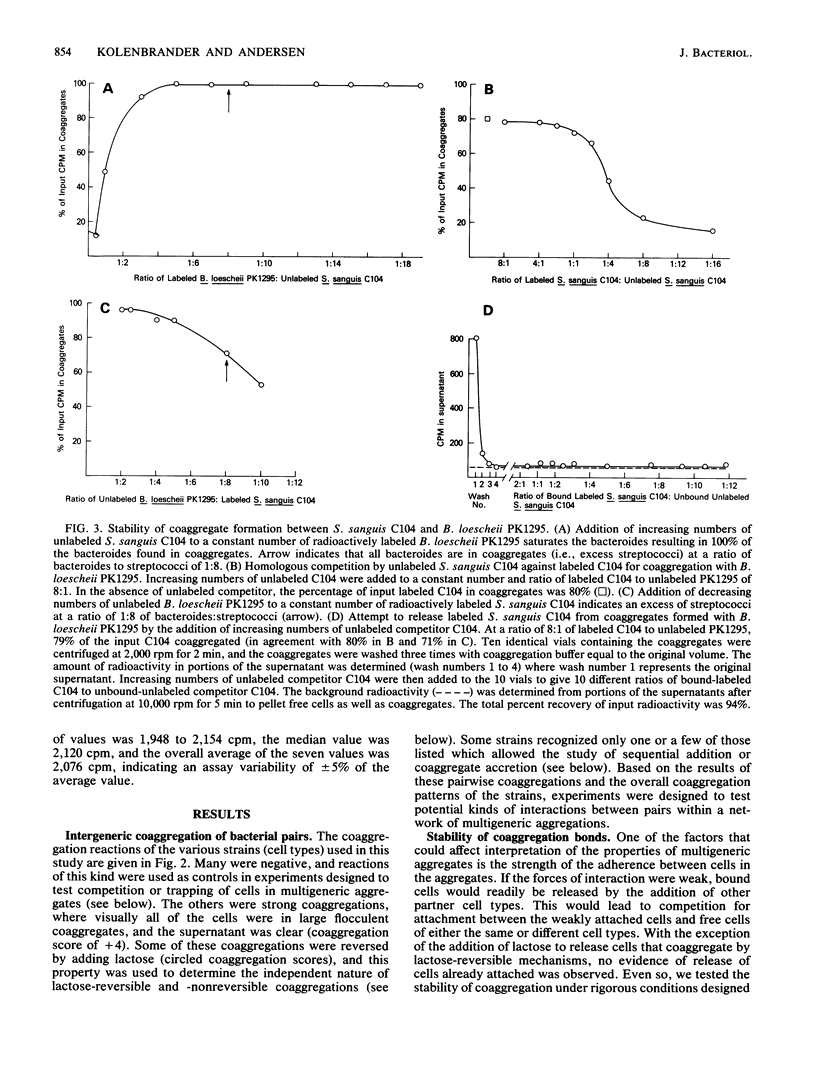
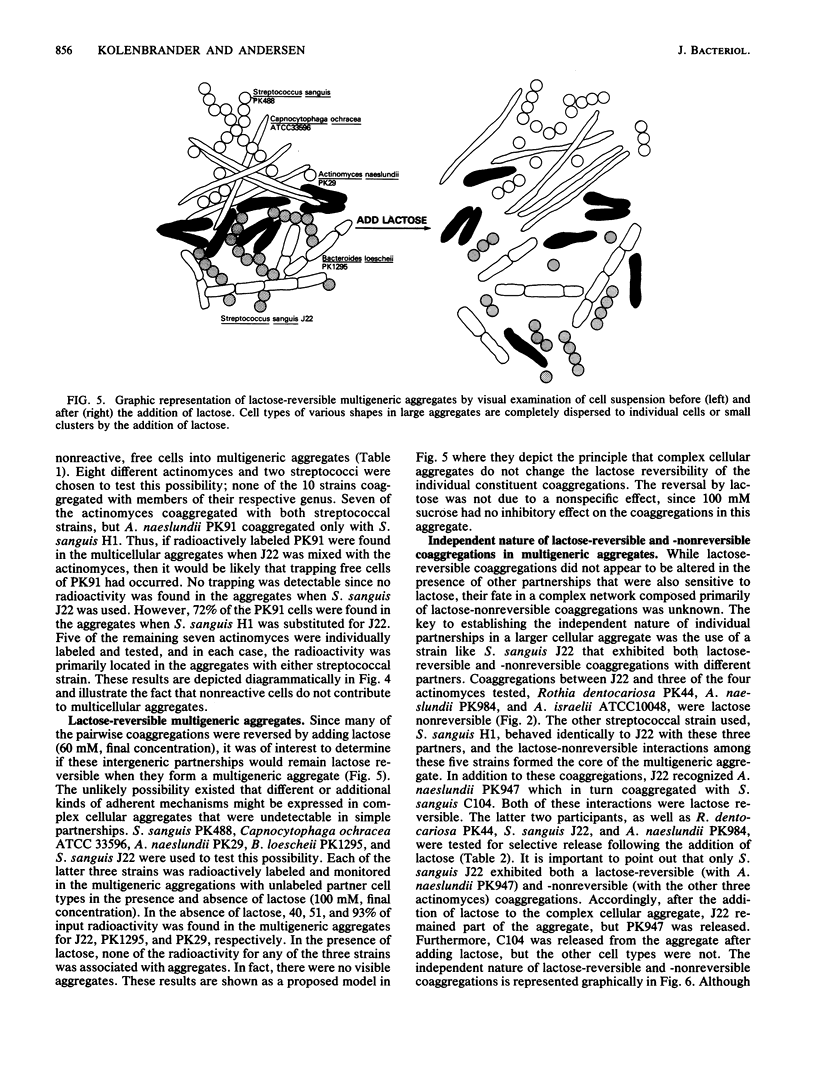
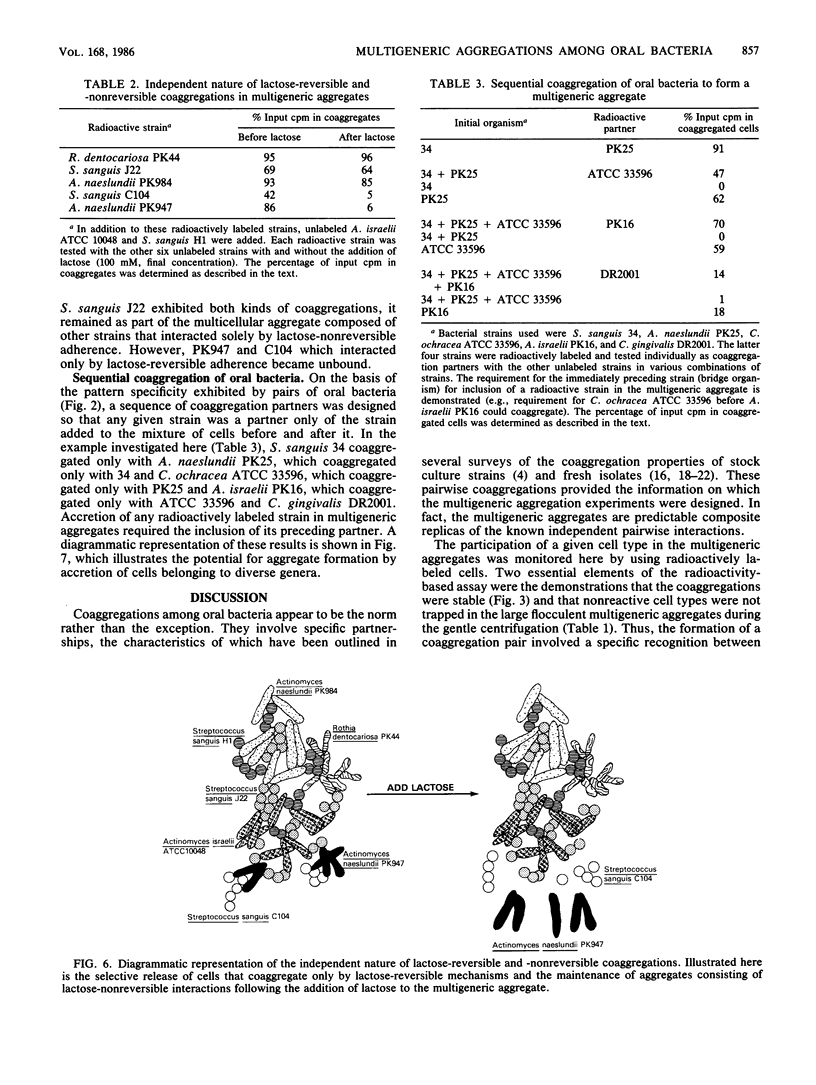
Abstract
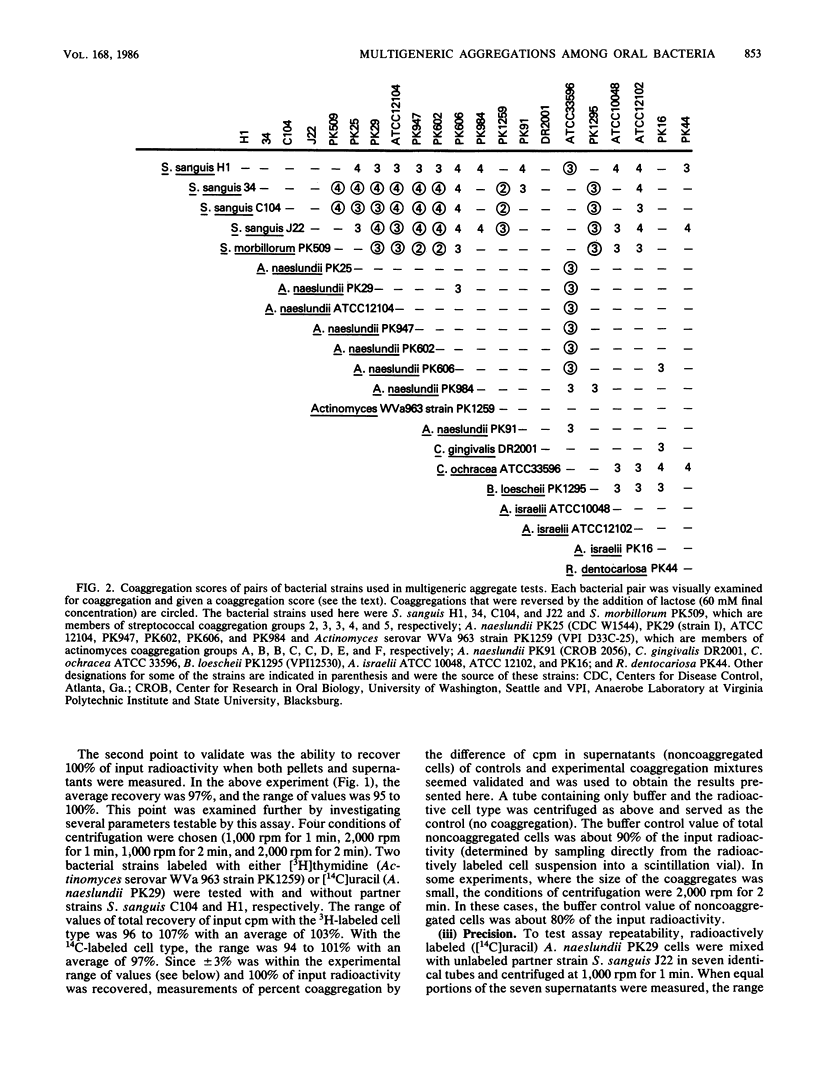
A radioactivity-based assay was developed to define the participation of radioactively labeled cell types within the milieu of unlabeled partners in multigeneric aggregates. The cell types in these multigeneric aggregations consisted of various combinations of 21 strains representing five genera of human oral bacteria. The coaggregation properties of each cell type, when paired individually with various strains, were delineated and were unchanged when the microbes took part in the more complex multigeneric aggregations. Competition between homologous labeled and unlabeled cells for binding to a partner cell type was achieved only when the homologous cells were mixed together before the addition of their partner cells. Attempts to displace a labeled cell type from an aggregate by subsequent addition of a large excess of the same unlabeled cell type were unsuccessful, which suggested that the forces that bound different cell types together were very strong and the cell-to-cell interactions were stable. However, a cell type that exhibited only lactose-reversible coaggregations with partners was easily and selectively released by the addition of lactose to multigeneric aggregates otherwise consisting solely of lactose-nonreversible cell-to-cell interactions. This not only indicates the independent nature of individual coaggregations but also suggests the involvement of lectinlike adhesins in these sugar-inhibitable coaggregations. Although the molecular mechanisms responsible for multigeneric aggregations are unknown, the principle of a common partner cell type serving as a bridge between two otherwise noncoaggregating cell types was firmly established by the observation of sequential addition of one cell type to another. Thus, competition, bridging, coaggregate stability, independent nature of interactions, and partner specificity are the key principles of adherence that form the framework for continued studies of multigeneric aggregates. While the human oral cavity is a prime example of a complex microbial community, collectively the community appears to consist of simple and testable individual interactions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baseman J. B., Banai M., Kahane I. Sialic acid residues mediate Mycoplasma pneumoniae attachment to human and sheep erythrocytes. Infect Immun. 1982 Oct;38(1):389–391. doi: 10.1128/iai.38.1.389-391.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breznak J. A., Pankratz H. S. In situ morphology of the gut microbiota of wood-eating termites [Reticulitermes flavipes (Kollar) and Coptotermes formosanus Shiraki]. Appl Environ Microbiol. 1977 Feb;33(2):406–426. doi: 10.1128/aem.33.2.406-426.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celesk R. A., London J. Attachment of oral Cytophaga species to hydroxyapatite-containing surfaces. Infect Immun. 1980 Aug;29(2):768–777. doi: 10.1128/iai.29.2.768-777.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Napoli C. A., Hubbell D. H. Adsorption of bacteria to roots as related to host specificity in the Rhizobium-clover symbiosis. Appl Environ Microbiol. 1976 Jul;32(1):166–171. doi: 10.1128/aem.32.1.166-171.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Balcerzak-Raczkowski I. B. Interbacterial aggregation of Actinomyces naeslundii and dental plaque streptococci. J Periodontal Res. 1977 Jan;12(1):11–20. doi: 10.1111/j.1600-0765.1977.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Geesey G. G., Richardson W. T., Yeomans H. G., Irvin R. T., Costerton J. W. Microscopic examination of natural sessile bacterial populations from an alpine stream. Can J Microbiol. 1977 Dec;23(12):1733–1736. doi: 10.1139/m77-249. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Interbacterial aggregation of plaque bacteria. Arch Oral Biol. 1970 Dec;15(12):1397–1400. doi: 10.1016/0003-9969(70)90031-2. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Freter R. Adhesive properties of Vibrio cholerae: nature of the interaction with isolated rabbit brush border membranes and human erythrocytes. Infect Immun. 1976 Jul;14(1):240–245. doi: 10.1128/iai.14.1.240-245.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagermeier A. S., London J., Kolenbrander P. E. Evidence for the participation of N-acetylated amino sugars in the coaggregation between Cytophaga species strain DR2001 and Actinomyces israelii PK16. Infect Immun. 1984 May;44(2):299–305. doi: 10.1128/iai.44.2.299-305.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagermeier A., London J. Identification and preliminary characterization of a lectinlike protein from Capnocytophaga gingivalis (emended). Infect Immun. 1986 Feb;51(2):490–494. doi: 10.1128/iai.51.2.490-494.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelstrup J., Funder-Nielsen T. D. Aggregation of oral streptococci with Fusobacterium and Actinomyces. J Biol Buccale. 1974 Dec;2(4):347–362. [PubMed] [Google Scholar]
- Kolenbrander P. E., Andersen R. N. Cell to cell interactions of Capnocytophaga and Bacteroides species with other oral bacteria and their potential role in development of plaque. J Periodontal Res. 1984 Nov;19(6):564–569. doi: 10.1111/j.1600-0765.1984.tb01315.x. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E., Andersen R. N., Holdeman L. V. Coaggregation of oral Bacteroides species with other bacteria: central role in coaggregation bridges and competitions. Infect Immun. 1985 Jun;48(3):741–746. doi: 10.1128/iai.48.3.741-746.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Celesk R. A. Coaggregation of human oral Cytophaga species and Actinomyces israelii. Infect Immun. 1983 Jun;40(3):1178–1185. doi: 10.1128/iai.40.3.1178-1185.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Inouye Y., Holdeman L. V. New Actinomyces and Streptococcus coaggregation groups among human oral isolates from the same site. Infect Immun. 1983 Aug;41(2):501–506. doi: 10.1128/iai.41.2.501-506.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Williams B. L. Lactose-reversible coaggregation between oral actinomycetes and Streptococcus sanguis. Infect Immun. 1981 Jul;33(1):95–102. doi: 10.1128/iai.33.1.95-102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Williams B. L. Prevalence of viridans streptococci exhibiting lactose-inhibitable coaggregation with oral actinomycetes. Infect Immun. 1983 Aug;41(2):449–452. doi: 10.1128/iai.41.2.449-452.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Källenius G., Svenson S., Möllby R., Cedergren B., Hultberg H., Winberg J. Structure of carbohydrate part of receptor on human uroepithelial cells for pyelonephritogenic Escherichia coli. Lancet. 1981 Sep 19;2(8247):604–606. doi: 10.1016/s0140-6736(81)92743-4. [DOI] [PubMed] [Google Scholar]
- LOE H., THEILADE E., JENSEN S. B. EXPERIMENTAL GINGIVITIS IN MAN. J Periodontol. 1965 May-Jun;36:177–187. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- Leffler H., Svanborg-Edén C. Glycolipid receptors for uropathogenic Escherichia coli on human erythrocytes and uroepithelial cells. Infect Immun. 1981 Dec;34(3):920–929. doi: 10.1128/iai.34.3.920-929.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Crosby L. K., Barlow J. J., Matta K. L. Structural preferences of beta-galactoside-reactive lectins on Actinomyces viscosus T14V and Actinomyces naeslundii WVU45. Infect Immun. 1983 Aug;41(2):848–850. doi: 10.1128/iai.41.2.848-850.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Crosby L. K., Vatter A. E. Inhibitors of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34: beta-galactosides, related sugars, and anionic amphipathic compounds. Infect Immun. 1982 Apr;36(1):371–378. doi: 10.1128/iai.36.1.371-378.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Cato E. P., Smibert R. M., Burmeister J. A., Ranney R. R. Bacteriology of moderate (chronic) periodontitis in mature adult humans. Infect Immun. 1983 Nov;42(2):510–515. doi: 10.1128/iai.42.2.510-515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Smibert R. M., Good I. J., Burmeister J. A., Palcanis K. G., Ranney R. R. Bacteriology of experimental gingivitis in young adult humans. Infect Immun. 1982 Nov;38(2):651–667. doi: 10.1128/iai.38.2.651-667.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Smibert R. M., Hash D. E., Burmeister J. A., Ranney R. R. Bacteriology of severe periodontitis in young adult humans. Infect Immun. 1982 Dec;38(3):1137–1148. doi: 10.1128/iai.38.3.1137-1148.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Paerl H. W., Gallucci K. K. Role of chemotaxis in establishing a specific nitrogen-fixing cyanobacterial-bacterial association. Science. 1985 Feb 8;227(4687):647–649. doi: 10.1126/science.227.4687.647. [DOI] [PubMed] [Google Scholar]
- Slots J., Gibbons R. J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978 Jan;19(1):254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilade E., Wright W. H., Jensen S. B., Löe H. Experimental gingivitis in man. II. A longitudinal clinical and bacteriological investigation. J Periodontal Res. 1966;1:1–13. doi: 10.1111/j.1600-0765.1966.tb01842.x. [DOI] [PubMed] [Google Scholar]
- Tylenda C. A., Enriquez E., Kolenbrander P. E., Delisle A. L. Simultaneous loss of bacteriophage receptor and coaggregation mediator activities in Actinomyces viscosus MG-1. Infect Immun. 1985 Apr;48(1):228–233. doi: 10.1128/iai.48.1.228-233.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerkamp A. H., McBride B. C. Identification of a Streptococcus salivarius cell wall component mediating coaggregation with Veillonella alcalescens V1. Infect Immun. 1981 May;32(2):723–730. doi: 10.1128/iai.32.2.723-730.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]