Abstract
The pED208 plasmid is a 90-kilobase conjugative plasmid which is the derepressed form of Fo lac plasmid (IncFV). A 3.3-kilobase HindIII-PstI fragment from the pED208 plasmid was cloned and sequenced and was found to contain four open reading frames which were highly homologous to the traA, traL, traE, and traY gene products of the F plasmid. The pED208 traA propilin protein was 119 amino acids in length, consisting of a leader sequence of 55 amino acids and a mature pilin subunit of 64 residues. The leader sequence contained a hydrophobic region followed by a classic signal peptidase cleavage site (Ala-Ser-Ala-55). F and pED208 pilin proteins shared 27 conserved residues and had similar predicted secondary structures. The pED208 traA and traL genes were separated by a single base pair, and no ribosome binding site preceded the traL gene. The pED208 traY gene contained an IS2 insertion element in orientation II 180 nucleotides (60 residues) upstream of the traY stop codon. This insertion of IS2 resulted in a predicted fusion peptide of 69 residues for traY which may provide the observed traY activity. Since IS2 is absent in the wild-type plasmid, Fo lac, derepression and concomitant multipiliation may be due to the insertion of IS2 providing constitutive expression of the pED208 tra operon.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Manning P. A., Edelbluth C., Herrlich P. Export without proteolytic processing of inner and outer membrane proteins encoded by F sex factor tra cistrons in Escherichia coli minicells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4837–4841. doi: 10.1073/pnas.76.10.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong G. D., Frost L. S., Sastry P. A., Paranchych W. Comparative biochemical studies on F and EDP208 conjugative pili. J Bacteriol. 1980 Jan;141(1):333–341. doi: 10.1128/jb.141.1.333-341.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S. A., Hall M. N., Silhavy T. J. Genetic analysis of protein export in Escherichia coli K12. Annu Rev Biochem. 1985;54:101–134. doi: 10.1146/annurev.bi.54.070185.000533. [DOI] [PubMed] [Google Scholar]
- Bradley D. E., Meynell E. Serological characteristics of pili determined by the plasmids R711b and F0lac. J Gen Microbiol. 1978 Sep;108(1):141–149. doi: 10.1099/00221287-108-1-141. [DOI] [PubMed] [Google Scholar]
- Falkow S., Baron L. S. EPISOMIC ELEMENT IN A STRAIN OF SALMONELLA TYPHOSA. J Bacteriol. 1962 Sep;84(3):581–589. doi: 10.1128/jb.84.3.581-589.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
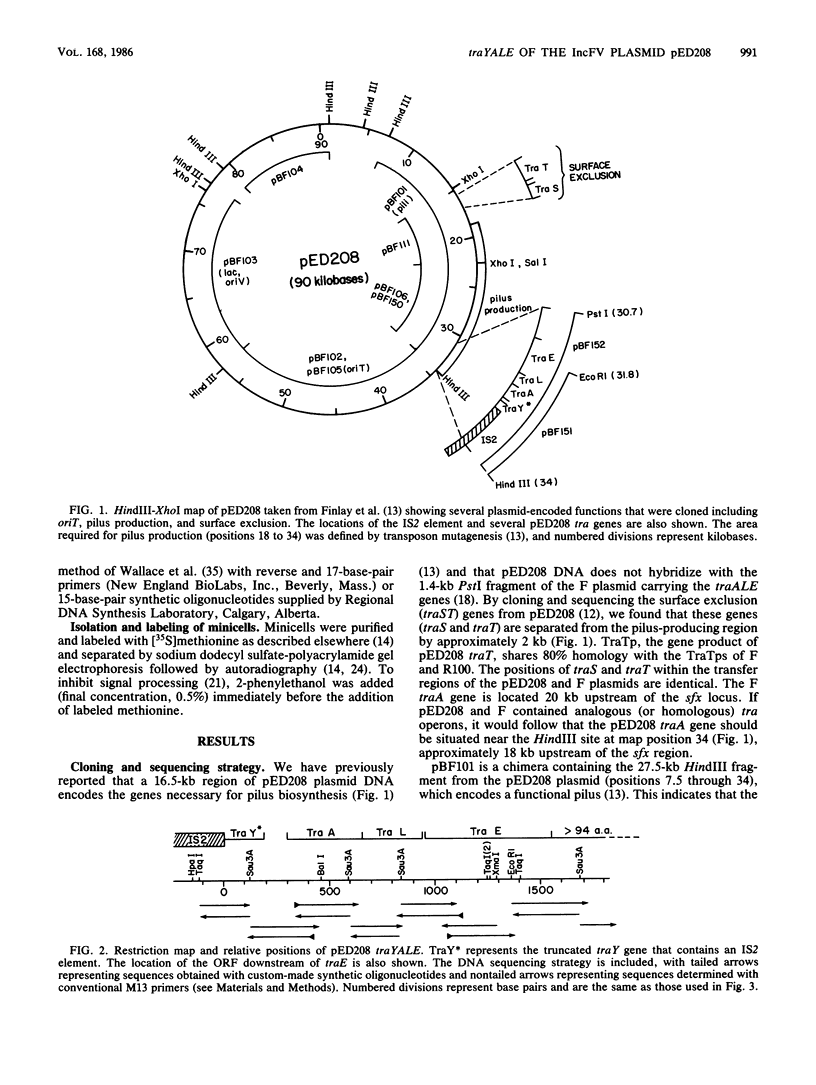
- Finlay B. B., Frost L. S., Paranchych W. Localization, cloning, and sequence determination of the conjugative plasmid ColB2 pilin gene. J Bacteriol. 1984 Oct;160(1):402–407. doi: 10.1128/jb.160.1.402-407.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Frost L. S., Paranchych W. Nucleotide sequences of the R1-19 plasmid transfer genes traM, finP, traJ, and traY and the traYZ promoter. J Bacteriol. 1986 May;166(2):368–374. doi: 10.1128/jb.166.2.368-374.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Frost L. S., Paranchych W., Parker J. M., Hodges R. S. Major antigenic determinants of F and ColB2 pili. J Bacteriol. 1985 Jul;163(1):331–335. doi: 10.1128/jb.163.1.331-335.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Paranchych W., Falkow S. Characterization of conjugative plasmid EDP208. J Bacteriol. 1983 Oct;156(1):230–235. doi: 10.1128/jb.156.1.230-235.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Paranchych W. Nucleotide sequence of the surface exclusion genes traS and traT from the IncF0 lac plasmid pED208. J Bacteriol. 1986 Jun;166(3):713–721. doi: 10.1128/jb.166.3.713-721.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Pasloske B. L., Paranchych W. Expression of the Pseudomonas aeruginosa PAK pilin gene in Escherichia coli. J Bacteriol. 1986 Feb;165(2):625–630. doi: 10.1128/jb.165.2.625-630.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkhard W., Leonard K. R., Malsey S., Marvin D. A., Dubochet J., Engel A., Achtman M., Helmuth R. X-ray diffraction and electron microscope studies on the structure of bacterial F pili. J Mol Biol. 1979 May 15;130(2):145–160. doi: 10.1016/0022-2836(79)90423-6. [DOI] [PubMed] [Google Scholar]
- Fowler T., Taylor L., Thompson R. The control region of the F plasmid transfer operon: DNA sequence of the traJ and traY genes and characterisation of the traY leads to Z promoter. Gene. 1983 Dec;26(1):79–89. doi: 10.1016/0378-1119(83)90038-0. [DOI] [PubMed] [Google Scholar]
- Frost L. S., Armstrong G. D., Finlay B. B., Edwards B. F., Paranchych W. N-terminal amino acid sequencing of EDP208 conjugative pili. J Bacteriol. 1983 Feb;153(2):950–954. doi: 10.1128/jb.153.2.950-954.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost L. S., Finlay B. B., Opgenorth A., Paranchych W., Lee J. S. Characterization and sequence analysis of pilin from F-like plasmids. J Bacteriol. 1985 Dec;164(3):1238–1247. doi: 10.1128/jb.164.3.1238-1247.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost L. S., Paranchych W., Willetts N. S. DNA sequence of the F traALE region that includes the gene for F pilin. J Bacteriol. 1984 Oct;160(1):395–401. doi: 10.1128/jb.160.1.395-401.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal D., Sommer H., Saedler H. Nucleotide sequence of the transposable DNA-element IS2. Nucleic Acids Res. 1979 Mar;6(3):1111–1122. doi: 10.1093/nar/6.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halegoua S., Inouye M. Translocation and assembly of outer membrance proteins of Escherichia coli. Selective accumulation of precursors and novel assembly intermediates caused by phenethyl alcohol. J Mol Biol. 1979 May 5;130(1):39–61. doi: 10.1016/0022-2836(79)90551-5. [DOI] [PubMed] [Google Scholar]
- Hinton D. M., Musso R. E. Specific in vitro transcription of the insertion sequence IS2. J Mol Biol. 1983 Sep 5;169(1):53–81. doi: 10.1016/s0022-2836(83)80175-2. [DOI] [PubMed] [Google Scholar]
- Kennedy N., Beutin L., Achtman M., Skurray R., Rahmsdorf U., Herrlich P. Conjugation proteins encoded by the F sex factor. Nature. 1977 Dec 15;270(5638):580–585. doi: 10.1038/270580a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laine S., Moore D., Kathir P., Ippen-Ihler K. Genes and gene products involved in the synthesis of F-pili. Basic Life Sci. 1985;30:535–553. doi: 10.1007/978-1-4613-2447-8_38. [DOI] [PubMed] [Google Scholar]
- Minkley E. G., Jr, Polen S., Brinton C. C., Jr, Ippen-Ihler K. Identification of the structural gene for F-pilin. J Mol Biol. 1976 Nov;108(1):111–121. doi: 10.1016/s0022-2836(76)80098-8. [DOI] [PubMed] [Google Scholar]
- Ogata R. T., Winters C., Levine R. P. Nucleotide sequence analysis of the complement resistance gene from plasmid R100. J Bacteriol. 1982 Aug;151(2):819–827. doi: 10.1128/jb.151.2.819-827.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- Queen C., Korn L. J. A comprehensive sequence analysis program for the IBM personal computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):581–599. doi: 10.1093/nar/12.1part2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Thompson R., Taylor L. Promoter mapping and DNA sequencing of the F plasmid transfer genes traM and traJ. Mol Gen Genet. 1982;188(3):513–518. doi: 10.1007/BF00330058. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Suggs S. V., Miyoshi K., Bhatt R., Itakura K. A set of synthetic oligodeoxyribonucleotide primers for DNA sequencing in the plasmid vector pBR322. Gene. 1981 Dec;16(1-3):21–26. doi: 10.1016/0378-1119(81)90057-3. [DOI] [PubMed] [Google Scholar]
- Willetts N., Maule J. Specificities of IncF plasmid conjugation genes. Genet Res. 1986 Feb;47(1):1–11. doi: 10.1017/s0016672300024447. [DOI] [PubMed] [Google Scholar]
- Willetts N., Skurray R. The conjugation system of F-like plasmids. Annu Rev Genet. 1980;14:41–76. doi: 10.1146/annurev.ge.14.120180.000353. [DOI] [PubMed] [Google Scholar]
- Willetts N., Wilkins B. Processing of plasmid DNA during bacterial conjugation. Microbiol Rev. 1984 Mar;48(1):24–41. doi: 10.1128/mr.48.1.24-41.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worobec E. A., Paranchych W., Parker J. M., Taneja A. K., Hodges R. S. Antigen-antibody interaction. The immunodominant region of EDP208 pili. J Biol Chem. 1985 Jan 25;260(2):938–943. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]