Abstract
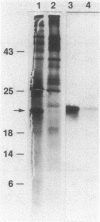
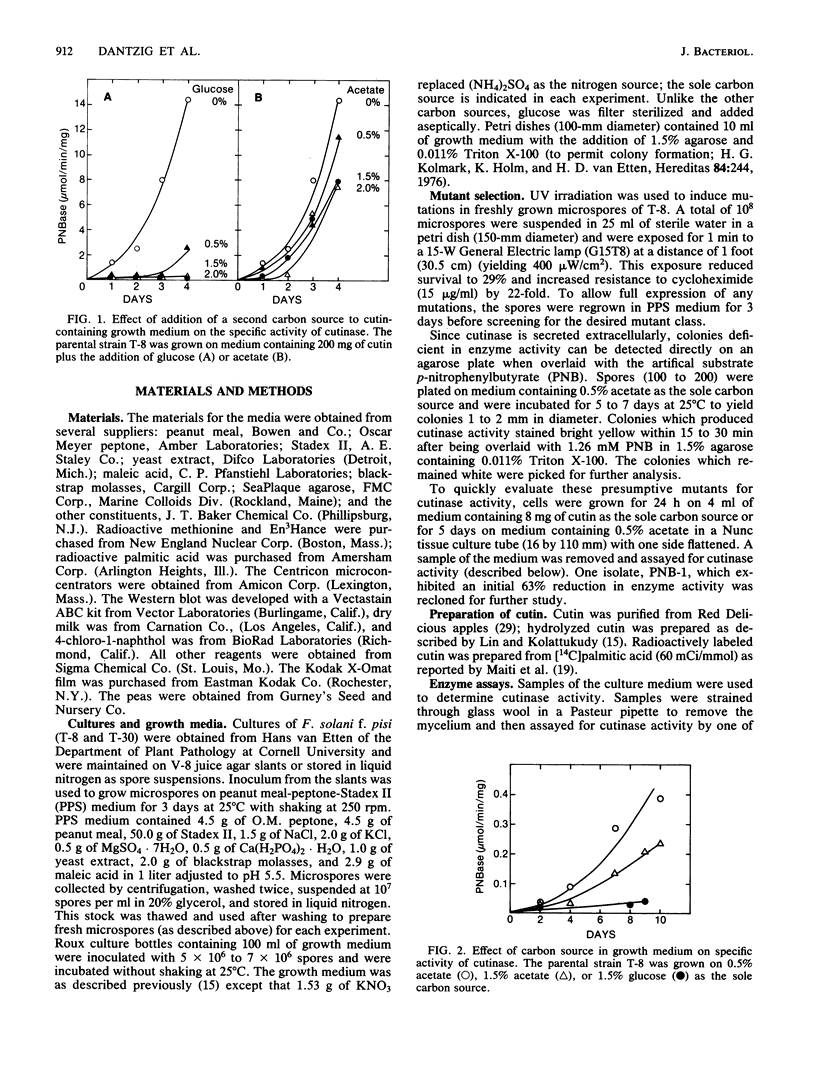
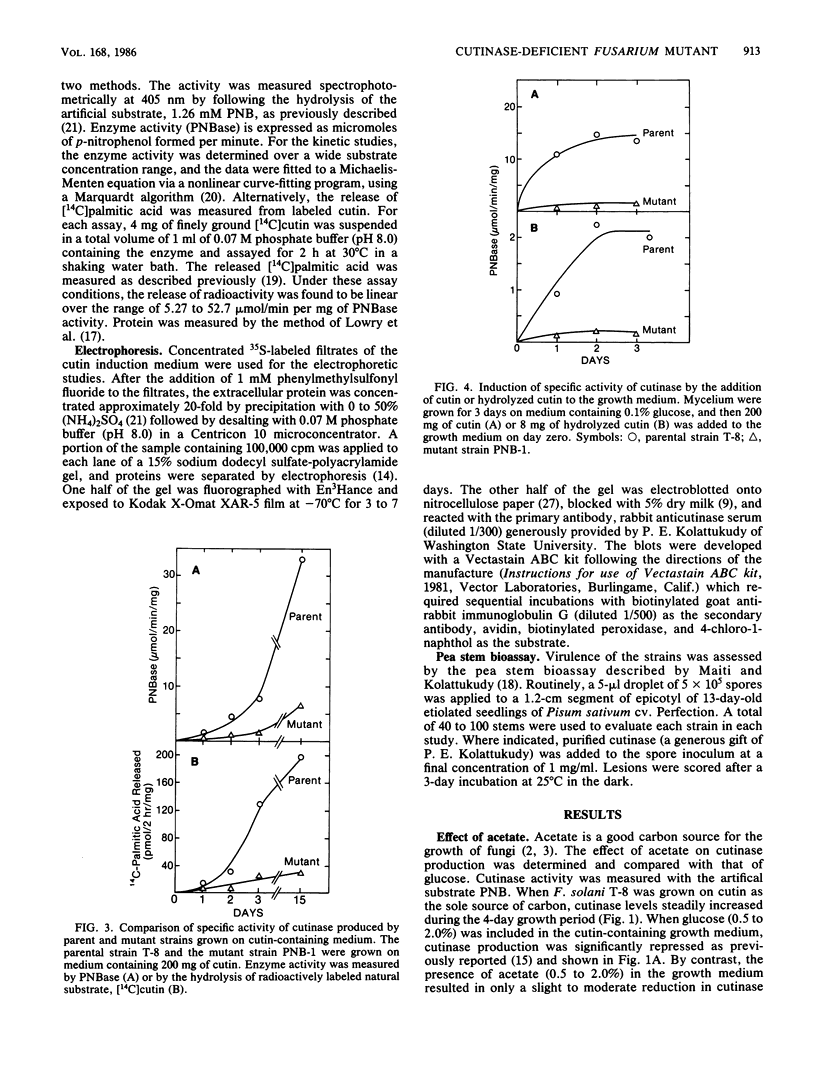
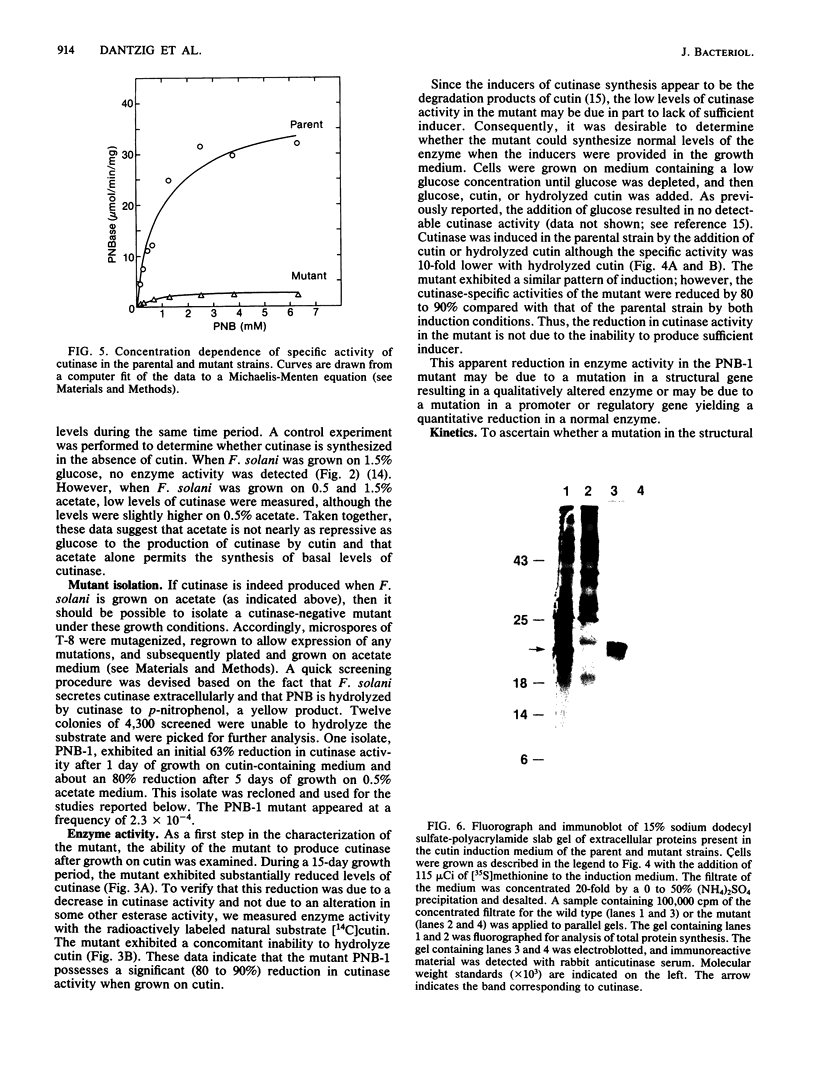
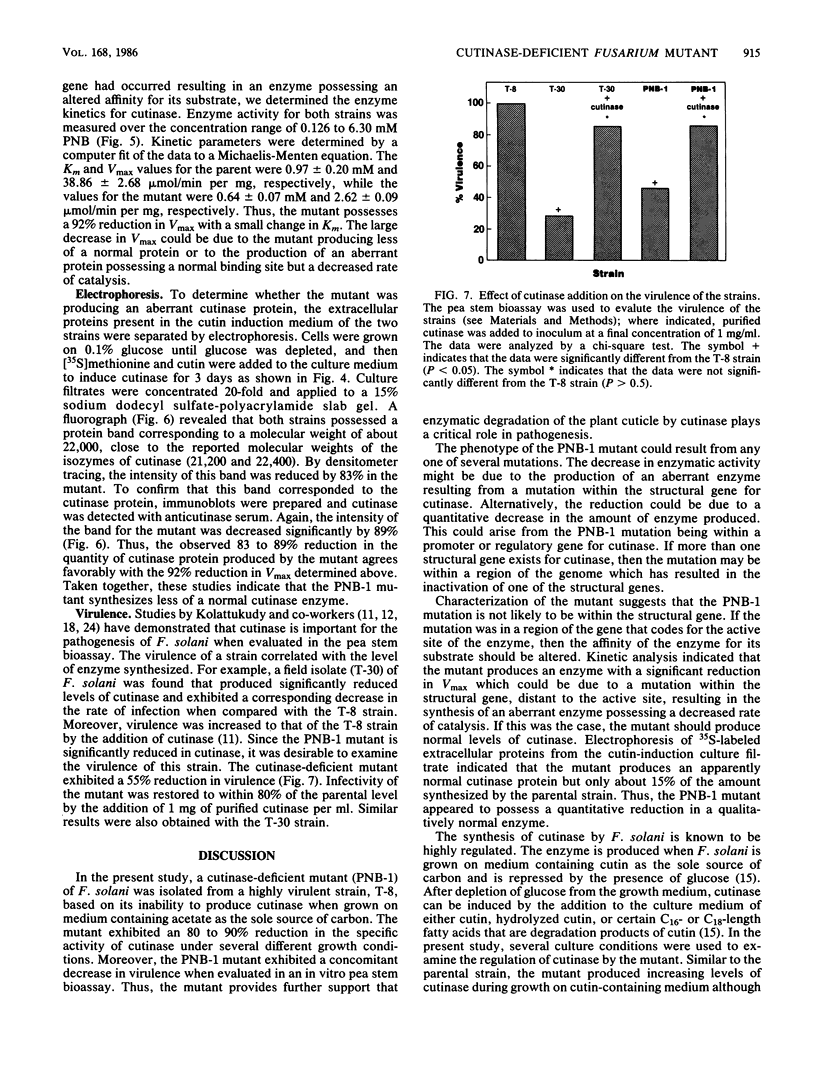
Fusarium solani isolate T-8 produces an extracellular enzyme, cutinase, which catalyzes the degradation of cutin in the plant cuticle. Cutinase activity can be measured by the hydrolysis of either the artifical substrate, p-nitrophenylbutyrate (PNB), or radioactive cutin containing [14C]palmitic acid. In the present study, the culture filtrate contained basal levels of cutinase when T-8 was grown on acetate as a sole source of carbon. After mutagenesis, a cutinase-defective mutant (PNB-1) was identified by screening acetate-grown colonies for a loss of PNBase activity. The mutant possessed an 80 to 90% reduction in cutinase activity when grown for 3 to 5 days on acetate- or cutin-containing medium. Induction of cutinase by cutin or hydrolyzed cutin after growth on glucose medium was similarly reduced. Kinetic analysis indicated that cutinase from the mutant possessed a near normal Km for PNB and a 92% reduction in Vmax. Fluorography and Western blotting of 15% sodium dodecyl sulfate-polyacrylamide gels of separated 35S-labeled proteins from cutin induction medium revealed that in the mutant the 22,000-molecular-weight band corresponding to cutinase was reduced approximately 85%. The virulence of the mutant in a pea stem bioassay was decreased by 55% and was restored to nearly the parental level by the addition of purified cutinase. The data suggest that the mutant synthesizes reduced quantities of a functional and immunoreactive cutinase enzyme and that cutinase plays a critical role in infection. The PNB1 mutation may be within a regulatory gene or a promoter for cutinase.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Flurkey W. H., Kolattukudy P. E. In vitro translation of cutinase mRNA: evidence for a precursor form of an extracellular fungal enzyme. Arch Biochem Biophys. 1981 Nov;212(1):154–161. doi: 10.1016/0003-9861(81)90354-4. [DOI] [PubMed] [Google Scholar]
- Köller W., Kolattukudy P. E. Mechanism of action of cutinase: chemical modification of the catalytic triad characteristic for serine hydrolases. Biochemistry. 1982 Jun 22;21(13):3083–3090. doi: 10.1021/bi00256a008. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin T. S., Kolattukudy P. E. Induction of a biopolyester hydrolase (cutinase) by low levels of cutin monomers in Fusarium solani f.sp. pisi. J Bacteriol. 1978 Feb;133(2):942–951. doi: 10.1128/jb.133.2.942-951.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti I. B., Kolattukudy P. E. Prevention of fungal infection of plants by specific inhibition of cutinase. Science. 1979 Aug 3;205(4405):507–508. doi: 10.1126/science.205.4405.507. [DOI] [PubMed] [Google Scholar]
- Maiti I. B., Kolattukudy P. E., Shaykh M. Purification and characterization of novel cutinase from nasturtium (Tropaeolum majus) pollen. Arch Biochem Biophys. 1979 Sep;196(2):412–423. doi: 10.1016/0003-9861(79)90292-3. [DOI] [PubMed] [Google Scholar]
- Purdy R. E., Kolattukudy P. E. Depolymerization of a hydroxy fatty acid biopolymer, cutin, by an extracellular enzyme from Fusarium solani f. pisi: isolation and some properties of the enzyme. Arch Biochem Biophys. 1973 Nov;159(1):61–69. doi: 10.1016/0003-9861(73)90429-3. [DOI] [PubMed] [Google Scholar]
- Purdy R. E., Kolattukudy P. E. Hydrolysis of plant cuticle by plant pathogens. Properties of cutinase I, cutinase II, and a nonspecific esterase isolated from Fusarium solani pisi. Biochemistry. 1975 Jul;14(13):2832–2840. doi: 10.1021/bi00684a007. [DOI] [PubMed] [Google Scholar]
- Purdy R. E., Kolattukudy P. E. Hydrolysis of plant cuticle by plant pathogens. Purification, amino acid composition, and molecular weight of two isozymes of cutinase and a nonspecific esterase from Fusarium solani f. pisi. Biochemistry. 1975 Jul;14(13):2824–2831. doi: 10.1021/bi00684a006. [DOI] [PubMed] [Google Scholar]
- Shaykh M., Soliday C., Kolattukudy P. E. Proof for the Production of Cutinase by Fusarium solani f. pisi during Penetration into Its Host, Pisum sativum. Plant Physiol. 1977 Jul;60(1):170–172. doi: 10.1104/pp.60.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliday C. L., Flurkey W. H., Okita T. W., Kolattukudy P. E. Cloning and structure determination of cDNA for cutinase, an enzyme involved in fungal penetration of plants. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3939–3943. doi: 10.1073/pnas.81.13.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliday C. L., Kolattukudy P. E. Primary structure of the active site region of fungal cutinase, an enzyme involved in phytopathogenesis. Biochem Biophys Res Commun. 1983 Aug 12;114(3):1017–1022. doi: 10.1016/0006-291x(83)90663-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton T. J., Kolattukudy P. E. Determination of the structures of cutin monomers by a novel depolymerization procedure and combined gas chromatography and mass spectrometry. Biochemistry. 1972 May 9;11(10):1885–1896. doi: 10.1021/bi00760a025. [DOI] [PubMed] [Google Scholar]