study characteristics, patient demographic information, enrollment criteria, therapy allocation, adverse effects, outcomes, and reasons for dropout were extracted independently by two reviewers. We focused on the efficacy of treatment for PBS/IC compared with placebo or active controls. Continuous measures included assessment of specific symptoms (pain, frequency and urgency) as well as O’Leary-Sant Interstitial Cystitis Symptom and Problem Index scores (OLS-SI and OLS-PI).19 Our dichotomous measure was patient-reported global improvement with treatment. Given the large placebo effect seen in IC trials, we provide only qualitative information about RCTs.
Quantitative assessment
Only trials for treatment of PBS/IC with pentosan polysulfate (PPS) had sufficient numbers to allow a pooled analysis of effect using a random-effects model.20 Heterogeneity was assessed using the Q and I2 statistic and publication bias was assessed using the Egger’s test.21 For the remaining treatment modalities, we decided not to attempt to pool the data because of the wide variety of designs; small sample sizes; many different treatments, with few studies on each specific treatment; broad classes of medications, raising the question of whether drugs within these broad classes can be pooled; different modes of drug administration (oral vs. intravesical); and considerable variation in the reporting of statistical details, such as exact P values and standard deviations.
For all trials, an attempt was made to abstract the data as a standardized mean difference. This produces measures of effect for each treatment trial on a similar metric. By convention, these standardized mean differences, also known as effect sizes, are considered small if less than 0.2, moderate if between 0.5 to 0.8 and large if greater than 0.8.22 For many trials, this was not possible and the studies were classified simply as positive or negative, in terms of efficacy, for that outcome.
For some study-specific characteristics, such as duration of studies or number of patients included, the Student t–test was used to compare continuous variables, and the chi–square test to compare binary variables between certain study subgroups. The Mann-Whitney U-test was used to compare median sample sizes between positive and negative studies and between high and low quality studies as sample sizes were skewed.
RESULTS
Out of 278 trials identified using our search criteria, 21 RCTs met the requirements for inclusion in our final analysis.. Those excluded (n = 257) did not address treatment of PBS/IC or did not report global or symptom–specific outcomes (n = 77); did not use a randomized, double–blind, placebo–controlled design (n = 55); included patients without a diagnosis of PBS/IC (n = 57); were incomplete or duplicate publications (n = 7); were not published in English (n = 34); or involved fewer than 10 patients (n = 27).
The 21 randomized controlled trials (RCTs) we analyzed are shown in table 1. A single agent was evaluated in 18 RCTs, and a combination of two agents were evaluated in 3trials. The 21 trials spanned 1987 to 2006, reporting on a total of 1470 adult patients. The studies averaged 70 patients enrolled (range 16–265), with ages ranging from 18 to 80 years (mean, 46.87 years); 90% were women. Eleven RCTs (52%) were conducted in North America and 10 (48%) in Europe. All studies were conducted in urological settings, either single (63%) or multiple (37%) practices. Three trials (14%) were published before 1989, 6 (29%) between 1990 and 1999, and 12 (57%) between 2000 and 2007.
Table 1.
RCTs for the Treatment of Painful Bladder Syndrome/Interstitial Cystitis*
| Study (Year) | N | Design | Mean Age |
Women,% | Duration in weeks |
NIDDK Criteria |
Symptom Outcomes SMD (95% CI) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Symptom Index |
Pain | Urgency | Frequency | |||||||
| Amitriptyline | ||||||||||
| van Ophoven34 (2004) | 50 | Parallel | 55 | 88% | 16 | Yes | −0.77 (−1.36, −0.18) | 1.12 ( 0.51, 1.73) | −2.61 (−3.38, −1.83) | −0.62 (−1.2, −0.04) |
| Antibiotics | ||||||||||
| Warren43(2000) | 50 | Parallel | 52 | 90% | 18 | Yes | NR | − | − | 0.15 (−0.68, 0.97) |
| Bacillus Calmette-Guerin (BCG) – Intravesical | ||||||||||
| Peters38 (1997) | 33 | Parallel | 42 | 100% | 6 | Yes | NR | −0.63 (−1.36, 0.11) | − | −0.4 (−1.17, 0.28) |
| Peeker32 (2000) | 21 | Cross over | 51 | 95% | 12 | Yes | NR | −0.13 (−0.74, 0.48) | − | −0.45 (−1.1, 0.17) |
| Mayer39 (2005) | 265 | Parallel | 48 | 82% | 6 | No† | −0.18 (−0.42, 0.07) | 0.22 (−0.02, 0.46) | −0.28 (−0.52, −0.04) | −0.13 (−0.38, 0.11) |
| Cimetidine | ||||||||||
| Thilagarajah42 (1998) | 36 | Parallel | 42 | 97% | 12 | No‡ | NR | −0.71 (−1.38,−0.03) | −0.35 (−1.02, 0.3) | −0.20 (−0.86, 0.46) |
| Cyclosporine | ||||||||||
| Sairanen29 (2005) | 64 | Parallel | 59 | 83% | 24 | Yes | −1.87 (−2.48, −1.25) | −1.34 (−1.92, −0.77) | + | −1.42 (−2.0, −0.85) |
| Dimethylsulfoxide (DMSO) – intravesical | ||||||||||
| Perez– Marrero33 (1988) | 33 | Cross Over | 48 | 91% | 8 | Yes | NR | + | + | − |
| Peeker32 (2000) | 21 | Cross Over | 51 | 95% | 12 | Yes | NR | −0.92 (−1.56, −0.29) | NR | −0.80 (−1.43, −0.17) |
| Hydroxyzine | ||||||||||
| Sant28 (2003) | 121 | Parallel | 45 | 89% | 24 | No† | −0.14 (−0.64, 0.36) | −0.28 (−0.78, 0.22) | 0.18 (−0.32, 0.68) | −0.11 (−0.61, 0.39) |
| L–Arginine | ||||||||||
| Korting44 (1999) | 53 | Parallel | 49 | 100% | 12 | Yes | −0.33 (−0.93, 0.27) | −0.60 (−1.19, −0.01) | −0.52 (−1.14, 0.08) | −0.36 (−0.96, 0.24) |
| Cartledge45 (2000) | 16 | Parallel | 51 | 75% | 4 | Yes | −0.13 (−0.82, 0.57) | NR | NR | 0.85 (0.13, 1.58) |
| Oxybutynin – intravesical | ||||||||||
| Barbalias46 (2000) | 36 | Parallel | 45 | 100% | 18 | NR | + | NR | NR | −1.59 (−2.4, −0.80) |
| Oxygen, hyperbaric | ||||||||||
| van Ophoven47 (2006) | 21 | Parallel | 65 | 100% | 36 | Yes | −0.93 (−1.89, 0.02) | −1.08 (−2.05, −0.11) | −0.47 (−1.38, 0.46) | −0.73 (−1.67, 0.21) |
| Pentosan Polysulfate | ||||||||||
| Holm– Bentzen24 (1987) | 115 | Parallel | 57 | 90% | 16 | Yes | NR | − | − | NR |
| Parsons26 (1987) | 75 | Cross Over | NR | 75% | 16 | Yes | NR | −0.74 (−1.3, −0.14) | −0.62 (−1.14, −0.10) | −0.51 (−1.06, 0.03) |
| Mulholland 25 (1990) | 110 | Parallel | 44 | NR | 12 | No || | NR | −0.15 (−0.53, 0.22) | − | NR |
| Parsons27 (1993) | 148 | Parallel | 43 | 97% | 12 | No || | NR | + | + | NR |
| Sant28 (2003) | 121 | Parallel | 45 | 89% | 18 | No† | −0.26 (−0.77, 0.25) | −0.11 (−0.62, 0.40) | 0.06 (−0.45, 0.56) | 0.06 (−0.45, 0.57) |
| Sairanen 29 (2005) | 64 | Parallel | 59 | 83% | 24 | Yes | −0.89 (−1.50, −0.28) | −0.49 (−1.01, 0.04) | NR | −0.27 (−0.85, 0.31) |
| Pentosan Polysulfate – Intravesical | ||||||||||
| Bade48 (1997) | 20 | Parallel | 51 | 100% | 12 | Yes | NR | NR | − | −0.30 (−1.19, 0.58) |
| Resiniferatoxin (RTX)– intravesical | ||||||||||
| Lazzeri49 (2000) | 18 | Parallel | 41 | 37% | Single application | Yes | NR | −4.01 (−5.67, −2.34) | 1.10 (0.10, 2.10) | −1.57 (−2.64, −0.50) |
| Chen50 (2005) | 22 | Parallel | 44 | 77% | Single application | Yes |
0.05 ugm
−0.27(−1.44, 0.89) 0.10 ugm −0.69 (−1.93, 0.55) |
0.05 ugm
0.05 (−1.15, 1.25) 0.10 ugm −0.32 (−1.48, 0.85) |
0.05 ugm
−0.26 (−1.43, 0.90) 0.10 ugm −0.84 (−2.10, 0.41) |
0.05 ugm
−0.19 (−1.35, 0.97) 0.10 ugm −0.68 (−1.92, 0.56) |
| Payne51 (2005) | 163 | Parallel | 47 | 86 | Single application | Yes | − |
0.01 ugm
−0.33 (−0.98, 0.33) 0.05 ugm : −0.26 (−0.91, 0.39) 0.10 ugm −0.52, −1.18,0.39) |
0.01 ugm
−0.20(−0.85, 0.45) 0.05 ugm −0.05 (−0.70, 0.60) 0.10 ugm 0.12 (−0.53, 0.77) |
0.01 ugm
0.00 (−0.42, 0.42) 0.05 ugm 0.06 (−0.37, 0.48) 0.10 ugm 0.19 (−0.25, 0.64) |
Abbreviations:
NR: Not Collected,
Reported as not effective, data not extractable,
Reported as effective, data not extractable
urodynamics not required,
urodynamics not required, chronic inflammation on bladder biopsy required, which is not part of the NIDDK criteria,
cystoscopy and urodynamics not required,
urodynamics not required, capacity under anesthesia <800 ml required, which is not part of the NIDDK criteria.
Patient population
Seventeen RCTs used the 1987 NIH/NIDDK Research Criteria for diagnosing IC (Table 1). Four studies based the diagnosis on operational criteria. All trials reported an adequate work–up to exclude organic disease, including history, physical examination, laboratory, radiologic, and cystoscopic evaluation.
Study design
Of the 21 RCTs, 17 employed parallel and 4 a crossover design. Length of the intervention ranged from a single treatment procedure to 36 weeks of treatment, with a mean of 15 and a median of 12 weeks. Symptom severity, as assessed within each individual trial, was similar at baseline between the intervention and control groups in all of the parallel RCTs. Treatment adherence was reported in only 4 RCTs and was measured by pill counts or patient interview. Adherence was similar between the intervention and control groups in these 4 trials, although actual adherence rates were not provided. Co–interventions, such as concurrent use of other medications to relieve IC symptoms and dietary changes during the intervention period, were assessed in 4 of the RCTs. In these 4 trials, patients were simply advised to avoid use of other medications. Despite relatively short trials with few patients enrolled, none of the trials with negative outcomes reported power analyses.
Outcome assessment
Both global as well as individual symptom improvement was reported in all of the studies. The definition of symptoms, such as pain, urgency and frequency, varied considerably across the trials. Data were collected by a daily voiding diary maintained by the patient. A standardized symptom questionnaire (OLS19) was used in 11 RCTs and was reportedly validated in 1 trial.23
Treatment efficacy
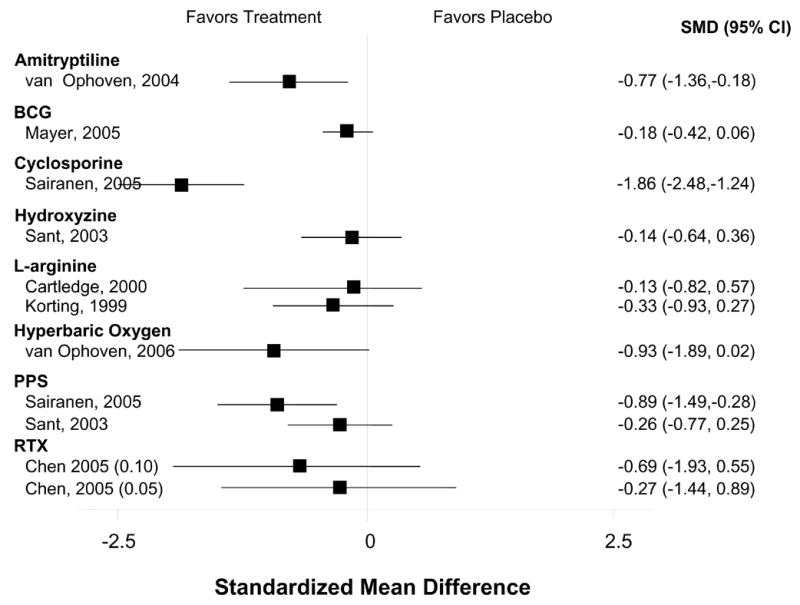
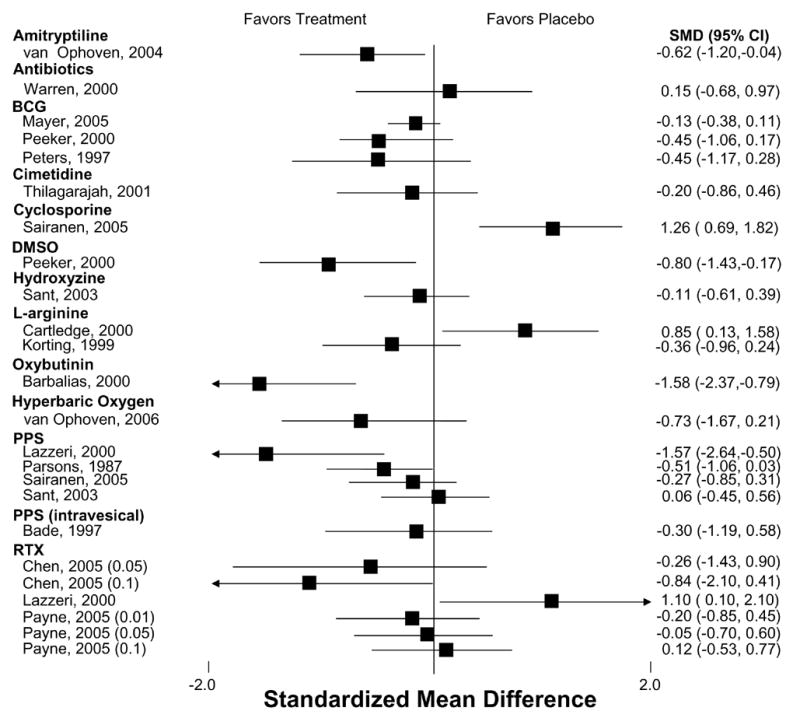
Table 1 shows the evidence for treatment efficacy of each pharmacologic agent in their respective RCT(s). Outcomes most frequently assessed—pain, urgency, frequency, and the OLS-SI—are itemized for each trial. The specific symptoms assessed and the measures used varied considerably among the different studies. Therefore, we viewed global improvement as the common metric across treatments which was usually reported as the number of patients reporting self-improvement in each group. The mean frequency of global improvement was 19% (range, 4% to 40%) among control groups and 49% (range, 28% to 89%) among treatment groups for all RCTs that reported this outcome. The effect size for the magnitude of improvement for pain, frequency, urgency and the OLS-SI among the studies reporting these outcomes was generally small (Figures 1–4).
Figure 1.
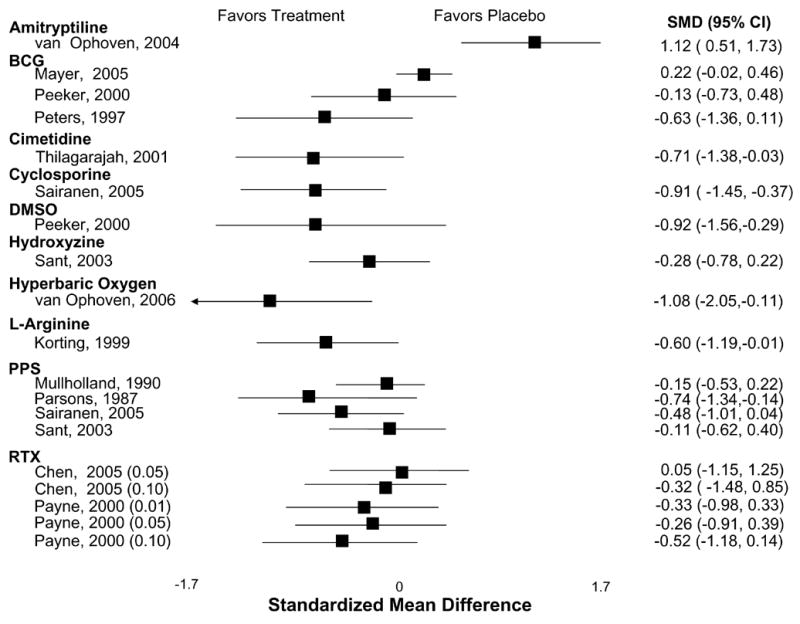
Effect on Patient-Reported Pain
Figure 4.
Effect on Patient-Reported O’Leary IC Symptom Index
Findings on specific agents
Six RCTs,24–29 and one meta-analysis30 examined treatment with oral pentosan polysulfate (PPS) (Table 1, Figs 1–5). The reported overall response rate varied between 15–67% at the 300 mg FDA-recommended dose. An industry-sponsored dose-ranging three-arm study comparing 300 mg, 600 mg and 900 mg failed to show dose-related efficacy; duration of administration was more important than dosage itself, although side-effects were dose-related.31 The most rigorous NIDDK-supported trial by the Interstitial Cystitis Clinical Trials Group (ICCGT) failed to demonstrate superiority of PPS over placebo although the study was underpowered.28 Our pooled analysis (Figure 5) suggested benefit, with a relative risk for patient-reported improvement in symptoms of 1.78 (95% CI: 1.34 – 2.35). This result was not heterogeneous (Q= 3.53, I2 = 0%, p= 0.47) and was without evidence of publication bias (Egger’s p= 0.18).
Figure 5.
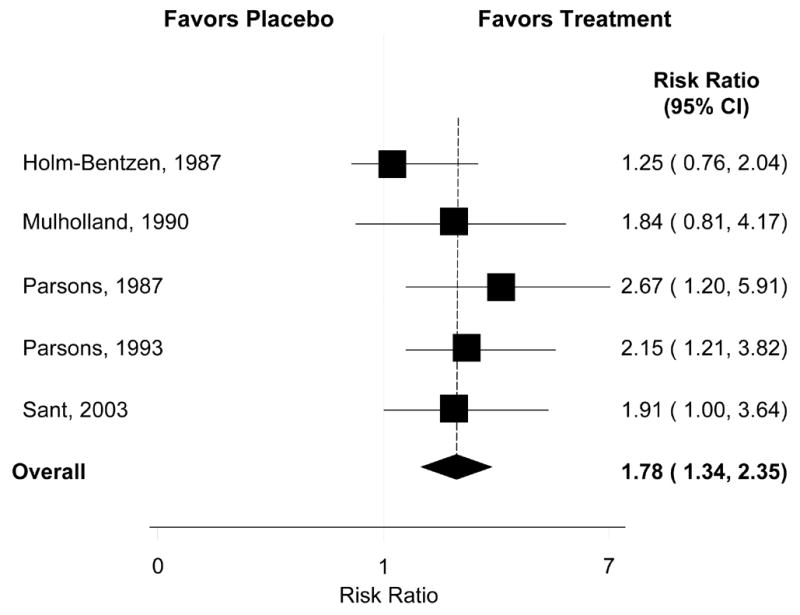
Relative Risk of Overall Improvement with PPS treatment
Intravesical 50% DMSO, the only FDA-approved intravesical treatment for IC, proved beneficial in two crossover RCTs.32, 33 One trial demonstrated a 93% objective improvement and 53% subjective improvement compared with 35% and 18%, respectively, for saline solution.33 Symptom alleviation has been demonstrated in up to 80% of patients with the usual treatment schedule of 6 weekly bladder instillations of 50% DMSO, followed by maintenance therapy every 2–4 weeks and then every 2–3 months. One important caveat here is that saline cannot really be considered an appropriate “placebo” in DMSO trials since the latter possesses prominent side effects (taste and smell). Furthermore, in these early studies, DMSO was administered once every two weeks rather than weekly, as is usually the case today.
Amitriptyline, a tricyclic antidepressant, provided symptomatic relief for 15 out of 24 patients in one RCT, although the study did not provide details regarding the use of active or inactive placebo.34 The median preferred dose was 75 mg in a range of 25 to 150 mg taken at dinner time rather than bedtime. As is the case with fibromyalgia and CFS, it is generally recommended that patients start at the lowest possible dose (10 mg) and titrate up to the dose which provides optimal symptom relief.35–37
The efficacy of intravesical Bacillus Calmette-Guerin (BCG) for the treatment of IC was evaluated in 3 RCTs.32, 38, 39 Sixty percent of the BCG-treated and 27% of the placebo-treated patients reported at least moderate improvement in one trial (P=0.065).38 The most recent NIDDK-sponsored RCT further supports those findings demonstrating benefit in 21% of the BCG-treated patients compared to 12% improvement in the placebo group (P=0.062).40 In a crossover trial of BGC vs. DMSO, none of the patients improved on BCG as first treatment, whereas 7 improved on DMSO (two when DMSO was the first treatment, and five when DMSO followed BCG).32 The findings from this study pose a special challenge to interpretation in light of the fact that there was no a priori outcome and, as a result, no estimated sample size for power calculation. Furthermore, the authors failed to observe an optimal washout period before crossing BCG patients over to DMSO. Thus, if BCG is followed by DMSO, the reported benefit might actually be a delayed BCG effect.
Hydroxyzine, an H1-blocker, failed to show efficacy as a single agent in the recent NIH/NIDDK study although the combination with PPS approached statistical significance (P=0.06).28 However, the study lacked the power to detect significant differences.
DISCUSSION
Evaluation of treatment efficacy in PBS/IC is challenging due to short duration of trials, heterogeneity of disease, and the lack of knowledge of the natural history of disease.
The RCTs we analyzed have not taken into consideration the variability of symptoms over time and regression to the mean. Most trials were short, with a mean duration of 15 weeks, which might not be optimal given the chronic nature of PBS/IC symptoms. As reflected in results from the largest observational IC study to date, the Interstitial Cystitis Database (ICDB)—patients who began with the most severe symptoms demonstrated the greatest initial improvement (i.e., their symptom scores moved toward the population mean).41 Conversely, patients who began with mild symptoms were more likely to worsen. Appropriately designed RCTs would have minimized such bias. Additionally, short duration of trials limits generalizability of findings.
Inadequate blinding (e.g., saline as placebo in DMSO trials32, 33), small number of patients (e.g., PPS24–26, hydroxyzine28, amitriptyline34), and nonstandardized outcome measures (e.g., bladder biopsy findings42) present additional challenges to analyzing treatment efficacy for PBS/IC. While most trials used the NIDDK diagnostic criteria for IC, very limited information was presented about participants who were ineligible or about symptomatic patients without bladder glomerulations (e.g. patients with painful bladder syndrome). It is well-known that strict use of NIDDK criteria would exclude 60% of patients with PBS/IC.6 Therefore, it is difficult to extrapolate how the findings from IC trials might relate to the larger majority of patients across the spectrum of PBS/IC symptoms.
The definition of symptoms—such as pain, urgency and frequency—varied considerably across the trials. Symptoms were measured using several different scales, making it inappropriate to pool the data for specific pharmacologic interventions investigated in more than one trial and also making it difficult to compare the findings in a qualitative synthesis. Consequently, it is not clear whether a positive result based on the pain scale of the OLS-SI, for example, is as good as, better than, or worse than a positive result on a different scale. Outcomes such as “global improvement,” in which participants were asked to rate themselves as better or worse than they were before the intervention began, were frequently reported. However, as has been shown to be the case with CFS and other chronic pain syndromes, the person may feel better able to cope with symptoms because they have reduced their expectations of what they should achieve, rather than because they have made any recovery as a result of the intervention. A more objective measure of the effect of any intervention would be whether participants have increased their working or waking hours, returned to work or school, or increased their physical or sexual activities.
The appropriate duration and follow-up of interventions used in the management of PBS/IC remains unknown. Given fluctuation of symptoms and the relapsing nature of PBS/IC we suggest that follow-up should continue for at least an additional 6 to 12 months after the intervention period has ended to confirm that any improvement observed was due to the intervention itself and not just to a naturally occurring fluctuation in the course of the illness or regression to the mean.
High dropout rates may be important indicators of the unacceptability of an intervention. This appears to be the case with cyclosporine,29 DMSO32, 33 and antibiotics,43 which had dropout rates of 55%, 19%, and 26%, respectively. High dropout rates may also indicate that the trial protocol is too rigid to accommodate any but a very specific group of participants, as might be the case with cyclosporine and DMSO. Again, this limits the generalizability of the findings.
Finally, many of the treatment response differences in IC clinical trials may be related to the heterogeneity of this illness. Identifying patient subsets based on response to specific treatments and biologic variables is one of the most challenging tasks in IC research. Patients with Hunner’s ulcer on cystoscopy form one such subgroup. This group, however, is relatively small since an ulcer is present in only about 15% of patients with IC. Hunner’s ulcer patients, therefore, should probably be enrolled as an isolated subset of IC patients.
Scant data are available from RCTs to confirm the efficacy of current pharmacological approaches to PBS/IC. What data do exist emerge from inadequately designed trials characterized by high dropout rates, restrictive protocols, variation in outcomes measures and definitional vagueness. Moreover, PBS/IC is a multifactorial and heterogeneous clinical symptom complex, yet RCTs designed to test pharmacologic agents have not taken into consideration the variability of symptoms over time or regression to the mean. Determining the optimal treatment strategy therefore remains elusive. Future treatments for PBS/IC will certainly be better informed by further unraveling the pathophysiologic mechanisms underlying the disease. Meanwhile, the key to developing evidence-based therapies is in establishing a consensus on standardized outcome measures and then designing and conducting appropriate RCTs that employ those standards.
Figure 2.
Effect on Patient-Reported Frequency
Figure 3.
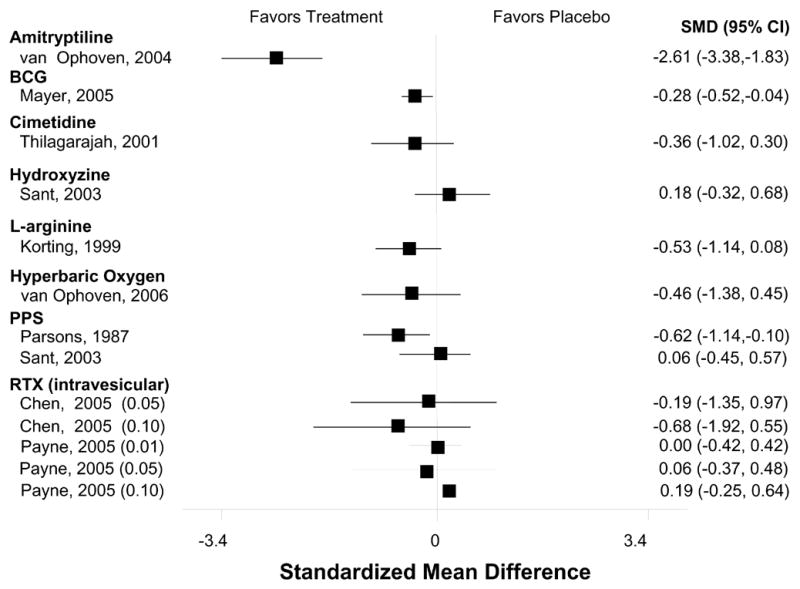
Effect on Patient-Reported Urgency
Acknowledgments
This work was supported by NIH/NIDDK grant R01 DK 065990 to Jordan Dimitrakov. The authors gratefully acknowledge Russell Reich for assistance in the preparation of the manuscript.
ABBREVIATIONS
- BCG
Bacillus Calmette-Guerin
- CFS
chronic fatigue syndrome
- CI
confidence interval
- DMSO
dimethylsulfoxide
- IBS
irritable bowel syndrome
- ICCTG
interstitial cystitis clinical trials group
- ICDB
interstitial cystitis database
- ITT
intention–to–treat
- NIH/NIDDK
National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases
- OLS
O’Leary-Sant questionnaire
- OLS-SI
O’Leary-Sant Symtpom Index
- PBS/IC
painful bladder syndrome/interstitial cystitis
- PPS
pentosan polysulfate
- RR
risk ratio
- RCT
randomized controlled trial
- RTX
resiniferatoxin
References
- 1.Vaughan E, Wilt T, Hanno P, Curhan GC. National Institutes of Diabetes and Digestive and Kidney Diseases/National Institutes of Health. Bethesda, MD: 2003. [Accessed February 26, 2007]. Interstitial Cystitis Epidemiology Task Force Meeting Executive Committee Summary. Available at http://www.niddk.nih.gov/fund/reports/ic/executive_summary.htm. [Google Scholar]
- 2.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37–49. doi: 10.1016/s0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- 3.Hunner GL. A rare type of bladder ulcer in women; report of cases. Boston Med Surg Journal. 1915;172:660–664. [Google Scholar]
- 4.Messing EM, Stamey TA. Interstitial cystitis: early diagnosis, pathology, and treatment. Urology. 1978;12:381–92. doi: 10.1016/0090-4295(78)90286-8. [DOI] [PubMed] [Google Scholar]
- 5.Gillenwater JY, Wein AJ. Summary of the National Institute of Arthritis, Diabetes, Digestive and Kidney Diseases Workshop on Interstitial Cystitis, National Institutes of Health, Bethesda, Maryland, August 28–29, 1987. J Urol. 1988;140:203–6. doi: 10.1016/s0022-5347(17)41529-1. [DOI] [PubMed] [Google Scholar]
- 6.Hanno PM, Landis JR, Matthews-Cook Y, Kusek J, Nyberg L., Jr The diagnosis of interstitial cystitis revisited: lessons learned from the National Institutes of Health Interstitial Cystitis Database study. J Urol. 1999;161:553–7. doi: 10.1016/s0022-5347(01)61948-7. [DOI] [PubMed] [Google Scholar]
- 7.Waxman JA, Sulak PJ, Kuehl TJ. Cystoscopic findings consistent with interstitial cystitis in normal women undergoing tubal ligation. J Urol. 1998;160:1663–7. [PubMed] [Google Scholar]
- 8.Erickson DR. Glomerulations in women with urethral sphincter deficiency: report of 2 cases. J Urol. 1995;153:728–9. corrected. [PubMed] [Google Scholar]
- 9.Tomaszewski JE, Landis JR, Russack V, et al. Biopsy features are associated with primary symptoms in interstitial cystitis: results from the interstitial cystitis database study. Urology. 2001;57:67–81. doi: 10.1016/s0090-4295(01)01166-9. [DOI] [PubMed] [Google Scholar]
- 10.Abrams P, Hanno P, Wein A. Overactive bladder and painful bladder syndrome: there need not be confusion. Neurourol Urodyn. 2005;24:149–50. doi: 10.1002/nau.20082. [DOI] [PubMed] [Google Scholar]
- 11.Roberts RO, Bergstralh EJ, Bass SE, Lightner DJ, Lieber MM, Jacobsen SJ. Incidence of physician-diagnosed interstitial cystitis in Olmsted County: a community-based study. BJU Int. 2003;91:181–5. doi: 10.1046/j.1464-410x.2003.04060.x. [DOI] [PubMed] [Google Scholar]
- 12.Clemens JQ, Meenan RT, Rosetti MC, Gao SY, Calhoun EA. Prevalence and incidence of interstitial cystitis in a managed care population. J Urol. 2005;173:98–102. doi: 10.1097/01.ju.0000146114.53828.82. discussion 102. [DOI] [PubMed] [Google Scholar]
- 13.Jones CA, Nyberg L. Epidemiology of interstitial cystitis. Urology. 1997;49:2–9. doi: 10.1016/s0090-4295(99)80327-6. [DOI] [PubMed] [Google Scholar]
- 14.Miller JL, Bavendam TG, Berger RE. Interstitial cystitis in men. In: Sant GR, editor. Interstitial Cystitis. Philadelphia, PA: Lippincott-Raven; 1997. pp. 165–168. [Google Scholar]
- 15.Leppilahti M, Sairanen J, Tammela TL, Aaltomaa S, Lehtoranta K, Auvinen A. Prevalence of clinically confirmed interstitial cystitis in women: a population based study in Finland. J Urol. 2005;174:581–3. doi: 10.1097/01.ju.0000165452.39125.98. [DOI] [PubMed] [Google Scholar]
- 16.Leppilahti M, Tammela TL, Huhtala H, Kiilholma P, Leppilahti K, Auvinen A. Interstitial cystitis-like urinary symptoms among patients with Sjogren’s syndrome: a population-based study in Finland. Am J Med. 2003;115:62–5. doi: 10.1016/s0002-9343(03)00257-2. [DOI] [PubMed] [Google Scholar]
- 17.Rovner E, Propert KJ, Brensinger C, et al. Treatments used in women with interstitial cystitis: the interstitial cystitis data base (ICDB) study experience. The Interstitial Cystitis Data Base Study Group. Urology. 2000;56:940–5. doi: 10.1016/s0090-4295(00)00845-1. [DOI] [PubMed] [Google Scholar]
- 18.Jailwala J, Imperiale TF, Kroenke K. Pharmacologic treatment of the irritable bowel syndrome: a systematic review of randomized, controlled trials. Ann Intern Med. 2000;133:136–47. doi: 10.7326/0003-4819-133-2-200007180-00013. [DOI] [PubMed] [Google Scholar]
- 19.O’Leary MP, Sant GR, Fowler FJ, Jr, Whitmore KE, Spolarich-Kroll J. The interstitial cystitis symptom index and problem index. Urology. 1997;49:58–63. doi: 10.1016/s0090-4295(99)80333-1. [DOI] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kazis LE, Anderson JJ, Meenan RF. Effect sizes for interpreting changes in health status. Med Care. 1989;27:S178–89. doi: 10.1097/00005650-198903001-00015. [DOI] [PubMed] [Google Scholar]
- 23.Lubeck DP, Whitmore K, Sant GR, Alvarez-Horine S, Lai C. Psychometric validation of the O’leary-Sant interstitial cystitis symptom index in a clinical trial of pentosan polysulfate sodium. Urology. 2001;57:62–6. doi: 10.1016/s0090-4295(01)01126-8. [DOI] [PubMed] [Google Scholar]
- 24.Holm-Bentzen M, Jacobsen F, Nerstrom B, et al. A prospective double-blind clinically controlled multicenter trial of sodium pentosanpolysulfate in the treatment of interstitial cystitis and related painful bladder disease. J Urol. 1987;138:503–7. doi: 10.1016/s0022-5347(17)43241-1. [DOI] [PubMed] [Google Scholar]
- 25.Mulholland SG, Hanno P, Parsons CL, Sant GR, Staskin DR. Pentosan polysulfate sodium for therapy of interstitial cystitis. A double-blind placebo-controlled clinical study. Urology. 1990;35:552–8. doi: 10.1016/0090-4295(90)80116-5. [DOI] [PubMed] [Google Scholar]
- 26.Parsons CL, Mulholland SG. Successful therapy of interstitial cystitis with pentosanpolysulfate. J Urol. 1987;138:513–6. doi: 10.1016/s0022-5347(17)43243-5. [DOI] [PubMed] [Google Scholar]
- 27.Parsons CL, Benson G, Childs SJ, Hanno P, Sant GR, Webster G. A quantitatively controlled method to study prospectively interstitial cystitis and demonstrate the efficacy of pentosanpolysulfate. J Urol. 1993;150:845–8. doi: 10.1016/s0022-5347(17)35629-x. [DOI] [PubMed] [Google Scholar]
- 28.Sant GR, Propert KJ, Hanno PM, et al. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. J Urol. 2003;170:810–5. doi: 10.1097/01.ju.0000083020.06212.3d. [DOI] [PubMed] [Google Scholar]
- 29.Sairanen J, Tammela TL, Leppilahti M, et al. Cyclosporine A and pentosan polysulfate sodium for the treatment of interstitial cystitis: a randomized comparative study. J Urol. 2005;174:2235–8. doi: 10.1097/01.ju.0000181808.45786.84. [DOI] [PubMed] [Google Scholar]
- 30.Hwang P, Auclair B, Beechinor D, Diment M, Einarson TR. Efficacy of pentosan polysulfate in the treatment of interstitial cystitis: a meta-analysis. Urology. 1997;50:39–43. doi: 10.1016/S0090-4295(97)00110-6. [DOI] [PubMed] [Google Scholar]
- 31.Nickel JC, Barkin J, Forrest J, et al. Randomized, double-blind, dose-ranging study of pentosan polysulfate sodium for interstitial cystitis. Urology. 2005;65:654–8. doi: 10.1016/j.urology.2004.10.071. [DOI] [PubMed] [Google Scholar]
- 32.Peeker R, Haghsheno MA, Holmang S, Fall M. Intravesical bacillus Calmette-Guerin and dimethyl sulfoxide for treatment of classic and nonulcer interstitial cystitis: a prospective, randomized double-blind study. J Urol. 2000;164:1912–5. doi: 10.1016/s0022-5347(05)66916-9. discussion 1915–6. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Marrero R, Emerson LE, Feltis JT. A controlled study of dimethyl sulfoxide in interstitial cystitis. J Urol. 1988;140:36–9. doi: 10.1016/s0022-5347(17)41478-9. [DOI] [PubMed] [Google Scholar]
- 34.van Ophoven A, Pokupic S, Heinecke A, Hertle L. A prospective, randomized, placebo controlled, double-blind study of amitriptyline for the treatment of interstitial cystitis. J Urol. 2004;172:533–6. doi: 10.1097/01.ju.0000132388.54703.4d. [DOI] [PubMed] [Google Scholar]
- 35.van Ophoven A, Hertle L. Long-term results of amitriptyline treatment for interstitial cystitis. J Urol. 2005;174:1837–40. doi: 10.1097/01.ju.0000176741.10094.e0. [DOI] [PubMed] [Google Scholar]
- 36.Hanno PM. Amitriptyline in the treatment of interstitial cystitis. Urol Clin North Am. 1994;21:89–91. [PubMed] [Google Scholar]
- 37.Hanno PM, Buehler J, Wein AJ. Use of amitriptyline in the treatment of interstitial cystitis. J Urol. 1989;141:846–8. doi: 10.1016/s0022-5347(17)41029-9. [DOI] [PubMed] [Google Scholar]
- 38.Peters K, Diokno A, Steinert B, et al. The efficacy of intravesical Tice strain bacillus Calmette-Guerin in the treatment of interstitial cystitis: a double-blind, prospective, placebo controlled trial. J Urol. 1997;157:2090–4. [PubMed] [Google Scholar]
- 39.Mayer R, Propert KJ, Peters KM, et al. A Randomized Controlled Trial Of Intravesical Bacillus Calmette-Guerin For Treatment Refractory Interstitial Cystitis. J Urol. 2005;173:1186–1191. doi: 10.1097/01.ju.0000152337.82806.e8. [DOI] [PubMed] [Google Scholar]
- 40.Mayer R, Propert KJ, Peters KM, et al. A randomized controlled trial of intravesical bacillus calmette-guerin for treatment refractory interstitial cystitis. J Urol. 2005;173:1186–91. doi: 10.1097/01.ju.0000152337.82806.e8. [DOI] [PubMed] [Google Scholar]
- 41.Propert KJ, Schaeffer AJ, Brensinger CM, Kusek JW, Nyberg LM, Landis JR. A prospective study of interstitial cystitis: results of longitudinal followup of the interstitial cystitis data base cohort. The Interstitial Cystitis Data Base Study Group. J Urol. 2000;163:1434–9. doi: 10.1016/s0022-5347(05)67637-9. [DOI] [PubMed] [Google Scholar]
- 42.Thilagarajah R, Witherow RO, Walker MM. Oral cimetidine gives effective symptom relief in painful bladder disease: a prospective, randomized, double-blind placebo-controlled trial. BJU Int. 2001;87:207–12. doi: 10.1046/j.1464-410x.2001.02031.x. [DOI] [PubMed] [Google Scholar]
- 43.Warren JW, Horne LM, Hebel JR, Marvel RP, Keay SK, Chai TC. Pilot study of sequential oral antibiotics for the treatment of interstitial cystitis. J Urol. 2000;163:1685–8. [PubMed] [Google Scholar]
- 44.Korting GE, Smith SD, Wheeler MA, Weiss RM, Foster HE., Jr A randomized double-blind trial of oral L-arginine for treatment of interstitial cystitis. J Urol. 1999;161:558–65. [PubMed] [Google Scholar]
- 45.Cartledge JJ, Davies AM, Eardley I. A randomized double-blind placebo-controlled crossover trial of the efficacy of L-arginine in the treatment of interstitial cystitis. BJU Int. 2000;85:421–6. doi: 10.1046/j.1464-410x.2000.00490.x. [DOI] [PubMed] [Google Scholar]
- 46.Barbalias GA, Liatsikos EN, Athanasopoulos A, Nikiforidis G. Interstitial cystitis: bladder training with intravesical oxybutynin. J Urol. 2000;163:1818–22. doi: 10.1016/s0022-5347(05)67551-9. [DOI] [PubMed] [Google Scholar]
- 47.van Ophoven A, Rossbach G, Pajonk F, Hertle L. Safety and efficacy of hyperbaric oxygen therapy for the treatment of interstitial cystitis: a randomized, sham controlled, double-blind trial. J Urol. 2006;176:1442–6. doi: 10.1016/j.juro.2006.06.065. [DOI] [PubMed] [Google Scholar]
- 48.Bade JJ, Laseur M, Nieuwenburg A, van der Weele LT, Mensink HJ. A placebo-controlled study of intravesical pentosanpolysulphate for the treatment of interstitial cystitis. Br J Urol. 1997;79:168–71. doi: 10.1046/j.1464-410x.1997.03384.x. [DOI] [PubMed] [Google Scholar]
- 49.Lazzeri M, Beneforti P, Spinelli M, Zanollo A, Barbagli G, Turini D. Intravesical resiniferatoxin for the treatment of hypersensitive disorder: a randomized placebo controlled study. J Urol. 2000;164:676–9. doi: 10.1097/00005392-200009010-00014. [DOI] [PubMed] [Google Scholar]
- 50.Chen TY, Corcos J, Camel M, Ponsot Y, Tu le M. Prospective, randomized, double-blind study of safety and tolerability of intravesical resiniferatoxin (RTX) in interstitial cystitis (IC) Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:293–7. doi: 10.1007/s00192-005-1307-4. [DOI] [PubMed] [Google Scholar]
- 51.Payne CK, Mosbaugh PG, Forrest JB, Evans RJ, Whitmore KE, Antoci JP, Perez-Marrero R, Jacoby K, Diokno AC. Intravesical Resiniferatoxin (RTX) Treatment of Interstitial Cystitis: A Randomized, Double-Blind, Placebo-Controlled Trial. J Urol. 2005:173. doi: 10.1097/01.ju.0000154631.92150.ef. [DOI] [PubMed] [Google Scholar]


