Abstract
Three radiation-induced alleles of the mouse p locus, p6H, p25H, and pbs, cause defects in growth, coordination, fertility, and maternal behavior in addition to p gene-related hypopigmentation. These alleles are associated with disruption of the p gene plus an adjacent gene involved in the disorders listed. We have identified this adjacent gene, previously named rjs (runty jerky sterile), by positional cloning. The rjs cDNA is very large, covering 15,264 nucleotides. The predicted rjs-encoded protein (4,836 amino acids) contains several sequence motifs, including three RCC1 repeats, a structural motif in common with cytochrome b5, and a HECT domain in common with E6-AP ubiquitin ligase. On the basis of sequence homology and conserved synteny, the rjs gene is the single mouse homolog of a previously described five- or six-member human gene family. This family is represented by at least two genes, HSC7541 and KIAA0393, from human chromosome 15q11–q13. HSC7541 and KIAA0393 lie close to, or within, a region commonly deleted in most Prader–Willi syndrome patients. Previous work has suggested that the multiple phenotypes in rjs mice might be due to a common neuroendocrine defect. In addition to this proposed mode of action, alternative functions of the rjs gene are evaluated in light of its known protein homologies.
Mutations of the mouse pink-eyed dilution locus, p, are defined by hypopigmentation, ranging from a minor diminution in coat color (with dark eyes) to a nearly complete lack of melanin pigment (with pink eyes) (1). The radiation-induced p alleles p6H, p25H, and pbs are associated with additional phenotypes including reduced growth, jerky gait, male sterility, female semisterility, and maternal behavior defects. The locus associated with these phenotypes has been previously named rjs [runty jerky sterile (2)] and jdf2 [juvenile development and fertility (3)].
The reduction in growth in rjs mice is apparent at birth and persists into adulthood, at which time homozygous mutants are ≈80% the size of heterozygotes or wild-type controls (4). Coordination is affected in rjs mice, as they have a jerky gait (2). The fertility problems associated with p6H, p25H, and pbs mutations include spermatocyte and oocyte abnormalities and possible endocrinological deficits (5–7). Males homozygous for p6H, p25H, or pbs are sterile and exhibit testicular hypoplasia, abnormal spermatogenesis, and abnormal sperm morphology manifested as multinucleated and multitailed sperm with acrosome abnormalities and disorganized mitochondria spirals (5, 7). Ovaries from mice homozygous for p6H, p25H, and pbs contain large numbers of developing follicles, but few, if any, corpora lutea or corpora hemorrhagica (6). The uterus in these mice is reduced in size and has been described as “thread like” (4). If bred early, mutant females may have a small litter, with a high incidence of neonatal death attributed to poor maternal behavior (8, 9).
Previous studies have suggested that disruption of a single gene underlies the rjs phenotypes of reduced growth, jerky gait, male sterility, female semisterility, and maternal behavior defects. Complementation studies using radiation-induced alleles (p6H, p25H, pbs, and several others) have failed to separate these diverse phenotypes—i.e., they form a single complementation group proximal to the p gene (2, 10). In addition, N-ethyl-N-nitrosourea-induced alleles (associated with point mutations) exhibit the same diverse phenotypes (3). To determine whether a single gene or multiple genes are involved in these phenotypes and to gain insights into the etiology of the underlying genetic defect, we set out to clone and sequence the gene or genes disrupted by the alleles p6H, p25H, and pbs. Here we report the identification of a single gene, rjs, mutations of which lead to these diverse phenotypes.
MATERIALS AND METHODS
Genomic and cDNA Cloning.
A 26-kb λ phage contig was constructed that included the 5′ end of the p gene and sequences proximal to it. Restriction maps of the clones were generated by using Southern blots of doubly digested DNA as previously described (11). λ clone fragments were isolated and used as hybridization probes on Southern blots of homozygous wild-type and pbs genomic DNA. Several unique sequence fragments were cloned that were present in wild-type DNA but absent from pbs DNA. These sequences were within an 8-kb segment that was proximal to the p gene and deleted in the pbs allele. The 8-kb region was found to be within the larger p6H deletion and within a chromosomal inversion of p25H (data not shown). Genomic fragments from within this 8-kb segment were used as probes for hybridization to DNA from other mammalian species—i.e., on a “zoo blot”—and also on Northern blots (data not shown). One of three fragments, BS2g (which includes at least three exons found between nucleotides 12661 and 14606 of the final rjs cDNA contig), with positive hybridization on both Northern and zoo blots was used to screen a randomly primed cDNA library from brain (CLONTECH, gift of J. Gregor Sutcliffe, Scripps Research Institute). A screen of 75,000 recombinant phage produced 3 independent clones. After confirmation by Southern and Northern blots, the insert from the largest of these clones (BS2c) was used to screen two additional randomly primed cDNA libraries prepared from testis [gift of T. Miki, National Institutes of Health (12)] and 16.5-day embryos (from Stratagene). Several rounds of screening identified 34 overlapping cDNA clones that covered 8,247 nucleotides of rjs cDNA. To obtain clones from the 5′ end of the rjs gene, mRNA was first isolated from wild-type mice by using the Fast-Track kit (Invitrogen) according to the manufacturer’s instructions. Five rounds of 5′ rapid amplification of cDNA ends (RACE) were subsequently performed by using the Marathon cDNA Amplification Kit (CLONTECH) according to the manufacturer’s instructions, using the following gene-specific primers (GSPs; numbering according to the position of the primers in the rjs cDNA contig reported here): MHB 181 (7615–7594, 5′-AAGGCTTCCACACCAGGCAAAC-3′), MHB 294 (6951–6932, 5′-CCGTGAAAGGCAAATTGTCT-3′), MHB 336 (2597–2580, 5′-CTTCTCTTGTGGTGGGGG-3′), and MHB 365 (634–615, 5′-CTGCTGCTTTTCCACTTAAC-3′). The sequence from within the most proximal cDNA clone was used to generate the first GSP used for 5′ RACE: MHB 282 (7397–7371, 5′-CCCTGGACTTGAAGGATGTGTGGATTC-3′). Subsequent GSPs were generated from sequenced cDNA clones obtained by 5′ RACE: MHB 302 (6767–6741, 5′-GCCATCAATCCCTCCAATGACAGCGAG-3′), MHB 316 (3805–3881, 5′-CAGAGCGACCACTGGATCTTCCCC-3′), MHB 334 (2401–2376, 5′-CTGTCCAGTCCTGGGAGTGCAGTCGG-3′), MHB 363 (344–317, 5′-TTCTTTCTTGGTTCCACTCTGAGCATCG-3′), and MHB 364 (564–539, 5′-CGAGGATCACCAGCCTCTGCTTCAAC-3′). After 5′ RACE amplification, PCR products were separated by agarose gel electrophoresis, gel purified, and cloned by using the TA Cloning Kit (Invitrogen). cDNA clones obtained by 5′ RACE were confirmed by reverse transcription–PCR (RT-PCR) and Southern and Northern blots using nucleic acids from wild-type and p6H/p6H mice as previously described (11). All of the cDNA clones were sequenced by cycle sequencing using an Applied Biosystems automated sequencer. Sequences were analyzed with the MacVector and Sequencher programs. The multiple-tissue Northern blot was obtained from CLONTECH and hybridized according to the supplier’s instructions.
Analysis of rjs Mutant Alleles.
Brain mRNA was isolated from p6H/p6H, p25H/p25H, and pbs/pbs mice by using a FastTrack kit from Invitrogen. 3′ RACE was done according to previously published methods (13), using Elongase (GIBCO/BRL) and the following GSPs: for p6H/p6H GSP1 = MHB 367 (892–909, 5′-CAACGAGGTTCCTCAGGT-3′) and GSP2 = MHB 369 (1447–1464, 5′-CGATCCAGTGTGAAAGCC-3′; note that the actual rjs sequence contains a G at position 1461), and for p25H/p25H, GSP1 = MHB 180 (7214–7236, 5′-TGACCTTGGGGATATGTCACCTG-3′) and GSP2 = MHB 154 (8513–8532, 5′-GGCTTCTCGTCTCATTGATG-3′). The pbs/pbs cDNA was isolated by RT-PCR using mRNA from brain and primers MHB 354 (12749–12772, 5′-TTCTGTCGCCCTTACCAAGTCTGG-3′) and MHB 250 (14597–14578, 5′-TGAAGCAAAAGAGTCGGCGTC-3′). Mutant allele-specific bands were isolated, cloned by using the TA cloning kit (Invitrogen), and sequenced.
Histology of Ovaries, Testis, and Mammary Tissue.
Tissues were dissected, embedded in paraffin, and sectioned. Sections were stained with hematoxylin and eosin and examined by light microscopy. Mammary tissue of wild-type and p6H/p6H mice was isolated from 8-week-old females several hours after they had given birth. Ovaries were isolated from 5- to 6-month old mice.
RESULTS
Isolation and Characterization of rjs cDNA clones.
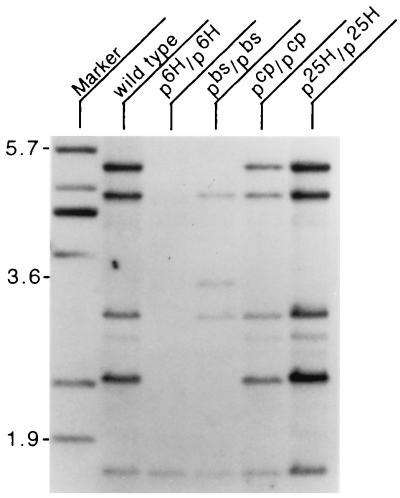
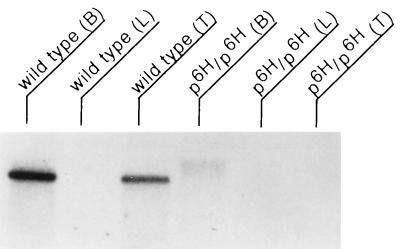
The noncomplementing alleles p6H, p25H, and pbs (2) were found to disrupt a common region of DNA located 5′ to the p gene. The smallest region associated with the phenotypes of this complementation group is delimited by the 8-kb pbs deletion, located 10 kb 5′ of the first exon of the p gene (data not shown). Several unique sequence fragments from within this region were identified, cloned, and found to hybridize to a very large, ≈15-kb, transcript from brain as well as to DNA from other mammalian species (data not shown). These fragments were then used to screen a randomly primed brain cDNA library. Three independent clones were isolated and subcloned. An insert from the longest of these clones, BS2c (corresponding to nucleotides 12661–14606 of the final rjs cDNA contig), was used as a hybridization probe on Southern and Northern blots prepared from mice homozygous for several genotypes, wild type, p6H, pbs, pcp, and p25H. Compared with wild type, homozygous p6H DNA lacks all but one hybridizing fragment; several hybridizing bands are deleted from pbs, with one novel band in evidence, and p25H has an additional hybridizing band (Fig. 1). pcp is complementing with rjs and, as expected, is not altered. No corresponding transcript is seen in p6H RNA with this probe (Fig. 2). The faint high molecular weight band in p6H brain total RNA probably represents nonspecific hybridization, since RT-PCR from p6H mRNA was negative with primers after nucleotide 1993 (data not shown). The expression level of rjs in the p25H and pbs alleles was also examined by Northern blotting with several probes upstream of the inversion and deletion breakpoints, respectively, and found to be similar to wild type (data not shown). To extend the cDNA contig, several additional rounds of screening were performed on a second randomly primed library derived from testes. Using the 5′-most ends of subsequently isolated cDNA clones, we isolated a total of 34 overlapping cDNA clones. These were sequenced and found to cover 8,247 nucleotides at the 3′ end of the rjs gene. Starting from the most 5′ end of the cDNA contig, we carried out five cycles of 5′ RACE, resulting in the isolation of overlapping clones covering an additional 7,017 nucleotides at the 5′ end of the rjs gene. The cDNA contig of this gene covers 15,264 nucleotides with a single large ORF predicting a protein of 4,836 amino acids (GenBank accession no. AF06529) (Fig. 3). The size of the transcript detected by Northern blotting matches the size predicted from the cDNA contig.
Figure 1.
Southern blot analysis of genomic DNA from wild type, p6H/p6H, pbs/pbs, pcp/pcp, and p25H/p25H homozygous mice digested with HindIII and hybridized with the BS2c probe (corresponding to nucleotides 12661–14606 of the final rjs cDNA contig that includes part of RLDc and all of the HECT domain). Marker lane contains size markers with position in kb indicated at left. Note deletion of all bands except lowest molecular weight band in p6H/p6H DNA.
Figure 2.
Northern blot of total RNA isolated from wild-type and p6H/p6H tissues (B, brain; L, liver; and T, testis) and hybridized with BS2c probe (corresponding to nucleotides 12661–14606 of the final rjs cDNA contig, which includes part of RLDc and all of the HECT domain). Markers (not shown) indicate this to be a very large transcript, ≥15 kb. Note the expression of the rjs gene in brain and testis of wild type and the absence of transcript in p6H/p6H total RNA. The faint high molecular weight band in p6H/p6H brain total RNA probably represents nonspecific hybridization since RT-PCR from p6H/p6H mRNA was negative with primers after nucleotide 1993.
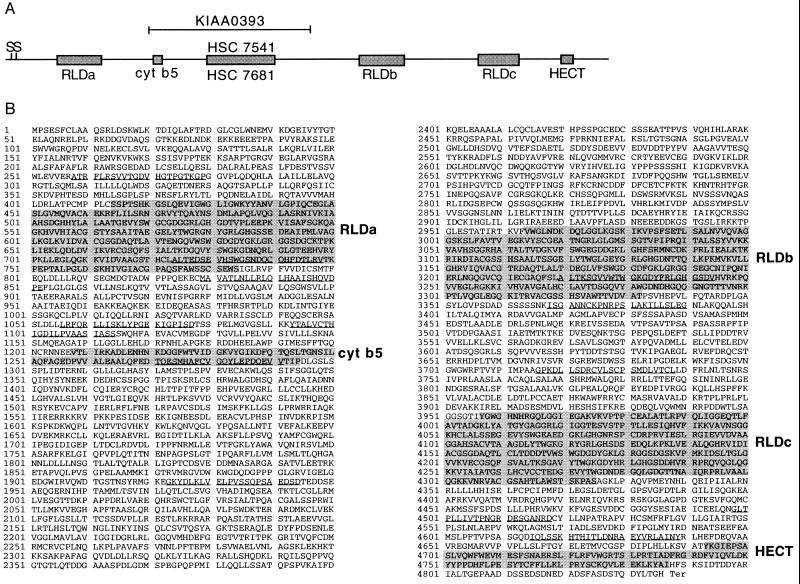
Figure 3.
(A) Graphic representation of the rjs nucleotide sequence and homologies. The solid line represents the rjs nucleotide sequence, with boxed regions indicating significant homologies. S denotes two possible methionine start codons. RLD indicates RCC1-like domains, and cyt b5 indicates homology to cytochrome b5. The HECT domain is found in proteins with homology to E6-AP in their carboxyl terminus. HSC 7541 indicates highly conserved homology (83% identity) to human chromosome 15 sequences related to MN7, and HSC 7681 indicates highly conserved homology (84% identity) to human chromosome 16 sequences related to MN7. KIAA0393 indicates high homology (85% identity) to a human cDNA that maps to chromosome 15, human homologs of the rjs gene (see text). (B) Predicted amino acid sequence encoded by the rjs gene, with regions of homology shaded and listed at right. Underlined amino acids are predicted to be transmembrane domains by the TM Pred program (19) accessible via http://www.isrec.isb-sib.ch/software/TMPRED_form.html.
GenBank database searches using the rjs cDNA and its predicted encoded protein uncovered several interesting homologies. The highest homologies were to three human cDNA clones, two of which, HSC7541 and HSC7681, were identified as homologues of a genomic clone MN7, originally isolated from chromosome 15 (14). The rjs gene has 83% identity with HSC7541 and 84% identity with HSC7681. A third cDNA sequence, KIAA0393, was isolated in a search of large cDNAs from brain (15). KIAA0393 cDNA is 85% identical to rjs. KIAA0393 is 97.1% identical to HSC7541 and 95.7% identical to HSC7681. Both cDNA clones HSC7541 and KIAA0393 map to chromosome 15q and are members of the MN7 gene family (14). However, we note that there appears to be a single rjs gene in the mouse on the basis of p6H Southern and Northern blots (Figs. 1 and 2). The regions of homology are indicated in Fig. 3A.
Searches with the predicted protein sequence revealed significant homology to several proteins, including RCC1 in three distinct regions (Fig. 3B). RCC1 (regulator of chromosome condensation), characterized by seven contiguous repeats, has been found in many different organisms to act as a guanine nucleotide exchange factor for Ran at the nuclear membrane (16). A large protein that contains two sets of RCC1 repeats, p532 [previously named p619 (17)], additionally contains homology to other parts of the rjs protein (see Discussion).
Although rjs has sequences homologous to cytochrome b5, the rjs-encoded protein lacks the two highly conserved histidine residues that act as heme-coordinating ligands in cytochrome b5 (Fig. 3B). The carboxyl terminus of the rjs-encoded protein also contains a well conserved HECT domain reminiscent of E3 ubiquitin-protein ligases (18). This domain utilizes a conserved cysteine residue at position 833 of the human E6-AP to catalyze the attachment of ubiquitin to specific proteins and target them for degradation through the ubiquitin-mediated proteolytic pathway. Significantly, the rjs-encoded protein has a cysteine at the homologous position in its HECT domain.
The rjs predicted polypeptide was examined for structural motifs by using the TM Pred program (19). This program uncovered clusters of hydrophobic amino acids and predicts 12 potential membrane-spanning regions in rjs (Fig. 3B). At least two of these regions may not be membrane spanning, because they overlap those regions with significant homology to RCC1 that, in RCC1, may not be membrane spanning (20).
Expression of the rjs Gene.
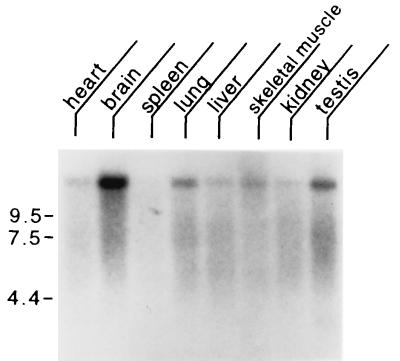
Northern blot analysis was conducted with poly(A)+ RNA from heart, brain, spleen, lung, liver, skeletal muscle, kidney, and testis (Fig. 4). We found that rjs is expressed at the highest levels in brain and testes, with lower levels of expression in heart, lung, liver, skeletal muscle, and kidney. Little expression was detected in the spleen.
Figure 4.
Mouse multiple tissue Northern blot. Each lane contains 2 μg of poly(A)+ RNA from the tissues indicated hybridized with a probe from the rjs gene (nucleotides 10180–11640). Marker sizes are indicated at left in kilobases. Control hybridization with β-actin (not shown) confirmed the integrity and equal loading of all RNAs.
cDNA and Predicted Protein Structure of p6H, p25H, and pbs Alleles.
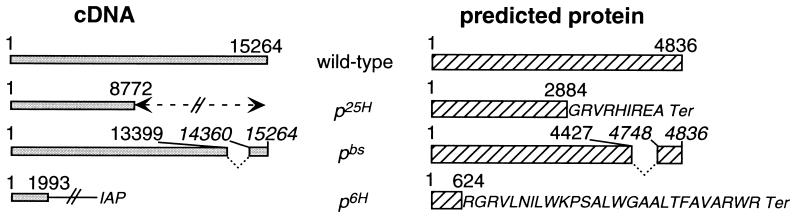
rjs cDNA clones were isolated from 3′ and 5′ RACE products produced from brain RNA of mice homozygous for the p6H and pbs deletion alleles and from the p25H inversion allele. The sequences of these clones were determined (Fig. 5) (GenBank accession nos.: AF06530 for pbs; AF06531 for p25H; and AF06532 for p6H). The p25H cDNA matches the wild-type rjs cDNA for the first 8,772 nucleotides prior to diverging. The protein predicted from the p25H cDNA contains the first 2,884 amino acids of the wild-type protein followed by 9 novel amino acids before a termination codon (Fig. 5). The pbs cDNA is internally deleted for 960 nucleotides (13400–14359, inclusive) but maintains the same ORF as wild type. This deletion results in a predicted peptide that lacks an internal stretch of 320 amino acids (4428–4747, inclusive), including much of the HECT domain. The effect of the p6H deletion is a loss of wild-type cDNA sequences after nucleotide 1,993. Interestingly, the sequences following that position matched those of the IAP elements. The predicted protein from this cDNA contains the amino-terminal 624 amino acids with an additional 27 novel amino acids (encoded by out-of-reading-frame IAP sequences) prior to a termination codon. Thus, all three alleles for the rjs gene were found to have disruptions in their cDNAs and predicted proteins.
Figure 5.
(Left) Graphical representation of cDNAs encoded by rjs alleles. (Right) Their corresponding proteins. The p25H inversion (indicated by double arrowhead) results in the loss of rjs cDNA sequences after nucleotide 8,772; the predicted p25H rjs peptide ends at amino acid number 2,884, followed by 9 novel amino acids before termination. The pbs deletion results in an internal loss of 960 nucleotides of rjs cDNA (13400–14359, inclusive); the predicted pbs rjs peptide lacks an internal 320 amino acids (4428–4747, inclusive). The p6H deletion results in a loss of rjs cDNA sequences after nucleotide 1,993, with the addition of sequences from an intracisternal A particle (IAP) element; the predicted p6H rjs peptide ends at amino acid 624, followed by 27 novel amino acids encoded by an out-of-reading-frame IAP element before termination.
Phenotypic Analysis of rjs Mutations.
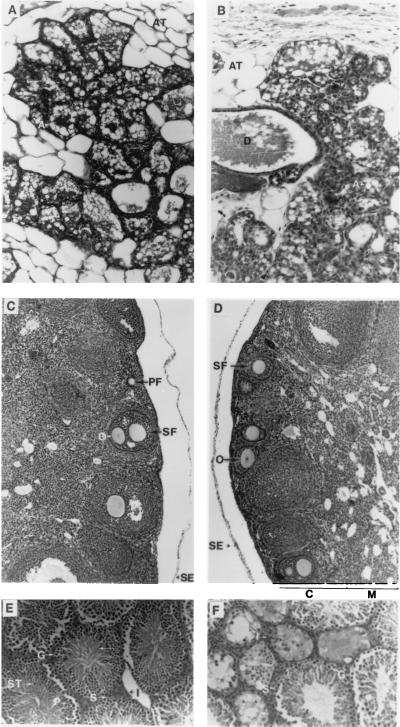
Mice homozygous for rjs mutations have been reported previously to have defects in reproduction—i.e., males were sterile and females were semisterile (5). We have confirmed that a failure in spermatogenesis causes male sterility (Fig. 6). Sertoli cells and spermatogonia were present but no normal mature sperm were evident. Our examination of female mice likewise confirmed previous studies (6): mutant ovaries contained maturing follicles; however, there was a decrease in the number of corpora lutea compared with wild type (Fig. 6).
Figure 6.
Light micrographs of sections from mouse mammary tissue (A and B), ovary (C and D), and testis (E and F) taken from wild-type (A, C, and E), p25H/p25H (B), and p6H/p6H (D and F) mice. Mammary tissue was taken from mice 1–2 months of age within hours of giving birth. Ovaries and testis were taken from adult mice 2–6 months of age. Letters on photos indicate important structures. Mammary tissue: D, duct; A, alveoli; and AT, adipose tissue. Ovary: SE, surface epithelium; M, medulla; C, cortex; PF, primary follicle; SF, secondary follicle; and O, oocytes. Testis: G, spermatogonium; I, interstitial cell; P, primary spermatocyte; S, Sertoli cell; and ST, spermatids; and arrow indicates developing spermatozoa. [Approximate final magnifications: ×50 (A and B), ×30 (C and D), and ×45 (E and F).]
Another previously reported phenotype was that rjs mutant mothers had a defect in maternal behavior (8, 9). Although female mutant mice were often fertile, they typically did not have more than one litter and that one litter was usually small. The pups born almost never survived more than 24 hr. However, pups of mutant mothers were successfully fostered to wild-type mothers, so their lack of survival is not intrinsic, but the exact reason for the pups’ death is not known. To determine whether mutant females were lactating normally, we examined mammary tissue from mothers sacrificed within hours after giving birth (Fig. 6). Mutant females were found to be lactating adequately. We have also found milk in the stomachs of some pups. Six mutant mothers and several controls were studied by using time-lapse videography of new mothers interacting with their pups over a 12- to 24-hr period after birth. We did not detect complete indifference (never picking up pups or cleaning them) or directed hostility toward pups (killing them) on the part of the mutant mothers. However, mutant mothers did not typically make a nest or keep their pups together (data not shown).
DISCUSSION
We have studied three alleles, p6H, p25H, and pbs, that share the same spectrum of phenotypes: reduced growth, jerky gait, male sterility, female semisterility, and maternal behavior defects. These mutations do not complement each other and are commonly disrupted for expression of the same gene—rjs (runty jerky sterile). We have isolated cDNAs encoded by the rjs gene covering 15,264 nucleotides. We determined the structure of rjs cDNAs from p6H, p25H, and pbs; all were found to contain disruptions. Because the pbs allele results from a relatively small intragenic deletion of the rjs gene (≈8 kb of genomic DNA, data not shown), leading to the loss of 320 amino acids internal to the protein (Fig. 5), it is very likely that disruption of this gene alone is sufficient to cause the rjs phenotypes. (The relatively mild hypopigmentation associated with the pbs deletion apparently reflects an effect of the deletion on expression of the closely linked p gene.) That a single gene underlies these phenotypes is also supported by earlier work (3) that demonstrated these same phenotypes in an N-ethyl-N-nitrosourea-induced series of noncomplementing alleles.
Database comparisons with the rjs nucleotide and predicted peptide sequence have revealed several interesting homologies. The highest homology was found between rjs cDNA and three human cDNAs, KIAA0393 (85%), HSC7541 (83%), and HSC7681 (84%). The latter two cDNAs were isolated by using the MN7 genomic DNA clone. MN7 was originally isolated by microdissection of human chromosome 15 to be used as a molecular probe for Prader–Willi syndrome (PWS) (21). Subsequently, MN7 was found to be homologous to cDNAs that mapped to both 15q11–q13 and 16p11.2, indicating that there are at least two homologous genes in humans and four or five additional MN7-related sequences on 15q11–q13 (14). KIAA0393 was identified in a screen for cDNA clones that encode large proteins (15). It was mapped to human chromosome 15 and shown to be expressed in several different tissues, including thymus and ovary. Although the isolated clone was obtained in an effort to isolate large full-length cDNAs, KIAA0393 is only 6 kb and may represent a partial or alternatively spliced cDNA of another member of the MN7 gene family on chromosome 15. The conservation of synteny between human and mouse predicts homology between the p region of mouse chromosome 7 and human chromosome 15q11–q13 (22, 23). Thus, the mouse rjs gene is the likely homolog of the MN7 gene family, originally isolated from the human PWS region (14, 21). Indeed, MN7 cross-hybridizing sequences were previously found to be deleted in p6H mice (24).
Approximately 70% of PWS patients lack a paternally derived region of 15q11–q13 (25), and it has been speculated that MN7 repeats may play a role in the genomic instability of this region (14). The remaining 30% of patients are uniparental disomic in this region or have a mutation in the imprinting center (26). Thus, critical sequences in this region in the human genome are imprinted. PWS patients exhibit common phenotypes, including small stature, mental retardation, behavior problems, hyperphagia, and sterility (27). PWS males have immature testes lacking germ cells and PWS females have small ovaries lacking follicular development or corpora lutea (28). Because of the phenotypic similarities with mice lacking the rjs gene, it is possible that the rjs gene will be important in understanding some aspects of PWS (22). Currently, at least five genes in the region are candidates for PWS on the basis of their imprinting status (26). We note that, unlike the situation in PWS, there is no evidence for imprinting of the rjs gene, as only homozygous mutant mice exhibit the mutant phenotypes (2).
The p6H/p6H deletion results in a loss of the carboxyl-terminal 4,212 amino acids and the addition of 27 novel amino acids from an IAP element. Among the ≈2,000 copies of IAP elements in the mouse genome (29), an IAP element was previously identified near the mouse IPW (Imprinted in Prader–Willi) syndrome gene (30). This IAP could be the same element found in the p6H/p6H mutant cDNA, or it could be an IAP from another part of chromosome 7.
The rjs-encoded amino acid sequence was found to contain several domains that may be of functional importance: RCC1 (regulator of chromosome condensation), cytochrome b5, and E6-AP ubiquitin ligase in the form of a HECT domain. There are three regions of rjs cDNA with homology to RCC1, whose structure was recently solved (20). RCC1 was originally isolated as the gene defective in the tsBN2 temperature-sensitive mutant cell line from hamster (31). Homologs of RCC1 have been isolated from many species and are characterized by seven homologous repeats of 50–60 amino acids (32). RCC1 associates with chromatin and also acts as a guanine nucleotide exchange factor (GEF) for a small GTP-binding protein, Ran. Ran, a nuclear localized homologue of Ras, is involved in nuclear membrane trafficking (33, 34). The chromatin binding and GEF activity of RCC1, along with genetic studies in which the loss of RCC1 uncoupled DNA replication from mitosis, suggest a role for RCC1 in the cell cycle communication between the cytoplasm and the chromatin status of the cell (16).
Cytochrome b5 is a membrane-bound protein that acts as an electron carrier for several membrane-bound oxygenases (35). There are two conserved histidine residues within its catalytic domain that serve as axial ligands for the heme group required for cytochrome b5 function. The rjs homology with the cytochrome b5 catalytic domain is not likely to indicate conserved function because the two critical histidine residues are not present in rjs. However, it is possible that this homology indicates conserved structure.
E6-AP is an E3 ubiquitin-protein ligase that accepts ubiquitin from E2 ubiquitin-conjugating enzyme and utilizes a conserved cysteine residue to transfer the ubiquitin to targeted substrates (18). E3 is believed to play an important role in the selection of proteins for degradation because E3 enzymes specifically bind protein substrates (36). Several proteins with HECT domains share function with E3 ubiquitin-protein ligase. The predicted carboxyl terminus of rjs contains a HECT domain (including the conserved cysteine at residue 4,765) that might play a role in the selection of proteins for degradation or in protein–protein interactions. The types of proteins targeted for degradation are diverse and include proteins that play a central role in cell cycle regulation (37). Roles for ubiquitination go beyond proteolysis to include possible roles in endocytosis (38) and phosphorylation (39). Recent reports suggest that UBE3, the human gene for E6-AP ubiquitin-ligase, is the 15q Angelman’s syndrome gene (40, 41). The ubiquitin pathway may play an important role in spermatogenesis. The ubiquitin carboxyl-terminal hydrolase Dffry gene is a candidate gene in the male sterility of human azoospermia factor a and the male sterility associated with the mouse Sxrb deletion (42). The HECT domain of rjs must be of critical functional importance, as it is this domain that is deleted in the pbs mutation.
A large protein, p532 (p619), has 24% overall identity with rjs and shares a similar structure. Although p532 is not a direct homolog of rjs, it does contain two regions of RCC1 homology and a HECT domain located at positions similar to those found in the rjs protein. Alignment of p532 and rjs reveals approximately 40% identity (60% similarity) between RCC1-like domains of rjs (RLDa and RLDc) and corresponding RCC1-like domains of p532 (RLD1 and RLD2), respectively. Conservation in the HECT domain is even higher, with 50% identity (66% similarity). The conservation may indicate a function for the rjs protein similar to what has been proposed for p532. The RLD1 region of p532 was found to possess guanine nucleotide exchange (GEF) activity on Ras superfamily members ARF1, Rab3A, and Rab5, suggesting a role for p532 in the regulation of membrane trafficking (17). This role was confirmed by the finding that RLD2 forms stable complexes with clathrin and the heat shock protein HSP70 (43). It was also found that cytosolic p532 is complexed with clathrin and HSP70, whereas Golgi vesicular-like membrane-associated p532 colocalizes with ARF1. Taken together, these results provide a model for the p532 protein in the membrane trafficking process. Structural similarities suggest that one or more of the RCC1-like domains of rjs may possess GEF activity, whereas the others might recruit interacting proteins.
Although the specific roles of the large rjs protein remain to be elucidated, its homologies suggest that different portions of the molecule may have diverse biochemical functions, with the protein as a whole acting to bring together and coordinate other proteins. The various phenotypes of the rjs mutants implies that multiple pathways are affected and that the rjs protein may have a distinct role in different pathways. Alternatively, the rjs protein may provide a physical link between interacting pathways. Especially intriguing is the potential role of rjs in cell cycle regulation. The cell cycle, including mitosis and meiosis, of male and female germ cells is tightly regulated during embryo development, with specific pauses in cell division. Neuronal development is also characterized by regulated cell division, with controlled cell division occurring at specific developmental check points. Thus, disruption of the timing of cell division in these tissues could cause changes in development resulting in the phenotypes seen in the rjs mutants. Alternatively, the homology to p532, believed to be involved in membrane trafficking, raises other possibilities. Membrane trafficking is critical for proper signaling in the nervous system. Disruption of normal trafficking could lead to aberrant levels of gonadotropins and altered signaling at neuromuscular junctions resulting in the defects observed in rjs mutants.
Early examinations of rjs mutants reported obvious morphological defects in the testes of males and fewer corpora lutea in the ovaries of females (6, 7). These defects were believed to be caused by endocrinological abnormalities in gonadotropins and structural defects in the pituitary (4, 6). Normal follicle development and spermatogenesis are both complex processes that require exogenous signals [follicle-stimulating hormone (FSH), luteinizing hormone (LH)] as well as timed programmed cell death (44–46). However, it is unlikely that FSH and LH are completely absent because females do get pregnant and do give birth. It is possible that FSH and LH decline later in development, so the estrous cycle is disrupted in older females and spermatogenesis is disrupted in males. Alternatively, the pituitary or hypothalamus could be defective, resulting in the multiple phenotypes seen in rjs mice.
The maternal behavior defect in the rjs mutants has been previously reported (8, 9). We observed the mutant mothers with their young and did not find they were hostile toward the young or completely indifferent. Mothers often cleaned the young after birth and spent time picking them up and moving them. Some mothers even spent time crouching over the young, although they did not make a nest. This led us to believe that the mothers might have defects in lactation leading to pup death. However, histological examination of mammary tissue taken from mothers within hours after birth revealed normal lactation. While the mothers are not completely indifferent, it is likely that they are not adequately attentive in caring for the pups to ensure their survival. These behaviors could be due to a hormonal defect or the result of a lack of a yet-unknown rjs activity.
Acknowledgments
The majority of this work was performed at The Fox Chase Cancer Center. We thank our colleagues John M. Gardner, Nobuko Hagiwara, Neelu Puri, Robert P. Erickson, Randall A. Heidenreich, Eva M. Eicher, and Muriel T. Davisson for helpful discussions. This work was supported by grants from the National Institutes of Health: GM22167 and GM/AR56181 (M.H.B.), CA09035 and CA06927 (Fox Chase Cancer Center), and HD08113 (A.L.L.).
ABBREVIATIONS
- GSP
gene-specific primer
- RACE
rapid amplification of cDNA ends
- RT-PCR
reverse transcription–PCR
- RLD
RCC1-like domains
- HECT
homology to E6-AP in their carboxyl terminus
- IAP
intracisternal A particle
- PWS
Prader–Willi syndrome
Footnotes
References
- 1.Silvers W K. The Coat Colors of Mice. New York: Springer; 1979. [Google Scholar]
- 2.Lyon M F, King T R, Gondo Y, Gardner J M, Nakatsu Y, Eicher E M, Brilliant M H. Proc Natl Acad Sci USA. 1992;89:6968–6972. doi: 10.1073/pnas.89.15.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rinchik E M, Carpenter D A, Handel M A. Proc Natl Acad Sci USA. 1995;92:6394–6398. doi: 10.1073/pnas.92.14.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson D R, Hunt D M. J Reprod Fertil. 1975;42:51–58. doi: 10.1530/jrf.0.0420051. [DOI] [PubMed] [Google Scholar]
- 5.Hunt D M, Johnson D R. J Embryol Exp Morphol. 1971;26:111–121. [PubMed] [Google Scholar]
- 6.Meldvold R W. Genet Res. 1974;23:319–325. doi: 10.1017/s0016672300014956. [DOI] [PubMed] [Google Scholar]
- 7.Wolfe H G. Genetics. 1977;85:303–308. doi: 10.1093/genetics/85.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollander W F, Bryan H D, Gowen J W. Genetics. 1960;45:413–418. doi: 10.1093/genetics/45.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolfe H G. Genetics. 1973;74:298s. (abstr.). [Google Scholar]
- 10.Russell L B, Montgomery C S, Cacheiro N L A, Johnson D K. Genetics. 1995;141:1547–1562. doi: 10.1093/genetics/141.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brilliant M H, Gondo Y, Eicher E. Science. 1991;252:566–569. doi: 10.1126/science.1673574. [DOI] [PubMed] [Google Scholar]
- 12.Lorenzi M V, Long J E, Miki T, Aaronson S A. Oncogene. 1995;10:2051–2055. [PubMed] [Google Scholar]
- 13.Frohman M A. In: The Polymerase Chain Reaction. Mullis K B, Ferre F, Gibbs R A, editors. Boston: Birkhauser; 1994. pp. 25–30. [Google Scholar]
- 14.Buiting K, Greger V, Brownstein B H, Mohr R M, Voiculescu I, Winterpacht A, Zabel B, Horsthemke B. Proc Natl Acad Sci USA. 1992;89:5457–5461. doi: 10.1073/pnas.89.12.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagase T, Ishikawa K, Nakajima D, Ohira M, Seki N, Miyajima N, Tanaka A, Kotani H, Nomura N, Ohara O. DNA Res. 1997;4:141–150. doi: 10.1093/dnares/4.2.141. [DOI] [PubMed] [Google Scholar]
- 16.Seki T, Hayashi N, Nishimoto T. J Biochem (Tokyo) 1996;120:207–214. doi: 10.1093/oxfordjournals.jbchem.a021400. [DOI] [PubMed] [Google Scholar]
- 17.Rosa J L, Casaroli-Marano R P, Buckler A J, Vilaro S, Barbacid M. EMBO J. 1996;15:4262–4273. [PMC free article] [PubMed] [Google Scholar]
- 18.Huibregtse J M, Scheffner M, Beaudenon S, Howley P M. Proc Natl Acad Sci USA. 1995;92:2563–2567. doi: 10.1073/pnas.92.7.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofmann K, Stoffel W. Biol Chem Hoppe-Seyler. 1993;374:166. doi: 10.1515/bchm3.1993.374.7-12.507. (abstr.). [DOI] [PubMed] [Google Scholar]
- 20.Renault L, Nassar N, Vetter I, Becker J, Klebe C, Roth M, Wittinghofer A. Nature (London) 1998;392:97–101. doi: 10.1038/32204. [DOI] [PubMed] [Google Scholar]
- 21.Buiting K, Neumann M, Ludecke H-J, Senger G, Claussen U, Antich J, Passarge E, Horsthemke B. Genomics. 1990;6:521–527. doi: 10.1016/0888-7543(90)90481-9. [DOI] [PubMed] [Google Scholar]
- 22.Brilliant M H. Mamm Genome. 1992;3:187–191. doi: 10.1007/BF00355717. [DOI] [PubMed] [Google Scholar]
- 23.Brilliant M H, Williams R W, Holdener B C, Angel J M, Stern M, Hunter K. Mamm Genome. 1997;7:s121–s142. doi: 10.1007/s003359900319. [DOI] [PubMed] [Google Scholar]
- 24.Nicholls R D, Gottlieb W, Russell L B, Davda M, Horsthemke B, Rinchik E M. Proc Natl Acad Sci USA. 1990;90:2050–2054. doi: 10.1073/pnas.90.5.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christian S L, Robinson W P, Huang B, Mutirangura A, Line M R, Nakao M, Surti U, Chakravarti A, Ledbetter D H. Am J Hum Genet. 1995;57:40–48. [PMC free article] [PubMed] [Google Scholar]
- 26.Horsthemke B. J Cell Physiol. 1997;173:237–241. doi: 10.1002/(SICI)1097-4652(199711)173:2<237::AID-JCP28>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 27.Cassidy S B. J Med Genet. 1997;34:917–923. doi: 10.1136/jmg.34.11.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bray G A, Dahms W T, Swerdloff R S, Fiser R H, Atkinson R L, Carrel R E. Medicine. 1983;62:59–80. [PubMed] [Google Scholar]
- 29.Kuff E L, Lueders K K. Adv Cancer Res. 1988;51:183–276. doi: 10.1016/s0065-230x(08)60223-7. [DOI] [PubMed] [Google Scholar]
- 30.Wevrick R, Francke U. Hum Mol Genet. 1997;6:325–332. doi: 10.1093/hmg/6.2.325. [DOI] [PubMed] [Google Scholar]
- 31.Kai R, Ohtsubo M, Sekiguchi M, Nishimoto T. Mol Cell Biol. 1986;6:2027–2032. doi: 10.1128/mcb.6.6.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohtsubo M, Yoshida T, Seino H, Nishitani H, Clark K L, Sprague G F, Jr, Frasch M, Nishimoto T. EMBO J. 1991;10:1265–1273. doi: 10.1002/j.1460-2075.1991.tb08068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoneda Y. J Biochem (Tokyo) 1997;121:811–817. doi: 10.1093/oxfordjournals.jbchem.a021657. [DOI] [PubMed] [Google Scholar]
- 34.Gorlich D. Curr Opin Cell Biol. 1997;9:412–419. doi: 10.1016/s0955-0674(97)80015-4. [DOI] [PubMed] [Google Scholar]
- 35.Ozols J. Biochim Biophys Acta. 1989;997:121–130. doi: 10.1016/0167-4838(89)90143-x. [DOI] [PubMed] [Google Scholar]
- 36.Ciechanover A. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 37.Nefsky B, Beach D. EMBO J. 1996;15:1301–1312. [PMC free article] [PubMed] [Google Scholar]
- 38.Hicke L, Riezman H. Cell. 1996;84:277–287. doi: 10.1016/s0092-8674(00)80982-4. [DOI] [PubMed] [Google Scholar]
- 39.Chen, Z. J., Parent, L. & Maniatis, T. Cell 84, 853–862. [DOI] [PubMed]
- 40.Kishino T, Lalande M, Wagstaff J. Nat Genet. 1997;15:70–73. doi: 10.1038/ng0197-70. [DOI] [PubMed] [Google Scholar]
- 41.Matsuura T, Sutcliffe J S, Fang P, Galjaard R J, Jiang Y, Benton C S, Rommens J M, Beaudet A L. Nat Genet. 1997;15:74–77. doi: 10.1038/ng0197-74. [DOI] [PubMed] [Google Scholar]
- 42.Brown G M, Furlong R A, Sargent C A, Erickson R P, Longepied G, Mitchell M, Jones M H, Hargreave T B, Cooke H J, Affara N A. Hum Mol Genet. 1998;7:97–107. doi: 10.1093/hmg/7.1.97. [DOI] [PubMed] [Google Scholar]
- 43.Rosa J L, Barbacid M. Oncogene. 1997;15:1–6. doi: 10.1038/sj.onc.1201170. [DOI] [PubMed] [Google Scholar]
- 44.Tapanainen J S, Tilly J L, Vihko K K, Hsueh A J W. Mol Endocrinol. 1993;7:643–650. doi: 10.1210/mend.7.5.8316250. [DOI] [PubMed] [Google Scholar]
- 45.Steinberger E. Physiol Rev. 1971;51:1–22. doi: 10.1152/physrev.1971.51.1.1. [DOI] [PubMed] [Google Scholar]
- 46.Hirshfield A N. Intl Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]