Abstract
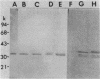
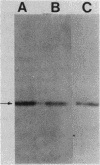
"Treponema phagedenis" periplasmic flagella (PF) have two major protein bands at molecular weights of 33,000 and 39,800 as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (R. J. Limberger and N. W. Charon, J. Bacteriol. 166:105-112, 1986). By use of Western blotting and a polyclonal antiserum directed toward the 33,000-molecular-weight PF protein, cell lysates of 12 species of spirochetes were surveyed for reactivity. Eight species of Treponema as well as Spirochaeta aurantia were positive. The results suggest that epitopes residing on the 33,000-molecular-weight PF protein of "T. phagedenis" are evolutionarily well conserved among the spirochetes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour A. G., Hayes S. F., Heiland R. A., Schrumpf M. E., Tessier S. L. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun. 1986 May;52(2):549–554. doi: 10.1128/iai.52.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharier M., Allis D. Purification and characterization of axial filaments from Treponema phagedenis biotype reiterii (the Reiter treponeme). J Bacteriol. 1974 Dec;120(3):1434–1442. doi: 10.1128/jb.120.3.1434-1442.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley D. B., Charon N. W. Axial filament involvement in the motility of Leptospira interrogans. J Bacteriol. 1979 Mar;137(3):1406–1412. doi: 10.1128/jb.137.3.1406-1412.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canale-Parola E. Motility and chemotaxis of spirochetes. Annu Rev Microbiol. 1978;32:69–99. doi: 10.1146/annurev.mi.32.100178.000441. [DOI] [PubMed] [Google Scholar]
- Carleton O., Charon N. W., Allender P., O'Brien S. Helix handedness of Leptospira interrogans as determined by scanning electron microscopy. J Bacteriol. 1979 Mar;137(3):1413–1416. doi: 10.1128/jb.137.3.1413-1416.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon N. W., Daughtry G. R., McCuskey R. S., Franz G. N. Microcinematographic analysis of tethered Leptospira illini. J Bacteriol. 1984 Dec;160(3):1067–1073. doi: 10.1128/jb.160.3.1067-1073.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs R. A., Pennington J., Uemura K., Scudder P., Goodfellow P. N., Evans M. J., Feizi T. High-molecular-weight glycoproteins are the major carriers of the carbohydrate differentiation antigens I, i and SSEA-1 of mouse teratocarcinoma cells. Biochem J. 1983 Dec 1;215(3):491–503. doi: 10.1042/bj2150491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Simon M. I. Variation in the primary structure of Bacillus subtilis flagellins. J Bacteriol. 1971 Jun;106(3):949–954. doi: 10.1128/jb.106.3.949-954.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Repesh L. A. Interactions of fibronectin with Treponema pallidum. Genitourin Med. 1985 Jun;61(3):147–155. doi: 10.1136/sti.61.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Greenberg E. P., Canale-Parola E. Chemotaxis in Spirochaeta aurantia. J Bacteriol. 1977 Apr;130(1):485–494. doi: 10.1128/jb.130.1.485-494.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanff P. A., Norris S. J., Lovett M. A., Miller J. N. Purification of Treponema pallidum, Nichols strain, by Percoll density gradient centrifugation. Sex Transm Dis. 1984 Oct-Dec;11(4):275–286. doi: 10.1097/00007435-198410000-00003. [DOI] [PubMed] [Google Scholar]
- Hardy P. H., Jr, Fredericks W. R., Nell E. E. Isolation and antigenic characteristics of axial filaments from the Reiter Treponeme. Infect Immun. 1975 Feb;11(2):380–386. doi: 10.1128/iai.11.2.380-386.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C. Anatomy and chemistry of spirochetes. Microbiol Rev. 1978 Mar;42(1):114–160. doi: 10.1128/mr.42.1.114-160.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joens L. A., Songer J. G., Harris D. L., Glock R. D. Experimental infection with Treponema hyodysenteriae in guinea pigs. Infect Immun. 1978 Oct;22(1):132–135. doi: 10.1128/iai.22.1.132-135.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberger R. J., Charon N. W. Treponema phagedenis has at least two proteins residing together on its periplasmic flagella. J Bacteriol. 1986 Apr;166(1):105–112. doi: 10.1128/jb.166.1.105-112.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao R., Fieldsteel A. H. Genetics of Treponema: relationship between Treponema pallidum and five cultivable treponemes. J Bacteriol. 1978 Jan;133(1):101–107. doi: 10.1128/jb.133.1.101-107.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskophidis M., Müller F. Molecular characterization of glycoprotein antigens on surface of Treponema pallidum: comparison with nonpathogenic Treponema phagedenis biotype Reiter. Infect Immun. 1984 Dec;46(3):867–869. doi: 10.1128/iai.46.3.867-869.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nell E. E., Hardy P. H., Jr Counterimmunoelectrophoresis of Reiter Treponeme Axial filaments as a diagnostic test for syphilis. J Clin Microbiol. 1978 Aug;8(2):148–152. doi: 10.1128/jcm.8.2.148-152.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paster B. J., Canale-Parola E. Involvement of periplasmic fibrils in motility of spirochetes. J Bacteriol. 1980 Jan;141(1):359–364. doi: 10.1128/jb.141.1.359-364.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N. S., Petersen C. S., Axelsen N. H. Enzyme-linked immunosorbent assay for detection of immunoglobulin M antibody against the Reiter treponeme flagellum in syphilis. J Clin Microbiol. 1982 Oct;16(4):608–614. doi: 10.1128/jcm.16.4.608-614.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N. S., Petersen C. S., Vejtorp M., Axelsen N. H. Serodiagnosis of syphilis by an enzyme-linked immunosorbent assay for IgG antibodies against the Reiter treponeme flagellum. Scand J Immunol. 1982 Apr;15(4):341–348. doi: 10.1111/j.1365-3083.1982.tb00657.x. [DOI] [PubMed] [Google Scholar]
- Penn C. W., Bailey M. J., Cockayne A. The axial filament antigen of Treponema pallidum. Immunology. 1985 Apr;54(4):635–641. [PMC free article] [PubMed] [Google Scholar]
- Petersen C. S., Pedersen N. S., Axelsen N. H. A simple method for the isolation of flagella from Treponema Reiter. Acta Pathol Microbiol Scand C. 1981 Dec;89(6):379–385. doi: 10.1111/j.1699-0463.1981.tb02716.x. [DOI] [PubMed] [Google Scholar]
- Simon M. I., Emerson S. U., Shaper J. H., Bernard P. D., Glazer A. N. Classification of Bacillus subtilis flagellins. J Bacteriol. 1977 Apr;130(1):200–204. doi: 10.1128/jb.130.1.200-204.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland F., Paul G., Sumper M. Halobacterial flagellins are sulfated glycoproteins. J Biol Chem. 1985 Dec 5;260(28):15180–15185. [PubMed] [Google Scholar]