Abstract
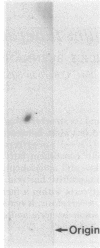
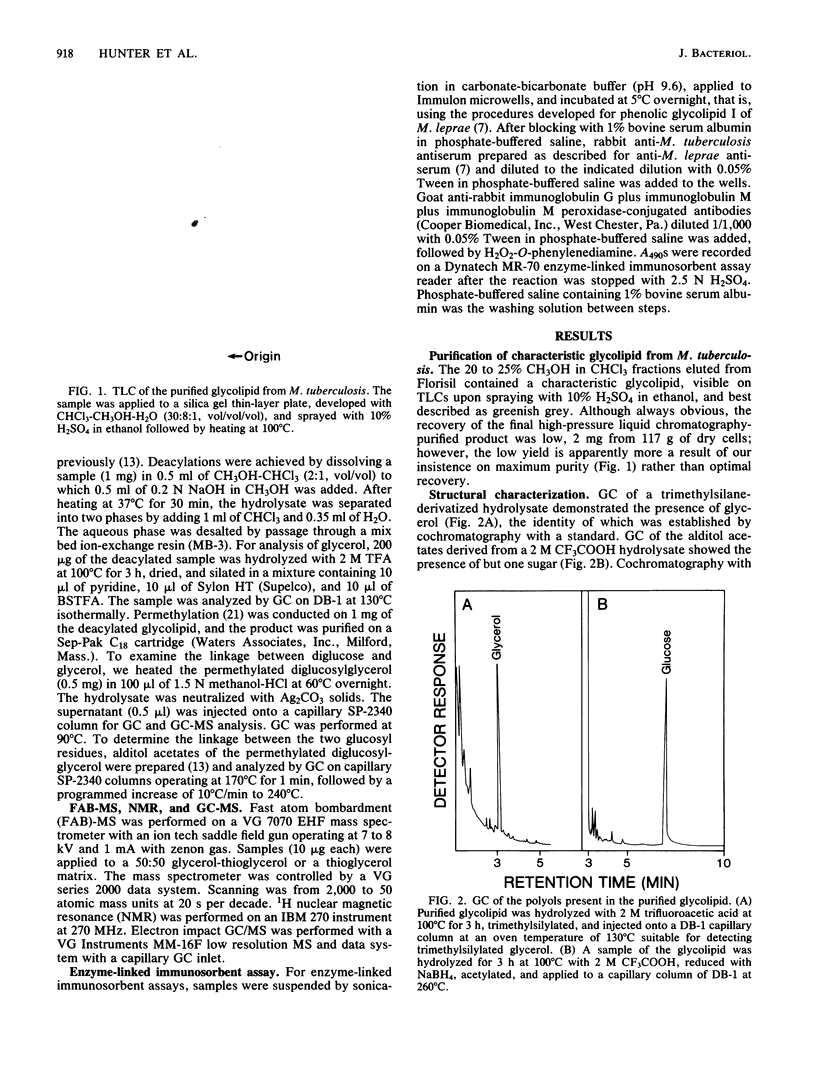
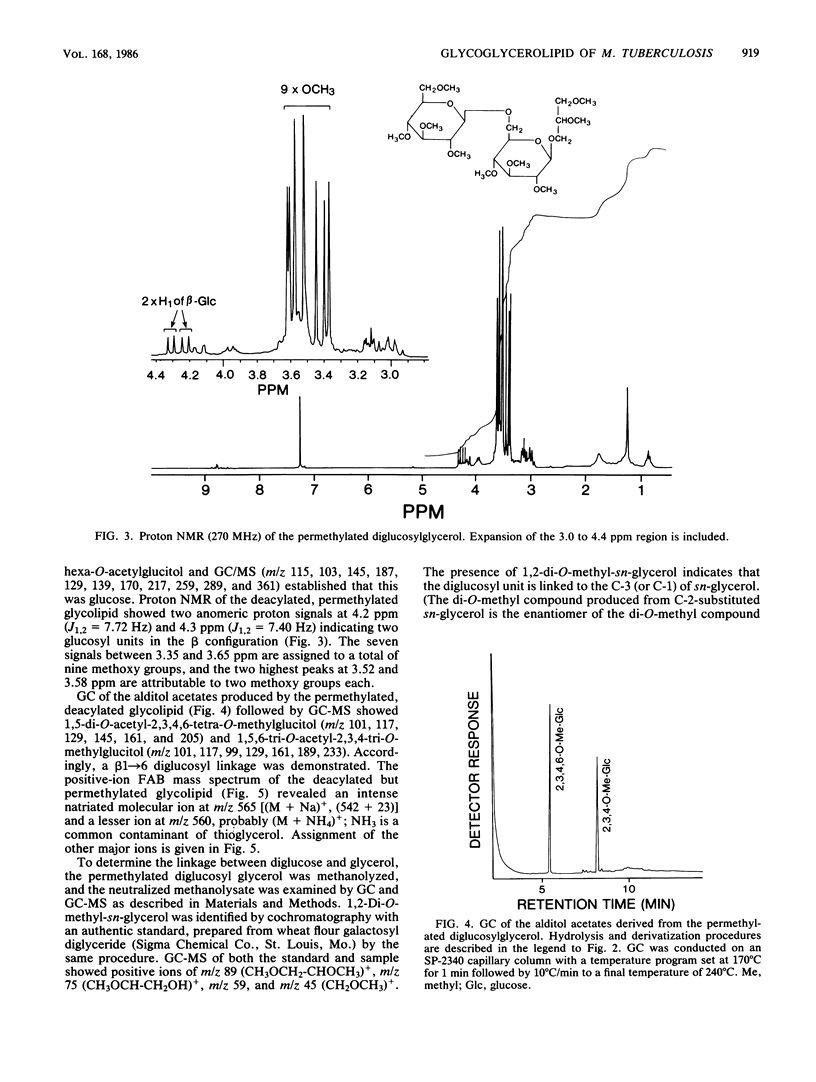
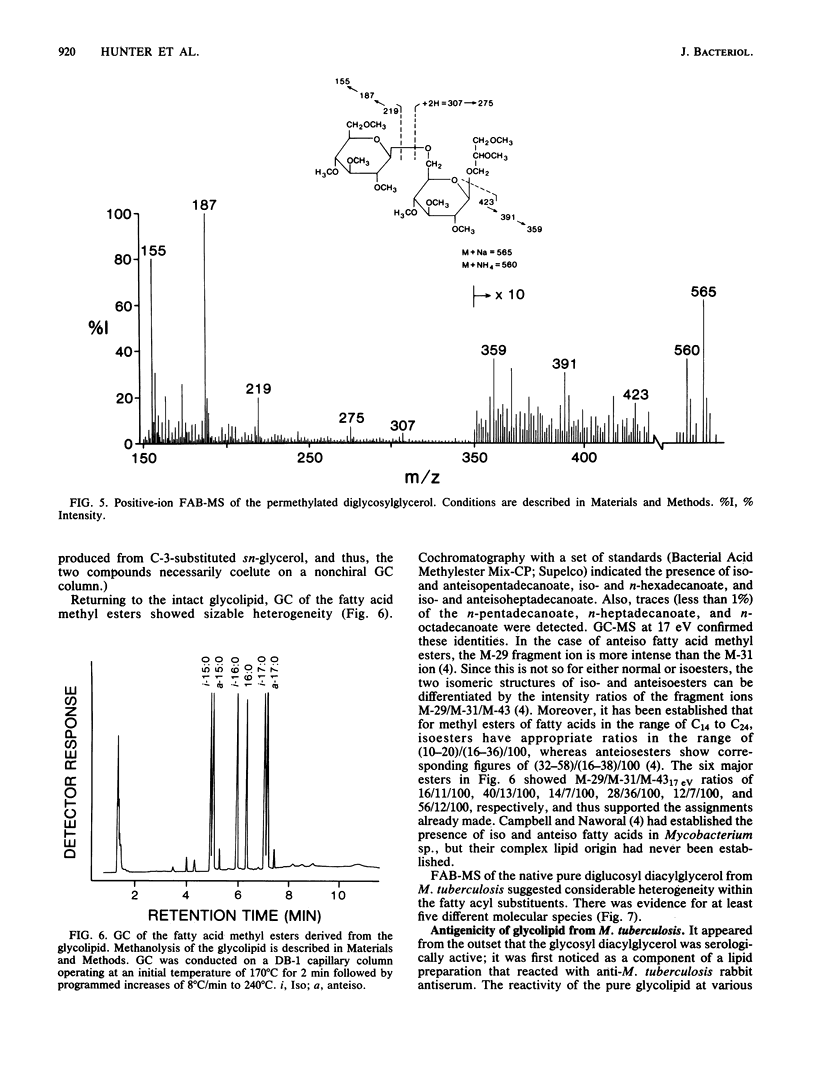
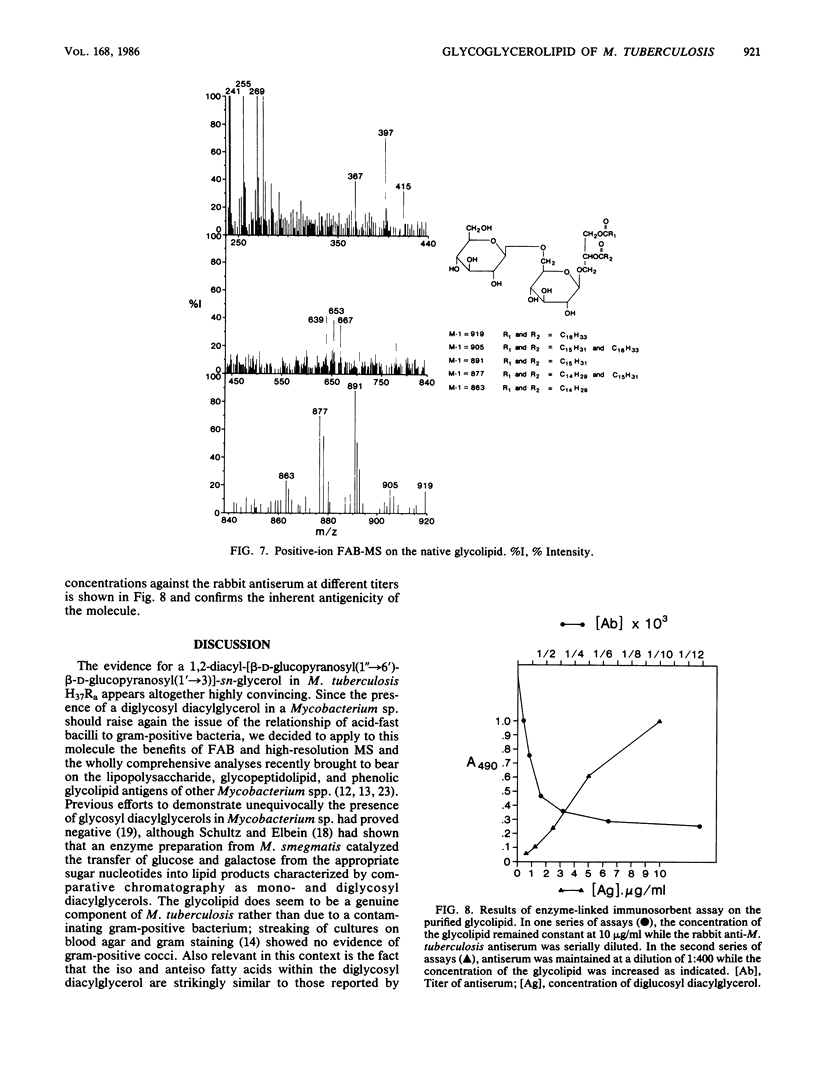
A diglycosyl diacylglycerol was isolated from Mycobacterium tuberculosis, and its structure was established by a combination of methylation analysis, 1H nuclear magnetic resonance, and fast atom bombardment-mass spectrometry. It is a 1,2-diacyl-[beta-D-glucopyranosyl(1"----6')-beta-D-glucopyranosyl(1'---- 3)]- sn-glycerol and exists in at least five molecular species differing in fatty acyl substituents. The major constituent fatty acids were identified as iso- and anteisopentadecanoate, iso- and n-hexadecanoate, and iso- and anteisoheptadecanoate. Although glycosyl diacylglycerols are common membrane components of gram-positive bacteria, this report represents the first substantial evidence for the presence of a glycosyl diacylglycerol within a member of the Mycobacterium genus. Although the glycolipid is not a major component of M. tuberculosis, it reacts readily in enzyme-linked immunosorbent assay against rabbit antibodies raised against whole bacteria and thus may be useful for the serodiagnosis of tuberculosis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brennan P. J., Goren M. B. Structural studies on the type-specific antigens and lipids of the mycobacterium avium. Mycobacterium intracellulare. Mycobacterium scrofulaceum serocomplex. Mycobacterium intracellulare serotype 9. J Biol Chem. 1979 May 25;254(10):4205–4211. [PubMed] [Google Scholar]
- Brett S. J., Draper P., Payne S. N., Rees R. J. Serological activity of a characteristic phenolic glycolipid from Mycobacterium leprae in sera from patients with leprosy and tuberculosis. Clin Exp Immunol. 1983 May;52(2):271–279. [PMC free article] [PubMed] [Google Scholar]
- Campbell I. M., Naworal J. Composition of the saturated and monounsaturated fatty acids of Mycobacterium phlei. J Lipid Res. 1969 Sep;10(5):593–598. [PubMed] [Google Scholar]
- Cho S. N., Fujiwara T., Hunter S. W., Rea T. H., Gelber R. H., Brennan P. J. Use of an artificial antigen containing the 3,6-di-O-methyl-beta-D-glucopyranosyl epitope for the serodiagnosis of leprosy. J Infect Dis. 1984 Sep;150(3):311–322. doi: 10.1093/infdis/150.3.311. [DOI] [PubMed] [Google Scholar]
- Cho S. N., Yanagihara D. L., Hunter S. W., Gelber R. H., Brennan P. J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect Immun. 1983 Sep;41(3):1077–1083. doi: 10.1128/iai.41.3.1077-1083.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fujiwara T., Hunter S. W., Cho S. N., Aspinall G. O., Brennan P. J. Chemical synthesis and serology of disaccharides and trisaccharides of phenolic glycolipid antigens from the leprosy bacillus and preparation of a disaccharide protein conjugate for serodiagnosis of leprosy. Infect Immun. 1984 Jan;43(1):245–252. doi: 10.1128/iai.43.1.245-252.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., Brennan P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J Bacteriol. 1981 Sep;147(3):728–735. doi: 10.1128/jb.147.3.728-735.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., Fujiwara T., Brennan P. J. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J Biol Chem. 1982 Dec 25;257(24):15072–15078. [PubMed] [Google Scholar]
- Hunter S. W., Jardine I., Yanagihara D. L., Brennan P. J. Trehalose-containing lipooligosaccharides from mycobacteria: structures of the oligosaccharide segments and recognition of a unique N-acylkanosamine-containing epitope. Biochemistry. 1985 May 21;24(11):2798–2805. doi: 10.1021/bi00332a030. [DOI] [PubMed] [Google Scholar]
- Hunter S. W., Murphy R. C., Clay K., Goren M. B., Brennan P. J. Trehalose-containing lipooligosaccharides. A new class of species-specific antigens from Mycobacterium. J Biol Chem. 1983 Sep 10;258(17):10481–10487. [PubMed] [Google Scholar]
- Minnikin D. E., Dobson G., Sesardic D., Ridell M. Mycolipenates and mycolipanolates of trehalose from mycobacterium tuberculosis. J Gen Microbiol. 1985 Jun;131(6):1369–1374. doi: 10.1099/00221287-131-6-1369. [DOI] [PubMed] [Google Scholar]
- Reggiardo Z., Aber V. R., Mitchison D. A., Devi S. Hemagglutination tests for tuberculosis with mycobacterial glycolipid antigens. Results in patients with active pulmonary tuberculosis before and during chemotherapy and in healthy tuberculosis contacts. Am Rev Respir Dis. 1981 Jul;124(1):21–25. doi: 10.1164/arrd.1981.124.1.21. [DOI] [PubMed] [Google Scholar]
- Reggiardo Z., Vazquez E., Schnaper L. ELISA tests for antibodies against mycobacterial glycolipids. J Immunol Methods. 1980;34(1):55–60. doi: 10.1016/0022-1759(80)90224-0. [DOI] [PubMed] [Google Scholar]
- Schultz J. C., Elbein A. D. Biosynthesis of glycosyldiglycerides in Mycobacterium smegmatis. J Bacteriol. 1974 Jan;117(1):107–115. doi: 10.1128/jb.117.1.107-115.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw N. Bacterial glycolipids. Bacteriol Rev. 1970 Dec;34(4):365–377. doi: 10.1128/br.34.4.365-377.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellner K., Saito H., Hakomori S. I. Determination of aminosugar linkages in glycolipids by methylation. Aminosugar linkages of ceramide pentasaccharides of rabbit erythrocytes and of Forssman antigen. Arch Biochem Biophys. 1973 Apr;155(2):464–472. doi: 10.1016/0003-9861(73)90138-0. [DOI] [PubMed] [Google Scholar]
- Takayama K., Schnoes H. K., Armstrong E. L., Boyle R. W. Site of inhibitory action of isoniazid in the synthesis of mycolic acids in Mycobacterium tuberculosis. J Lipid Res. 1975 Jul;16(4):308–317. [PubMed] [Google Scholar]
- Ward J. B., Perkins H. R. The chemical composition of the membranes of protoplasts and L-forms of Staphylococcus aureus. Biochem J. 1968 Jan;106(2):391–400. doi: 10.1042/bj1060391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara D. L., Barr V. L., Knisley C. V., Tsang A. Y., McClatchy J. K., Brennan P. J. Enzyme-linked immunosorbent assay of glycolipid antigens for identification of mycobacteria. J Clin Microbiol. 1985 Apr;21(4):569–574. doi: 10.1128/jcm.21.4.569-574.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. B., Buchanan T. M. A serological test for leprosy with a glycolipid specific for Mycobacterium leprae. Science. 1983 Sep 9;221(4615):1057–1059. doi: 10.1126/science.6348948. [DOI] [PubMed] [Google Scholar]