Abstract
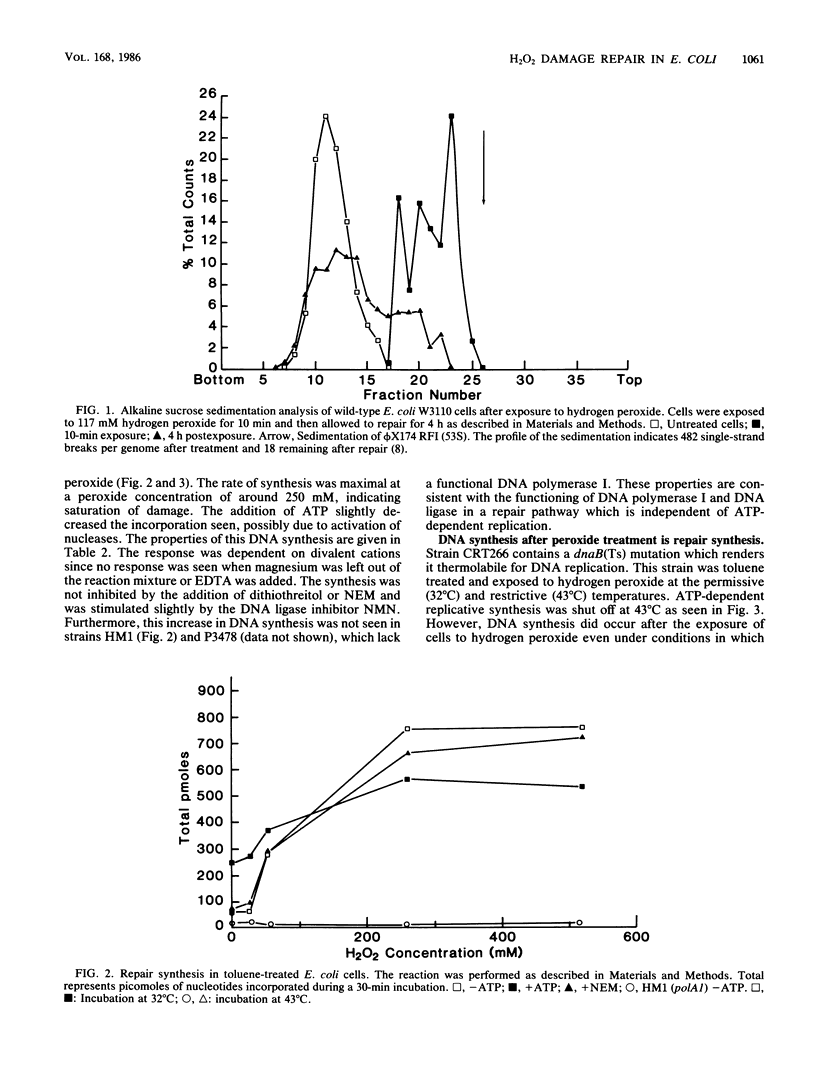
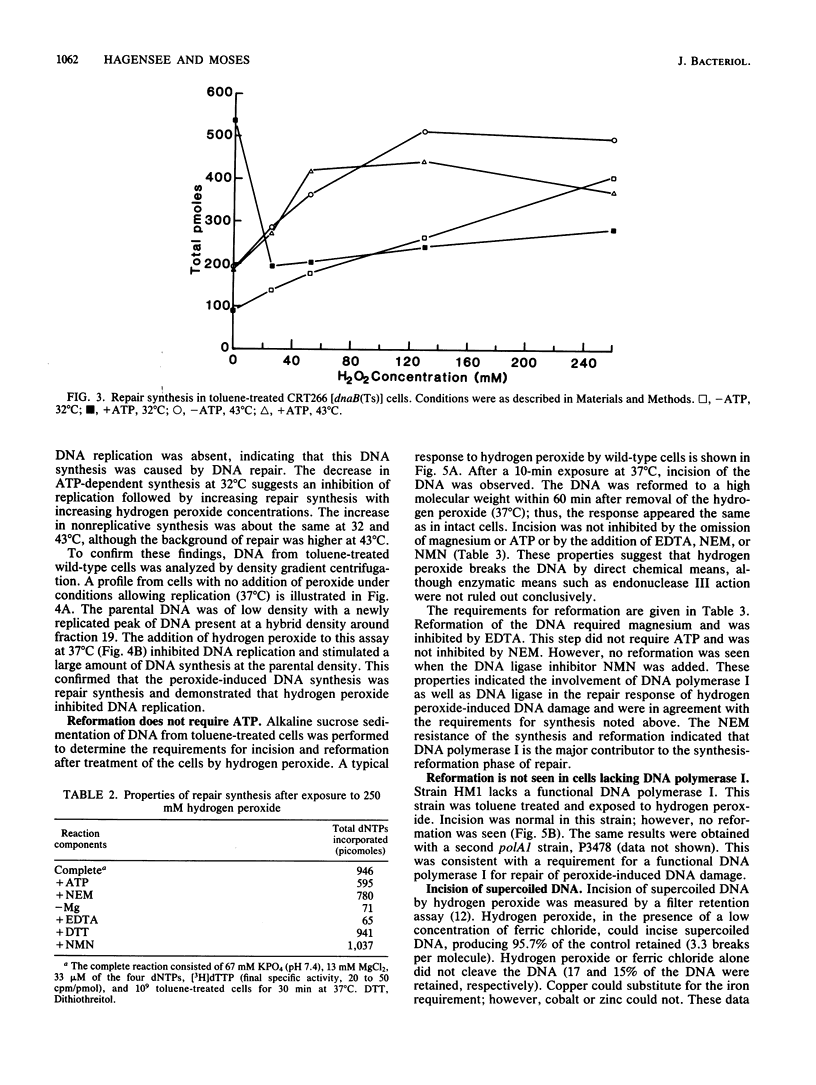
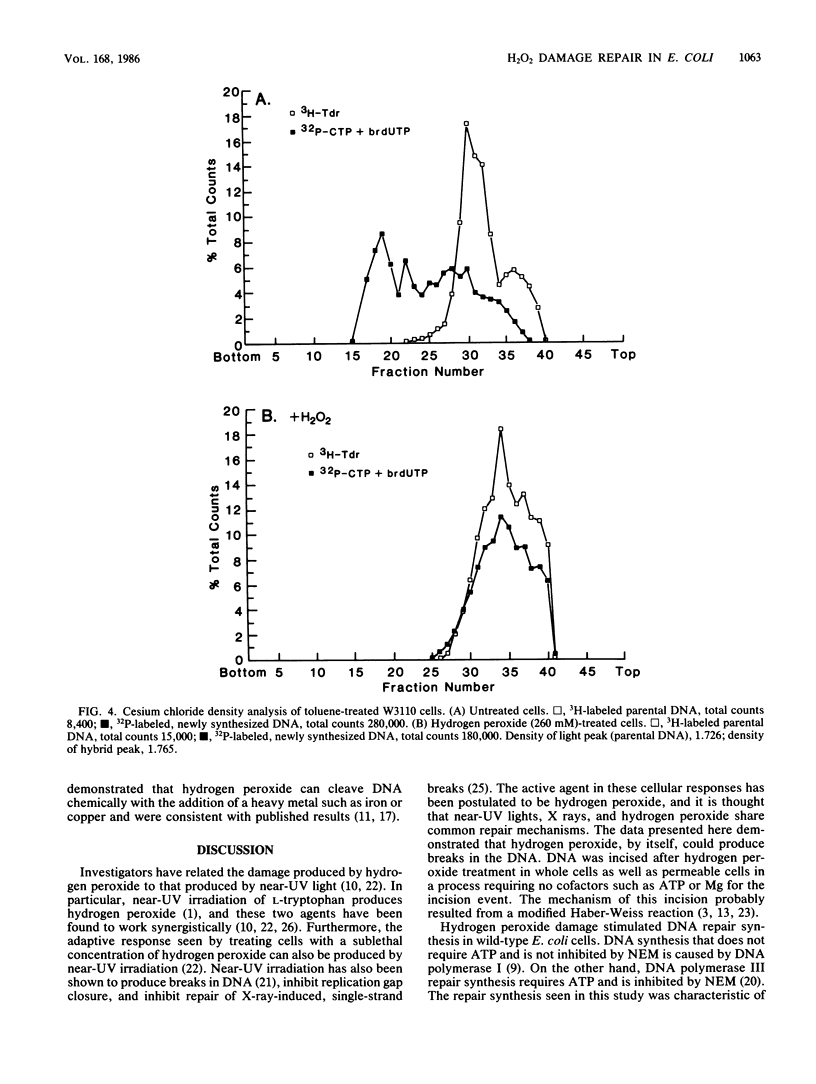
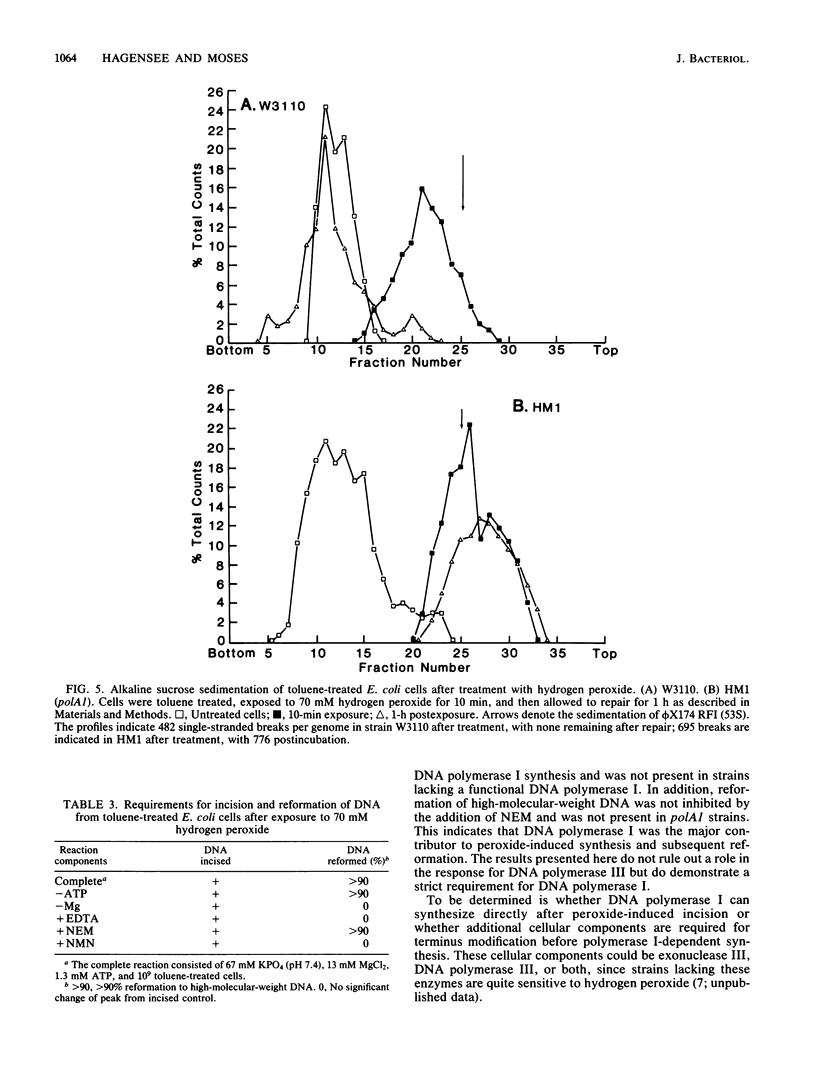
The repair response of Escherichia coli to hydrogen peroxide-induced DNA damage was investigated in intact and toluene-treated cells. Cellular DNA was cleaved after treatment by hydrogen peroxide as analyzed by alkaline sucrose sedimentation. The incision step did not require ATP or magnesium and was not inhibited by N-ethylmaleimide (NEM). An ATP-independent, magnesium-dependent incorporation of nucleotides was seen after the exposure of cells to hydrogen peroxide. This DNA repair synthesis was not inhibited by the addition of NEM or dithiothreitol. In dnaB(Ts) strain CRT266, which is thermolabile for DNA replication, normal levels of DNA synthesis were found at the restrictive temperature (43 degrees C), showing that DNA replication was not necessary for this DNA synthesis. Density gradient analysis also indicated that hydrogen peroxide inhibited DNA replication and stimulated repair synthesis. The subsequent reformation step required magnesium, did not require ATP, and was not inhibited by NEM, in agreement with the synthesis requirements. This suggests that DNA polymerase I was involved in the repair step. Furthermore, a strain defective in DNA polymerase I was unable to reform its DNA after peroxide treatment. Chemical cleavage of the DNA was shown by incision of supercoiled DNA with hydrogen peroxide in the presence of a low concentration of ferric chloride. These findings suggest that hydrogen peroxide directly incises DNA, causing damage which is repaired by an incision repair pathway that requires DNA polymerase I.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ananthaswamy H. N., Eisenstark A. Near-UV-induced breaks in phage DNA: sensitization by hydrogen peroxide (a tryptophan photoproduct). Photochem Photobiol. 1976 Nov;24(5):439–442. doi: 10.1111/j.1751-1097.1976.tb06851.x. [DOI] [PubMed] [Google Scholar]
- Ananthaswamy H. N., Eisenstark A. Repair of hydrogen peroxide-induced single-strand breaks in Escherichia coli deoxyribonucleic acid. J Bacteriol. 1977 Apr;130(1):187–191. doi: 10.1128/jb.130.1.187-191.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawn K., Fridovich I. DNA strand scission by enzymically generated oxygen radicals. Arch Biochem Biophys. 1981 Feb;206(2):414–419. doi: 10.1016/0003-9861(81)90108-9. [DOI] [PubMed] [Google Scholar]
- Bryan S. K., Moses R. E. Map location of the pcbA mutation and physiology of the mutant. J Bacteriol. 1984 Apr;158(1):216–221. doi: 10.1128/jb.158.1.216-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Carpenter V. S. The recA+ gene product is more important than catalase and superoxide dismutase in protecting Escherichia coli against hydrogen peroxide toxicity. J Bacteriol. 1980 Apr;142(1):319–321. doi: 10.1128/jb.142.1.319-321.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B., Halbrook J. Inducible repair of oxidative DNA damage in Escherichia coli. Nature. 1983 Aug 4;304(5925):466–468. doi: 10.1038/304466a0. [DOI] [PubMed] [Google Scholar]
- Demple B., Halbrook J., Linn S. Escherichia coli xth mutants are hypersensitive to hydrogen peroxide. J Bacteriol. 1983 Feb;153(2):1079–1082. doi: 10.1128/jb.153.2.1079-1082.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorson J. W., Deutsch W. A., Moses R. E. Role of DNA polymerases in excision repair in Escherichia coli. J Biol Chem. 1978 Feb 10;253(3):660–664. [PubMed] [Google Scholar]
- Dorson J. W., Moses R. E. DNA polymerase I-mediated ultraviolet repair synthesis in toluene-treated Escherichia coli. J Biol Chem. 1978 Feb 10;253(3):665–670. [PubMed] [Google Scholar]
- Hartman P. S., Eisenstark A. Synergistic killing of Escherichia coli by near-UV radiation and hydrogen peroxide: distinction between recA-repairable and recA-nonrepairable damage. J Bacteriol. 1978 Feb;133(2):769–774. doi: 10.1128/jb.133.2.769-774.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi H., Takagi M., Yano K. Relaxation of supercoiled plasmid DNA by oxidative stresses in Escherichia coli. J Bacteriol. 1984 Dec;160(3):1017–1021. doi: 10.1128/jb.160.3.1017-1021.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnlein U., Penhoet E. E., Linn S. An altered apurinic DNA endonuclease activity in group A and group D xeroderma pigmentosum fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1169–1173. doi: 10.1073/pnas.73.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Day E. D., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978 Feb 1;86(1):139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Reconstruction in vivo of irradiated Escherichia coli deoxyribonucleic acid; the rejoining of broken pieces. Nature. 1966 Oct 29;212(5061):534–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- Moses R. E., Richardson C. C. Replication and repair of DNA in cells of Escherichia coli treated with toluene. Proc Natl Acad Sci U S A. 1970 Oct;67(2):674–681. doi: 10.1073/pnas.67.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard E. C., Weller P. K. Chain scission of ribonucleic acid and deoxyribonucleic acid by ionizing radiation and hydrogen peroxide in vitro and in Escherichia coli cells. Radiat Res. 1967 Nov;32(3):417–440. [PubMed] [Google Scholar]
- Repine J. E., Pfenninger O. W., Talmage D. W., Berger E. M., Pettijohn D. E. Dimethyl sulfoxide prevents DNA nicking mediated by ionizing radiation or iron/hydrogen peroxide-generated hydroxyl radical. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1001–1003. doi: 10.1073/pnas.78.2.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhaese H. J., Freese E. Chemical analysis of DNA alterations. I. Base liberation and backbone breakage of DNA and oligodeoxyadenylic acid induced by hydrogen peroxide and hydroxylamine. Biochim Biophys Acta. 1968 Feb 26;155(2):476–490. [PubMed] [Google Scholar]
- Ross S. L., Moses R. E. Two actions of bleomycin on superhelical DNA. Biochemistry. 1978 Feb 21;17(4):581–586. doi: 10.1021/bi00597a004. [DOI] [PubMed] [Google Scholar]
- Ross S. L., Sharma S., Moses R. E. DNA polymerase III-dependent repair synthesis in response to bleomycin in toluene-treated Escherichia coli. Mol Gen Genet. 1980;179(3):595–605. doi: 10.1007/BF00271750. [DOI] [PubMed] [Google Scholar]
- Tuveson R. W., Peak J. G., Peak M. J. Single-strand DNA breaks induced by 365 NM radiation in Escherichia coli strains differing in sensitivity to near and far UV. Photochem Photobiol. 1983 Jan;37(1):109–112. doi: 10.1111/j.1751-1097.1983.tb04442.x. [DOI] [PubMed] [Google Scholar]
- Tyrrell R. M. A common pathway for protection of bacteria against damage by solar UVA (334 nm, 365 nm) and an oxidising agent (H2O2). Mutat Res. 1985 May;145(3):129–136. doi: 10.1016/0167-8817(85)90019-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto N. Damage, repair, and recombination. II. Effect of hydrogen peroxide on the bacteriophage genome. Virology. 1969 Jul;38(3):457–463. doi: 10.1016/0042-6822(69)90158-5. [DOI] [PubMed] [Google Scholar]
- Yoakum G., Eisenstark A., Webb R. B. Near-UV photoproduct(s) of L-typtophan: an inhibitor of medium-dependent repair of X-ray-induced single-strand breaks in DNA which also inhibits replication-gap closure in Escherichia coli DNA. Basic Life Sci. 1975;5B:453–458. doi: 10.1007/978-1-4684-2898-8_5. [DOI] [PubMed] [Google Scholar]
- Yoakum G., Ferron W., Eisenstark A., Webb R. B. Inhibition of replication gap closure in Escherichia coli by near-ultraviolet light photoproducts of L-tryptophan. J Bacteriol. 1974 Jul;119(1):62–69. doi: 10.1128/jb.119.1.62-69.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



