Abstract
Electrophysiological studies of H441 human distal airway epithelial cells showed that thapsigargin caused a Ca2+-dependent increase in membrane conductance (GTot) and hyperpolarization of membrane potential (Vm). These effects reflected a rapid rise in cellular K+ conductance (GK) and a slow fall in amiloride-sensitive Na+ conductance (GNa). The increase in GTot was antagonized by Ba2+, a nonselective K+ channel blocker, and abolished by clotrimazole, a KCNN4 inhibitor, but unaffected by other selective K+ channel blockers. Moreover, 1-ethyl-2-benzimidazolinone (1-EBIO), which is known to activate KCNN4, increased GK with no effect on GNa. RT-PCR-based analyses confirmed expression of mRNA encoding KCNN4 and suggested that two related K+ channels (KCNN1 and KCNMA1) were absent. Subsequent studies showed that 1-EBIO stimulates Na+ transport in polarized monolayers without affecting intracellular Ca2+ concentration ([Ca2+]i), suggesting that the activity of KCNN4 might influence the rate of Na+ absorption by contributing to GK. Transient expression of KCNN4 cloned from H441 cells conferred a Ca2+- and 1-EBIO-sensitive K+ conductance on Chinese hamster ovary cells, but this channel was inactive when [Ca2+]i was <0.2 μM. Subsequent studies of amiloride-treated H441 cells showed that clotrimazole had no effect on Vm despite clear depolarizations in response to increased extracellular K+ concentration ([K+]o). These findings thus indicate that KCNN4 does not contribute to Vm in unstimulated cells. The present data thus establish that H441 cells express KCNN4 and highlight the importance of GK to the control of Na+ absorption, but, because KCNN4 is quiescent in resting cells, this channel cannot contribute to resting GK or influence basal Na+ absorption.
Keywords: airway Na+ transport, cloning, Chinese hamster ovary cell expression, 1-ethyl-2-benzimidazolinone
The epithelia that line the airways spontaneously absorb Na+ from the overlying film of surface liquid, and this process, which is vital to the integrated function of the respiratory tract (see, for example, Refs. 3, 26), occurs via a “leak-pump” mechanism in which Na+ first crosses the apical membrane by diffusing down the inwardly directed electrochemical gradient and is then extruded from the cell by the basolateral Na+ pump (37). An important feature of this model is that the transepithelial Na+ transport rate is restricted by the rate of apical entry, implying that Na+ absorption can be controlled by agents that regulate apical Na+ conductance (GNa). However, early studies of absorptive tissues also revealed a correlation between the Na+ transport rate and the magnitude of the basolateral K+ conductance (GK), and it is now clear that changes in apical GNa and basolateral GK are coordinated to prevent large excursions in membrane potential (Vm). This interrelationship allows the driving force for Na+ entry (VNa) to be maintained, thus permitting sustained Na+ absorption, and also implies that Na+ absorption can be controlled by agents that regulate basolateral GK (6, 9, 10). However, despite this importance, the K+ channels underlying basolateral GK in absorptive airway epithelia have not been identified, although 1-ethyl-2-benzimidazolinone (1-EBIO), which activates intermediate-conductance Ca2+-dependent K+ channels (KCNN4) (9), has been shown to stimulate Na+ absorption in such tissues. This suggests that these channels, which are widely expressed in epithelial tissues (see, for example, Refs. 5, 7, 14, 28, 34), might be involved in the maintenance of VNa, and so the aim of the present study was to establish the extent to which KCNN4 activity can influence the rate of Na+ absorption in a cell line derived from the distal airway epithelium (H441; see, e.g., Refs. 4, 24, 32, 33).
METHODS
Cell culture
Standard techniques were used to maintain stocks of H441 cells in RPMI medium supplemented with 8.5% fetal bovine serum (FBS), 8.5% newborn calf serum (NCS), 2 mM glutamine, 5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml selenium, and an antibiotic-antimycotic mixture (Sigma, Poole, UK; catalog no. A5955). For experiments, cells were removed from culture flasks with trypsin-EDTA, resuspended in standard medium, and plated onto glass coverslips or Costar Snapwell culture membranes (Corning, Schipol-Rijk, The Netherlands) (∼106 cells/cm2). For experiments in which short-circuit current (ISC) and [Ca2+]i were measured simultaneously, the Snapwell membranes were cut into small pieces that were glued to Perspex disks with 1-mm holes drilled though them to form small wells into which the cells were seeded (21). Once cellular attachment occurred (2-3 h), the plating medium was replaced with medium identical to that described above except that FBS and NCS were replaced with 8.5% FBS that had been dialyzed to remove hormones and growth factors. Unless otherwise stated, this medium was supplemented with 0.2 μM dexamethasone, a synthetic glucocorticoid known to induce a Na+-absorbing phenotype in these cells (4, 30, 32). Stocks of Chinese hamster ovary (CHO) cells were maintained in Ham’s F-12 Glutamax medium (GIBCO, Paisley, UK) containing antibiotics and 10% FBS.
Membrane currents in H441 cells
Membrane currents were recorded (∼22°C) from single cells or small groups of H441 cells with the perforated-patch recording technique (Axopatch 200B amplifier, Digidata 1322A data acquisition board; Axon Instruments, Foster City, CA), in which electrical access to the cell interior is gained by including nystatin (0.5 mg/ml) in the pipette filling solution to render the patch of membrane spanning the pipette tip permeable to K+, Na+, and Cl- (15). This allows experimental control over the internal concentrations of these ions but prevents the loss of higher-molecularweight substances and allows [Ca2+]i to be regulated by normal physiological mechanisms. The pipette filling solution contained (in mM) 10 NaCl, 18 KCl, 92 K gluconate, 0.5 MgCl2, 1 EGTA, and 10 HEPES; pH was adjusted to 7.2 with KOH, which brought K+ concentration ([K+]) to 113.3 mM. The standard bath solution contained (in mM) 140 NaCl, 4.5 KCl, 1 MgCl2, 2.5 CaCl2, 10 HEPES, and 5 glucose; pH was adjusted to 7.4 with NaOH, which brought Na+ concentration ([Na+]) to 144.4 mM. These solutions were designed to maintain quasi-physiological ionic gradients, and the equilibrium potentials for Na+, K+, and Cl- (ENa, EK, and ECl, respectively) were normally +68, -82, and -42 mV, respectively. Modifications to the bath solution are detailed below. The recording pipettes had resistances of 2-5 MΩ, and once seals were obtained the nystatin-induced fall in access resistance (Ra) was monitored with the standard features of pCLAMP 9 and experiments were initiated once Ra had fallen to a stable value below 35 MΩ. In each experiment Ra and input capacitance (Cm) were noted and necessary compensations were applied to the recording circuitry. These parameters were monitored carefully, and the development of a large change in Ra or Cm led to the discontinuation of the experiment; therefore, all data are from preparations in which these parameters were stable. In studies of cells held under voltage clamp, Vm was inferred from the reversal potential [i.e., the value of holding potential (VHold) at which membrane current was zero], but in some instances Vm was measured directly by monitoring the zero-current potential in cells held under current clamp. Cited values of VHold and Vm have been corrected for liquid junction potentials (1), and values of membrane current and conductance have been normalized to the value of Cm associated with a single cell (39 pF). These data are therefore presented as picoamperes or nanoamperes per cell and picosiemens or nanosiemens per cell, respectively.
Electrophysiological properties of cultured epithelia
Assays of transepithelial ion transport were undertaken (37°C), using confluent cells on Snapwell membranes (see above) mounted in standard Ussing chambers and bathed with physiological saline (composition in mM: 117 NaCl, 25 NaHCO3, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 2.5 CaCl2, and 11 d-glucose; pH 7.3-7.5 when bubbled with 5% CO2) that was continually circulated with gas lifts. Transepithelial potential (Vt) was initially monitored under open-circuit conditions, and once this stabilized (20-30 min), Vt was clamped at 0 mV (DVC 1000 Voltage/Current Clamp; World Precision Instruments, Stevenage, UK) and the current needed to hold this potential (ISC) was monitored and recorded (4 Hz) with a PowerLab interface (AD Instruments, Hastings, UK). Transepithelial resistance (Rt) was determined from the expression Rt = Vt/ISC. To measure ISC and [Ca2+]i simultaneously, confluent cells were loaded with fura-2 by incubation (∼40 min, 37°C) in medium containing the acetoxymethyl ester form (3 μM) of this Ca2+-sensitive fluorescent dye together with Pluronic F127 (1.8 μM), a nonionic detergent, and probenecid (2.5 mM), an inhibitor of organic cation extrusion systems. Confluent H441 cells take up little dye under standard conditions, and inclusion of these compounds was necessary for adequate dye loading, but we (3a) have shown that these substances have no effect on Rt or Vt. The fura-2-loaded epithelia were mounted in a miniature Ussing chamber (21, 23) in which the basolateral and apical sides of the cell layer were independently superfused (∼3 ml/min) with physiological saline (see above). Solenoid-operated valves allowed these solutions to be independently switched, while thermostatically controlled in-line heaters (Warner Instrument Dual Automatic Temperature Control System) maintained the temperature at 37°C. The chamber was mounted on the stage of a Nikon inverted microscope equipped with extra long-working-distance fluorescence optics (Nikon CFI Plan Fluor ELWD, 0.6 numerical aperture) and a Cairn Research (Faversham, UK) Optoscan UV light source so that the cells could be alternatively illuminated at 340 and 380 nm. The intensity of fluorescence (510 nm) evoked at these wavelengths (F340 and F380, respectively) was monitored and recorded (4 Hz) in parallel with ISC (VCC600 voltage clamp; Physiologic Instruments, San Diego, CA.). Although the F340-to-F380 ratio is often used as an indicator of [Ca2+]i, most such experiments are undertaken with cells plated onto glass coverslips while confluent cells take up less dye than single cells, and a substantial amount of light is scattered by the culture membrane. The fractions of F340 and F380 due to background can thus be substantial under these conditions, and, because fura-2 fluorescence declines as the dye is bleached or extruded from the cell (12), the relative contribution of this background will change throughout the experiments. To ensure that this did not confound analysis of the present data, cells were exposed to thapsigargin (1 μM, bilateral) at the end of each experiment. This evokes a substantial rise in [Ca2+]i (see Ref. 36), and once this response was fully established the cells were exposed to 10 mM MnCl2. Because the thapsigargin-activated Ca2+-influx pathway is Mn2+ permeable (see, e.g., Ref. 18), and because Mn2+ rapidly quenches fura-2 fluorescence, Mn2+ application caused a rapid fall in F340 and F380 and the Mn2+-resistant component of each signal was assumed to indicate the cation-insensitive background fluorescence present throughout the preceding experiment. This allowed background corrections to be made before F340/F380 was calculated.
Cloning and characterization of KCNN4
To obtain cDNA encoding the human intermediate-conductance Ca2+-activated K+ channel (KCNN4), RNA was extracted from H441 cells with an SV total RNA isolation Kit (Promega) and reverse transcribed with oligo(dT) primers (Sigma) and avian myeloblastosis virus reverse transcriptase (Sigma). First-strand cDNA was subjected to PCR amplification using primers (5′-GTGCCTCAGAGCAAAAGTCC and 5′-CTACTTGGACTGCTGGCTGGG) designed with the Primer 3 PCR primer design program (Whitehead Institute for Biomedical Research). The 1,416-bp product was cloned into the pCR2.1 plasmid (Invitrogen) and clones were sequenced at the DNA Analysis Facility, Ninewells Hospital and Medical School, University of Dundee. This plasmid was then digested with EcoRI, and a 1,432-bp fragment containing the KCNN4 sequence was subcloned into the same site in pCDNA3 (Invitrogen). To characterize this cloned channel, CHO cells were plated onto glass coverslips on six-well plates and, after 24-48 h, Lipofectamine transfection reagent (Invitrogen) was used to cotransfect the cells with pcDNA3-KCNN4 (1-1.5 μg) in conjunction with a second plasmid (pEGFP, 0.1 μg) encoding green fluorescent protein (GFP). After a further 20-48 h, coverslips bearing transfected cells were mounted into a small chamber attached to the stage of an inverted microscope, where the cells were superfused with standard bath solution (composition in mM: 140 NaCl, 1 CaCl2, 5 KCl, 5 d-glucose, 1 MgCl2, and 10 HEPES; pH adjusted to 7.4 with NaOH) and viewed with fluorescence optics. Transfected cells were identified by GFP fluorescence, and the properties of the expressed channel were characterized by comparing the electrophysiological properties of KCNN4-transfected cells with those of cells transfected with GFP alone. Such experiments were undertaken with the standard whole cell recording configuration in which the patch of membrane spanning the pipette tip is physically ruptured by gentle suction. This allows the diffusible components of the cytoplasm and pipette filling solution to equilibrate, and [Ca2+]i was therefore under experimental control in these studies (13). The pipette filling solution used in these experiments contained (in mM) 10 NaCl, 20 KCl, 110 K gluconate, 5 EGTA, and 2 Mg-ATP; pH was adjusted to 7.2 with KOH. Sufficient CaCl2 (determined with REACT software; see Ref. 8) was added to this solution to raise [Ca2+] to 0.05, 0.2, or 0.5 μM. Recorded currents were normalized to the mean value of Cm associated with single cells (19 pF).
RESULTS
Thapsigargin-evoked membrane currents in H441 cells
Initial experiments explored the effects of thapsigargin on the conductive properties of H441 cells by recording membrane currents from groups of two to four voltage-clamped cells (see methods). In these experiments VHold was normally -40 mV, but every 15 s this potential was driven through a “staircase” containing eight distinct voltage steps (-92, -82, -62, -42, -13, 17, 47, and 68 mV) of 0.5-s duration. The mean membrane current flowing at each such value of VHold was subsequently determined and plotted against time. These data show that acute application of thapsigargin (1 μM) increased the membrane current flowing at values of VHold more positive than approximately -80 mV (Fig. 1A), and analysis of data collected once this response was fully developed (2-10 min) showed that GTot had increased approximately sixfold (Fig. 1B) while Vm had shifted to a value close to EK (Fig. 1C). These effects could be sustained for at least 15 min (not shown), but withdrawal of extracellular Ca2+ caused GTot and Vm to fall to their respective control levels (Fig. 1, A-C) and readmitting extracellular Ca2+ restored the stimulated values within 1-2 min (not shown). In subsequent studies of thapsigargin-stimulated cells membrane currents were successively recorded under standard conditions and after [K+]o had been raised to 31, 57, 84, and 110 mM by isosmotically replacing Na+. Such increases in [K+]o had no effect on GTot but depolarized Vm by 52.5 ± 1.2 mV per tenfold rise in [K+]o, which is essentially identical to the shift predicted by the Nernst equation for a selective K+ conductance (Fig. 1D). Ba2+ (5 mM) caused ∼75% inhibition of the thapsigargin-evoked increase in GTot, whereas clotrimazole (1 μM) essentially abolished the response (Fig. 1E). Chromanol 293B, iberiotoxin, and apamin, which block cAMP-activated K+ channels, large-conductance Ca2+-activated K+ channels (KCNM1), and small-conductance Ca2+-activated K+ channels (KCNN1-KCNN3), respectively, were without effect (Fig. 1E).
Fig. 1.
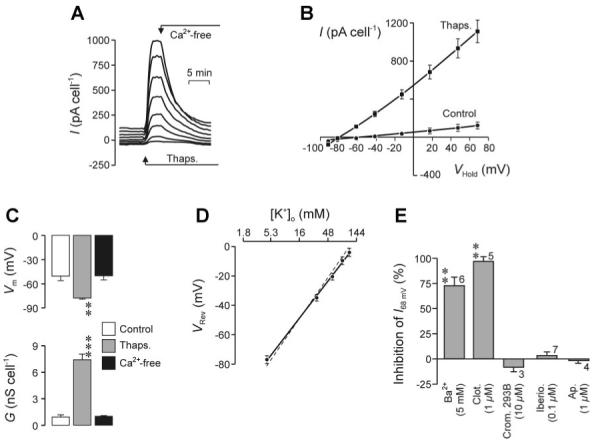
Thapsigargin-evoked membrane currents in H441 cells. A: cells were continuously superfused with physiological saline and, every 15 s, holding potential (VHold) was driven thorough a staircase containing 8 distinct voltage steps (-92, -82, -62, -42, -13, 17, 47, and 68 mV). The mean current flowing at each such value of VHold was then determined and plotted against time; cells were exposed to thapsigargin (1 μM) and nominally Ca2+-free saline as indicated. I, current. B: pooled data (n = 9) showing the relationship between I (means ± SE) and VHold during superfusion with standard saline and at the peak of the response to thapsigargin; data averaged from 4-6 consecutive pulse protocols. C: means ± SE values of membrane conductance (GTot) and membrane potential (Vm) determined from regression analysis of data collected under control conditions, at the peak of the response to thapsigargin, and after 10- to 15-min exposure to Ca2+-free solution. Statistically significant deviations from control (Student’s paired t-test): ***P < 0.001, **P < 0.02. D: relationship between extracellular K+ concentration ([K+]o) and Vm (inferred from VRev) in thapsigargin-stimulated cells (n = 5, means ± SE); the solid line was fitted to the data by least-squares regression (R2 = 0.997, P < 0.001), whereas the dashed line shows the solution to the Nernst equation for a selective K+ conductance. E: cells were stimulated with thapsigargin as shown in A and, once the characteristic rise in GTot and hyperpolarization of Vm were fully developed, exposed to putative K+ channel antagonists (Ba2+, clotrimazole, cromakalim 293B, iberiotoxin, or apamin). The effects of these compounds of were quantified (% inhibition) by determining the extent to which they blocked the current flowing at 68 mV (I68mV), a potential chosen because it equates to the equilibrium potential for Na+ (ENa), implying that I68mV will not contain a component carried by Na+. The results of this analysis are shown as means ± SE for values of n indicated beside each column. Statistically significant inhibitory effects (Student’s paired t-test): **P < 0.005.
Lowering [Na+]o to 10 mM by isosmotically substituting NMDG+ hyperpolarized unstimulated cell Vm by ∼35 mV (control -33 ± 8 mV, NMDG+ -66 ± 7 mV; n = 4, P < 0.05) but had no effect once the thapsigargin-evoked (1 μM, ∼4 min) hyperpolarization (Vm = -79 ± 2 mV; P < 0.02) was fully developed, suggesting that increased [Ca2+]i might also inhibit GNa. We therefore explored the effects of thapsigargin (1 μM) on the membrane currents that persisted in the presence of sufficient clotrimazole (3 μM) to block the rise in GTot described above. Vm was approximately -25 mV under these conditions, and, as anticipated (4, 33), amiloride (10 μM) reduced inward current and hyperpolarized Vm (Fig. 2A); analysis of these data showed that GTot and GNa were ∼850 and ∼300 pS/cell, respectively. Once these measurements were completed, amiloride was washed from the bath and the cells were exposed to 1 μM thapsigargin for ∼4 min before the measurements were repeated. Analysis of these data revealed a rise in GTot (unstimulated 853 ± 169, thapsigargin stimulated 1,207 ± 174 pS/cell; P < 0.001), but this was <5% of control (see Fig. 1) and occurred with no change in Vm (Fig. 2B), confirming that the thapsigargin-evoked increase in GTot is essentially abolished by clotrimazole. The physiological basis of this small, clotrimazole-resistant rise in GTot was not investigated further. The effects of amiloride (10 μM) on the thapsigargin-stimulated cells were essentially identical to control, indicating that thapsigargin has no effect on GNa (control 323 ± 173, thapsigargin 410 ± 184 pS/cell) over the timescale of this experiment. Subsequent experiments therefore studied the effects of thapsigargin (1 μM) over a longer time period by measuring GNa at 2- to 4-min intervals over 16 min. These experiments revealed a progressive fall in GTot (control 1,031 ± 335, 16-min thapsigargin 785 ± 137 pS/cell; P < 0.01) that occurred with no change in the amiloride-resistant component of GTot (control 596 ± 289, 16-min thapsigargin 578 ± 44 pS/cell) but could be attributed to a decline in GNa (Fig. 2, C and D).
Fig. 2.
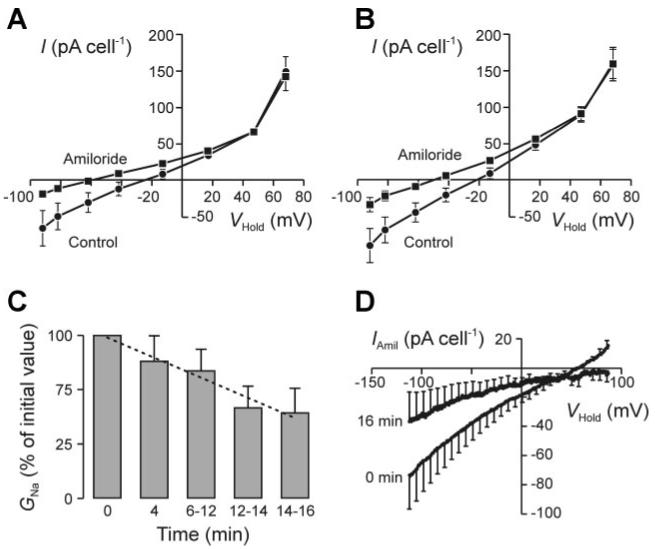
Effects of thapsigargin on Na+ conductance (GNa). A: membrane currents (n = 9) recorded from clotrimazole-treated (3 μM) H441 cells under control conditions and after application of 10 μM amiloride with the “staircase” protocol described in Fig. 1. B: analogous data derived from the same cells after ∼4-min exposure to 1 μM thapsigargin. C: data from experiments in which membrane currents were evoked by driving VHold through ramps from -113 to 87 mV in 1.75 s. At the onset of each experiment such data were collected both under standard conditions and after 30- to 40-s exposure to amiloride (10 μM). This drug was then washed from the bath, the cells exposed to thapsigargin (1 μM), and the entire protocol repeated at 2- to 4-min intervals over the following 16 min. Values of GNa were determined by analysis of these data, normalized to the initial value measured under control conditions at the onset of the experiment, and plotted (means ± SE) against the duration of exposure to thapsigargin; the dashed line was fitted to the data by least-squares regression and thus shows the relationship between GNa and time (R2 = 0.955, P < 0.001). D: relationship between the amiloride-sensitive component of the total membrane current (IAmil) and VHold determined (n = 6; means ± SE) under control conditions and after 16-min exposure to thapsigargin.
1-EBIO-evoked membrane currents in H441 cells
1-EBIO (1 mM) increased GTot and hyperpolarized Vm, although these effects were not as well sustained as the response to thapsigargin because the increase in GTot decayed back toward the starting value in the continued presence of 1-EBIO (Fig. 3). Analysis of data collected at the peak of this response (15-30 s) showed that the current recorded from 1-EBIO-stimulated cells reversed at a potential close to EK (Fig. 3). Parallel studies of clotrimazole-treated (3 μM) cells showed that 1-EBIO had no effect on GNa (control 495 ± 145, 1-EBIO 339 ± 104 pS/cell; n = 7).
Fig. 3.
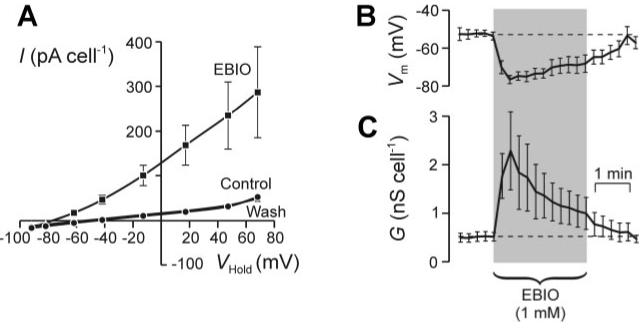
1-ethyl-2-benzimidazolinone (1-EBIO)-evoked membrane currents. A: I-VHold relationships (means ± SE; n = 6) determined by using the staircase protocol (see Fig. 2) to record membrane currents under control conditions, after 15- to 30-min exposure to 1-EBIO (1 mM), and 2-3 min after this substance had been washed from the bath. B: time course showing the 1-EBIO-evoked change in Vm. C: time course showing the 1-EBIO-induced change in GTot.
Effects of 1-EBIO on polarized H441 cells
Experiments in which ISC and [Ca2+]i were recorded simultaneously from confluent cells showed that 1-EBIO (1 mM, bilateral) evoked a reversible rise in ISC (ΔISC = 4.2 ± 0.8 μA/cm2; n = 6, P < 0.01) but had no effect on [Ca2+]i (Fig. 4). This effect was confirmed in further studies of cells mounted in standard Ussing chambers in which 1-EBIO (1 mM) increased ISC from 36.4 ± 3.6 to 40.9 ± 3.6 μA/cm2 (ΔISC = 4.5 ± 0.5 μA/cm2; n = 10, P < 0.001). Parallel studies of age-matched cells at identical passage confirmed that 10 μM amiloride (n = 10), an epithelial Na+ channel antagonist, caused ∼95% inhibition of this spontaneous ISC (see also, e.g., Refs. 30, 32) and showed that 1 mM 1-EBIO had no effect on the small ISC that persisted under these conditions (control 2.7 ± 2.2, 1-EBIO 3.5 ± 2.5 μA/cm2; n = 10).
Fig. 4.
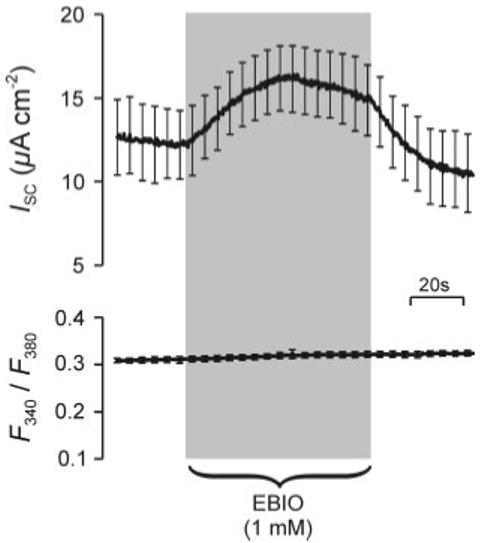
Effects of 1-EBIO on short-circuit current (ISC) and intracellular Ca2+ concentration ([Ca2+]i) in polarized H441 cells. Top: ISC recorded from confluent H441 cells under control conditions and during exposure to a pulse of 1-EBIO (1 mM). Bottom: simultaneously measured records of ratio of fura-2 fluorescence at 340 and 380 nm (F340/F380), which provides an indicator of [Ca2+]i. Both records are means ± SE (n = 6).
Expression of mRNA encoding Ca2+-activated K+ channels in H441 cells
RT-PCR based analysis of RNA extracted from polarized H441 cells showed that these cells expressed mRNA transcripts encoding KCNN4 but provided no evidence for the expression of mRNA encoding large (KCNMA1)- or small (KCNN1)-conductance Ca2+-activated K+ channels (Fig. 5).
Fig. 5.
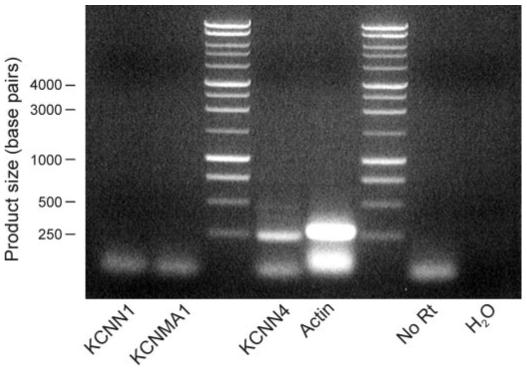
Expression of mRNA encoding different K+ channel subunits. RT-PCR-based analyses (n = 4) of RNA isolated from confluent, dexamethasone-treated cells were undertaken with primers designed to amplify sequences specific to KCNN1 (small-conductance Ca2+-activated K+ channel), KCNMA1 (large-conductance Ca2+-activated K+ channel), and KCNN4 (intermediate-conductance Ca2+-activated K+ channel). In each such experiment efficacy of the amplification procedure was verified with primers specific for actin, while omission of the reverse transcriptase step (No RT) and inclusion of a water control ensured that positive results could not be attributed to contamination from genomic DNA or contamination of the reagents, respectively. All products were isolated from the gels and sequenced to verify their origin.
Functional characteristics of KCNN4
To establish the extent to which the expression of KCNN4 (Fig. 5) could account for the Ca2+ (Fig. 2)- and 1-EBIO (Fig. 3)-evoked currents described above, this channel was cloned from H441 cells and its properties were studied by recording membrane currents from CHO cells transfected with this cDNA sequence (see methods). In these experiments the background currents attributable to endogenous CHO cell conductances were first characterized in cells expressing GFP because this fluorescent protein served as a marker of transfection in all such experiments. Data recorded with pipette filling solutions designed to hold [Ca2+]i at 0.5 μM (Fig. 6A) or 0.05 μM (not shown) showed that conductance of control cells was so low that only negligible currents were recorded at all test potentials. Moreover, irrespective of [Ca2+]i, 1-EBIO (1 mM, 1-2 min) had no effect on the currents recorded from such cells, indicating that this substance does not activate an endogenous conductance. Subsequent studies of KCNN4-expressing cells using a pipette solution designed to maintain [Ca2+]i at 0.5 μM showed that channel expression was associated with large membrane currents that reversed at a potential close to EK (Fig. 6A). Clotrimazole (10 μM) caused substantial inhibition of this current (Fig. 6B), and analysis of these data showed that KCNN4-transfection conferred ∼40 nS/cell of clotrimazole-sensitive conductance on the cells (Fig. 6B). Subsequent experiments using a wider range of putative K+ channel blockers confirmed the inhibitory effect of clotrimazole and showed that Ba2+ also caused substantial inhibition of the KCNN4-associated K+ conductance; iberiotoxin and apamin were both ineffective (Fig. 6C). Experiments in which Na+ was isosmotically replaced by K+ showed that increasing [K+]o depolarized Vm in KCNN4-expressing CHO cells in the manner predicted (Nernst equation) for a selective K+ conductance (Fig. 6D), demonstrating that the current associated with KCNN4 expression is K+ selective.
Fig. 6.
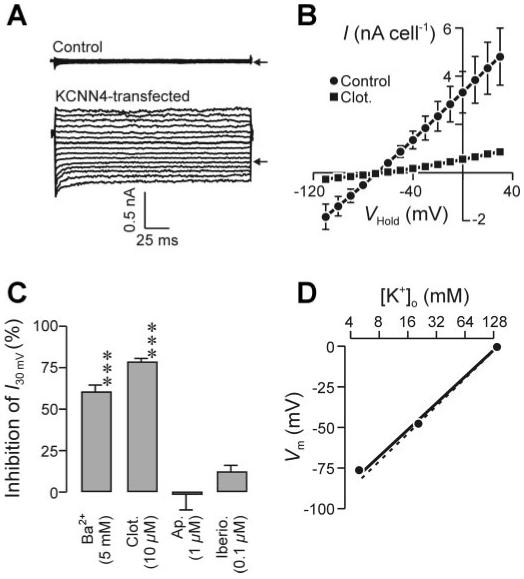
Membrane currents associated with heterologous expression of KCNN4. A: whole cell membrane currents recorded from control [i.e., green fluorescent protein (GFP) transfected] and KCNN4-transfected Chinese hamster ovary (CHO) cells under quasi-physiological ionic conditions. Currents were evoked by stepping (200 ms) VHold from 0 mV to a series of values between -110 and 30 mV; pipette [Ca2+] was 0.5 μM, and arrows indicate the zero-current level. B: relationship between I (means ± SE) and VHold for KCNN4-expressing cells with the pipette filling solution in which [Ca2+] was buffered to 0.5 μM. Data were first recorded under control conditions and then after the application of 10 μM clotrimazole (n = 6). C: extent to which Ba2+, clotrimazole, apamin, and iberiotoxin blocked the currents recorded from KCNN4-associated conductance CHO was determined by quantifying the inhibition of the membrane current required to hold Vm at 30 mV (I30mV). ***P < 0.005. D: data (n = 3; mean values, errors lie within symbols) from KCNN4-expressing cells showing the relationship between Vm and [K+]o; the solid line was fitted to the experimental data by linear regression, whereas the dashed line shows the relationship predicted (Nernst equation) for a selective K+ conductance.
Only small currents could be recorded from such KCNN4-expressing cells when pipette solutions designed to hold [Ca2+]i at 0.05 or 0.2 μM were used, although 1-EBIO consistently caused clear increases in membrane conductance in these cells (Fig. 7). The currents recorded from 1-EBIO-stimulated cells were qualitatively similar to those described above and reversed at potentials close to EK (Fig. 7, A and B), although 1-EBIO had no statistically significant effect on the spontaneous membrane current seen when [Ca2+]i was 0.5 μM (Fig. 7C). Further experiments explored the effects of 1-EBIO on the membrane current needed to clamp Vm at -40 mV (I-40mV); this potential equates to ECl, implying that K+ is the only ion able to carry outward current. Only very small currents were recorded during the initial period of superfusion with control saline when [Ca2+]i was 0.05 or 0.2 μM (Fig. 7D), confirming that KCNN4 expression has no overt effect on the conductive properties of CHO cells when [Ca2+]i is low. However, in both groups of cells acute application of 1-EBIO caused a rapid increase in I-40mV that became apparent with no discernible latency and reached a peak after ∼45 s. This increase in outward K+ current was maintained throughout a 2-min exposure to 1-EBIO, but the response decayed back toward its control value once this drug was withdrawn. Although this drug seemed to evoke a slightly larger and more rapidly developing response when [Ca2+]i was 0.2 μM (Fig. 7D), these effects were not statistically significant.
Fig. 7.
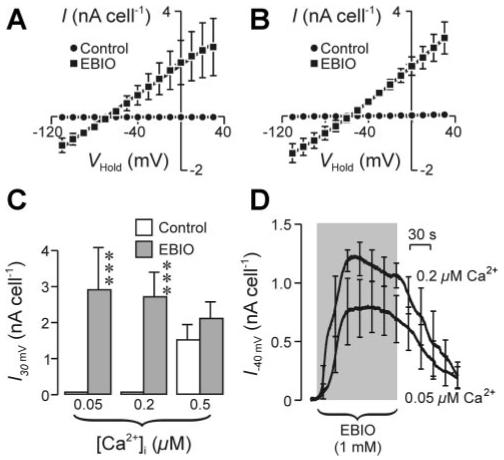
1-EBIO-evoked membrane currents in KCNN4-expressing CHO cells. A: relationships between VHold and I derived from studies of KCNN4-transfected cells undertaken with a pipette filing solution designed to hold [Ca2+]i at 0.05 μM. Data (means ± SE; n = 5) were obtained during superfusion with control saline and after 1- to 2-min exposure to 1 mM 1-EBIO. B: data from directly analogous experiments (n = 6) undertaken with a pipette filling solution designed to hold [Ca2+]i at 0.2 μM. C: pooled data showing the effects of [Ca2+]i and 1-EBIO on I30mV. Statistically significant effects of 1-EBIO (Student’s paired t-test): ***P < 0.005. D: continuous records (means ± SE) showing the effects of 1-EBIO on the current required to hold Vm at -40 mV (I-40mV) with pipette filling solutions designed to hold [Ca2+]i at 0.2 (n = 4) or 0.05 (n = 5) μM. This potential was chosen as it equates to the equilibrium potential for Cl- (ECl), implying that K+ is the only ion able to carry outward current under the present conditions.
Ionic basis of Vm in H441 cells
Previous studies (4) showed that Vm in unstimulated H441 cells is influenced by GNa and established that cell-to-cell variability in the magnitude of this conductance leads to considerable variability in Vm. The present experiments sought to identify the other membrane conductances that contribute to Vm, and, to ensure that this variability in GNa did not confound analysis of these data, the cells used in these experiments were treated with 10 μM amiloride to block this conductance (4, 33). Analysis of zero-current potentials recorded from cells held under current clamp showed that clotrimazole had no effect on Vm under these conditions (control 47.5 ± 6.7, clotrimazole 48.0 ± 6.8 mV), although subsequently raising [K+]o from 4.7 to 113 mM, the concentration in the pipette filling solution, consistently caused depolarization (control 52.8 ± 6.8, high K+ 25.7 ± 4.0 mV; P < 0.001). Anion substitution experiments showed that lowering [Cl-]o from 152.5 to 29 mM depolarized Vm if nominally impermeant Cl- substituents were used but caused hyperpolarization when Cl- was replaced with I- (Table 1). The anionic selectivity of the channels underlying the anion conductance in H441 cells is thus I- < Cl- < gluconate < methanesulfonate. Diphenylamine carboxylate (DPC; 1 mM), a relatively nonselective anion channel blocker, depolarized amiloride-treated cells by ∼6 mV (control -42 ± 12.6, DPC -36.2 ± 12.2 mV; n = 6, P < 0.05), whereas glibenclamide (100 μM), another anion channel blocker, had no effect (control -48.7 ± 4.0, glibenclamide -48.4 ± 4.0 mV; n = 6).
Table 1.
Effects of lowering [Cl-]o on Vm
|
Vm, mV |
||||
|---|---|---|---|---|
| Replacement Anion | n | Control | Low Cl- | ΔVm, mV |
| Gluconate | 17 | -40.6±3.4 | -30.9±4.8‡ | 9.7±2.5 |
| Methanesulfonate | 7 | -53.3±5.5 | -37.5±4.5* | 15.8±5.2 |
| I | 7 | -33.5±6.9 | -44.1±9.1† | -10.6±2.8 |
Data are means ± SE for n cells in each group. Values of membrane potential (Vm) measured under zero current clamp from amiloride-treated (10 μM) cells superfused successively with control saline [Cl- concentration ([Cl-]) = 151.5 mM] and with saline in which extracellular [Cl-] ([Cl-]o) had been lowered to 29 mM by replacing this anion with gluconate, methanesulfonate, or I. Shifts in Vm (ΔVm) were quantified by subtracting the value measured at low [Cl-]o from that measured in the same cells under control conditions. Significant effects of low Cl- (Student’s paired t-test):
P < 0.05
P < 0.005
P < 0.002
DISCUSSION
Thapsigargin-evoked rise in GTot
Because KCNN4 can be regulated via [Ca2+]i (see, e.g., Refs. 2, 28) we first explored the possibility that this K+ channel might be present in H441 cells by studying the effects of thapsigargin, which characteristically causes a large and sustained rise in [Ca2+]i (see, e.g., Ref. 36) on the membrane currents recorded from single cells or small groups of cells. These experiments revealed a Ca2+-dependent increase in GTot accompanied by a hyperpolarization of Vm, and once these effects were established increasing [K+]o depolarized the stimulated cells in the manner predicted for a selective K+ conductance. Ba2+, a nonselective K+ channel blocker, caused ∼75% inhibition of the response to thapsigargin, whereas clotrimazole, a relatively selective KCNN4 blocker, caused essentially complete blockade. Chromanol 293, apamin, and iberiotoxin, which inhibit cAMP-dependent K+ channels, large-conductance Ca2+-activated channels (KCNMA1), and small-conductance Ca2+-activated channels (KCNN1, KCNN2, KCNN3), respectively, were ineffective, and these data indicate that the thapsigargin-evoked increase in GTot reflects KCNN4 activation.
Effects of thapsigargin on GNa
Whereas replacing [Na+]o with a nominally impermeant cation (NMDG+) hyperpolarized unstimulated cells (see also Refs. 4, 33), this maneuver had no such effect after ∼4-min exposure to thapsigargin, indicating that the conductive properties of thapsigargin-stimulated cells are dominated by a K+ conductance. This contrasts with the situation in unstimulated cells, where Vm is clearly influenced by GNa (4, 33), and subsequent experiments therefore explored the effects of thapsigargin on GNa by characterizing the membrane current that persisted when KCNN4 was blocked with clotrimazole. These studies revealed a substantial (∼50%) but slowly developing fall in GNa. Spontaneous rundown of ENaC has been documented in experiments in which GNa was monitored by the standard whole cell recording technique, but this was inhibited by including nucleotides in the pipette filling solution (16) and our previously published data (4) show that, when the perforated-patch recording technique is used, GNa is stable over the time course of this experiment. The present data thus indicate that, as well as increasing GK, thapsigargin causes a slowly developing fall in GNa, and this is consistent with data from several different systems, including lung and airway epithelia, which show that increases in [Ca2+]i inhibit epithelial Na+ channels (ENaC) (17) and reduce Na+ absorption (11, 25, 27, 29, 38). The slow onset of this response was surprising because the hyperpolarizing response to NMDG+-containing saline was lost after only ∼4 min. However, the present data show that GTot rises rapidly in thapsigargin-stimulated cells, and once this response is established, GK would be 20- to 25-fold greater than GNa. Examination of this problem with the Goldman-Hodgkin-Katz Equation predicts that, even before a fall in GNa had occurred, exposure to the NMDG+-containing solution would hyperpolarize Vm by only ∼4 mV. Such a small response may not have been detected under the present conditions.
Effects of 1-EBIO
1-EBIO mimicked the effects of thapsigargin on GTot and Vm, and analysis of data collected at the peak of this response showed that the 1-EBIO- and thapsigargin-evoked membrane currents were qualitatively similar. Because 1-EBIO is known to activate KCNN4 (28), this result provides further evidence for the expression of these K+ channels in H441 cells. The response to 1-EBIO was less well sustained than the response to thapsigargin, and, although a clear fall in GTot occurred in the presence of this drug, Vm was still hyperpolarized by 10-15 mV after 3- to 4-min exposure to 1-EBIO. 1-EBIO had no effect on GNa, because this parameter remained at ∼300 pS/cell throughout the exposure to this drug, and these findings therefore predict that the 1-EBIO-induced hyperpolarization would potentiate cellular Na+ current by -4 to -6 pA/cell [because INa + ΔINa = (GNa + ΔGNa)·(VNa + ΔVNa)]. The confluent cultures used in the present study contained ∼106 cells/cm, and so this implies a 4 to 6 μA/cm2 stimulation of ISC, which almost exactly matches the response observed in our studies of polarized cells. The effects of 1-EBIO on the conductive properties of single cells can thus explain the stimulation of Na+ transport seen in cultured epithelia, and these data highlight the central importance of GK to the control of epithelial Na+ absorption (6, 9, 10). Moreover, our studies of polarized cells also show that the 1-EBIO-induced increase in ISC occurs with no change in [Ca2+]i, and this is consistent with the view that 1-EBIO activates KCNN4 by sensitizing these channels to Ca2+ (28).
Role of KCNN4 in unstimulated cells
Analysis of RNA extracted from H441 cells revealed mRNA transcripts encoding KCNN4 but indicated that two related K+ channels, one of which (KCNN1) is also activated by 1-EBIO, were not present. In subsequent experiments KCNN4 was therefore cloned from H441 cells and the corresponding cDNA sequence was expressed in CHO cells so that we could directly explore the physiological features of this gene product. Heterologous expression of KCNN4 was associated with a large and highly selective K+ conductance when [Ca2+]i was 0.5 μM but had no overt effect on the conductive properties of CHO cells when [Ca2+]i was 0.05 or 0.2 μM. However, 1-EBIO consistently caused a rapid increase in GK under these conditions, although this substance had little further effect on the large currents recorded when [Ca2+]i was 0.5 μM. These findings are therefore consistent with the observation that 1-EBIO sensitizes KCNN4 to [Ca2+]i (28) and thus confirm that KCNN4 expression confers a Ca2+- and 1-EBIO-sensitive K+ current on the plasma membrane.
The responses to thapsigargin and 1-EBIO seen in H441 cells can thus be attributed to activation of KCNN4, but the fact that expression of this K+ channel gene had no effect on the conductive properties of the membrane unless [Ca2+]i was >0.2 μM suggests that KCNN4 is inactive at the values of [Ca2+]i typically found in resting cells. This accords with the data from experiments in which KCNN4 was expressed in oocytes (35), although studies using an alternative mammalian expression system (HEK 293 cells) indicate that KCNN4 displays significant activity when [Ca2+]i is ∼0.1 μM (28). The discrepancy between these studies may reflect the fact that Ca2+ controls KCNN4 by activating calmodulin rather than by directly regulating channel gating, and the activity of this signaling pathway can be modulated via other signaling pathways such as calmodulin kinase (20). It is therefore possible that there may be differences between the concentrations of [Ca2+]i needed to activate KCNN4 in different cell types. We therefore undertook further studies of H441 cells that showed that increasing [K+]o consistently depolarized cells that had been treated with amiloride, establishing that GK is significant under these conditions. However, these experiments also showed that a concentration of clotrimazole sufficient to block KCNN4 had no effect on Vm, and this finding, in common with our data from the cloned channel, suggests that KCNN4 does not contribute to GK in unstimulated cells.
The K+-rich bath solution used in the present study was designed to shift EK to 0 mV, but the experiments that explored the effects of this solution on amiloride-treated cells showed that Vm never reached this potential, and so at least one other ionic conductance must contribute to Vm under these conditions. Subsequent experiments showed that lowering [Cl-]o, and thus shifting ECl to 0 mV, caused depolarization when nominally impermeant Cl- substituents were used, and this shows that Vm is also influenced by the cellular Cl- conductance (GCl). Although the channels underlying GCl are still to be identified, the fact that the I--rich solution caused hyperpolarization establishes that these channels are more permeable to I- than to Cl- [i.e., permeability (P)I > PCl], and channels with this property have been described in rat cortical lens fiber cells (40). At least one earlier study has suggested that H441 cells express the cAMP-regulated anion channels (CFTR) encoded by the gene that is mutated in cystic fibrosis (22), but these channels characteristically display a lower permeability to I- than to Cl- (i.e., PCl > PI) (see, e.g., Ref. 19), which does not accord with the present data. It thus appears that H441 cells do not express functional CFTR under the present experimental conditions, and further evidence of this came from the fact that DPC, a nonselective Cl- channel blocker, caused depolarization whereas glibenclamide, which displays some selectivity for CFTR, did not (see, e.g., Ref. 19).
H441 cells have now been used in several recent studies of human airway epithelial Na+ transport (4, 24, 30-33), and the present data show clearly that these cells express KCNN4 and establish that the presence of this K+ channel can account for the thapsigargin- and 1-EBIO-evoked increases in GTot seen in single cells and the 1-EBIO-evoked stimulation Na+ transport seen in cultured epithelia (see also Ref. 9). These findings highlight the importance of GK to the control of Na+ absorption (6, 9, 10) and show that physiological activators of KCNN4 might contribute to the neurohormonal modulation of this physiologically important ion transport process (see also Ref. 9). However, our data also show that KCNN4 is inactive at resting [Ca2+]i, and this is reminiscent of the situation described in the erythrocyte and colon, where KCNN4 expression confers a “latent” conductance on the membrane that can be activated by increases in [Ca2+]i or perturbations in cell volume (2, 39). In H441 cells, KCNN4 could well play an important role in mediating absorptive responses to hormones/neurotransmitters or in the control of cell volume during active ion transport. However, because this channel does not influence Vm in resting cells, KCNN4 cannot be part of the mechanism underlying spontaneous Na+ absorption. Indeed, our data show that VNa is dependent on unidentified K+ and Cl- channels and, given the importance of airway Na+ transport to lung function (see, for example, Refs. 3, 26), it is now important to identify the channels underlying these physiologically relevant conductances.
ACKNOWLEDGMENTS
We are grateful to the Wellcome Trust and Tenovus Scotland for the financial support that made this study possible, to Maree Constable (Maternal and Child Health Sciences, University of Dundee) for help with some of the experiments, and to Ray Caldwell (Cystic Fibrosis/Pulmonary Research and Treatment Center, University of North Carolina) for helpful comments on the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Barry PH, Lynch JW. Liquid junction potentials and small cell effects in patch-clamp analysis. J Membr Biol. 1991;121:101–117. doi: 10.1007/BF01870526. [DOI] [PubMed] [Google Scholar]
- 2.Begenisich T, Nakamoto T, Ovitt CE, Nehrke K, Brugnara C, Alper S, Melvin JE. Physiological roles of the intermediate conductance Ca2+-activated potassium channel Kcnn4. J Biol Chem. 2004;279:47681–47687. doi: 10.1074/jbc.M409627200. [DOI] [PubMed] [Google Scholar]
- 3.Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004;23:146–158. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- 3a.Chambers LA, Constable MJ, Clunes MT, Ko WH, Olver RE, Inglis SK, Wilson SM. Adenosine-evoked Na+ transport in airway epithelial cells. Br J Pharmacol. 2006 doi: 10.1038/sj.bjp.0706822. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clunes MT, Butt AG, Wilson SM. A glucocorticoid-induced Na+ conductance in human airway epithelial cells identified by perforated patch recording. J Physiol. 2004;557:809–819. doi: 10.1113/jphysiol.2004.061143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuthbert AW, Hickman ME, Thorn P, MacVinish L. Activation of Ca2+- and cAMP-sensitive K+ channels in murine colonic epithelia by 1-ethyl-2-benzimidazolone. Am J Physiol Cell Physiol. 1999;277:C111–C120. doi: 10.1152/ajpcell.1999.277.1.C111. [DOI] [PubMed] [Google Scholar]
- 6.Dawson DC, Richards NW. Basolateral K conductance: role in regulation of NaCl absorption and secretion. Am J Physiol Cell Physiol. 1990;259:C181–C195. doi: 10.1152/ajpcell.1990.259.2.C181. [DOI] [PubMed] [Google Scholar]
- 7.Devor DC, Singh AK, Frizzell RA, Bridges RJ. Modulation of Cl- secretion by benzimidazolones. I. Direct activation of a Ca2+-dependent K+ channel. Am J Physiol Lung Cell Mol Physiol. 1996;271:L775–L784. doi: 10.1152/ajplung.1996.271.5.L775. [DOI] [PubMed] [Google Scholar]
- 8.Duncan L, Burton FL, Smith GL. REACT: calculation of free metal and ligand concentrations using a Windows-based computer program (Abstract) J Physiol. 1999;517:2. [Google Scholar]
- 9.Gao L, Yankaskas JR, Fuller CM, Sorscher EJ, Matalon S, Forman HJ, Venglarik CJ. Chlorzoxazone or 1-EBIO increases Na+ absorption across cystic fibrosis airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1123–L1129. doi: 10.1152/ajplung.2001.281.5.L1123. [DOI] [PubMed] [Google Scholar]
- 10.Gordon LGM, MacKnight A. Application of membrane potential equations to tight epithelia. J Membr Biol. 1991;120:155–163. doi: 10.1007/BF01872398. [DOI] [PubMed] [Google Scholar]
- 11.Graham A, Steel DM, Alton EWFW, Geddes DM. Second messenger regulation of sodium transport in mammalian airway epithelia. J Physiol. 1992;453:475–491. doi: 10.1113/jphysiol.1992.sp019240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;265:3440–3450. [PubMed] [Google Scholar]
- 13.Hamill OP, Marty A, Neher E, Sakman B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton KL, Meads L, Butt AG. 1-EBIO stimulates Cl- secretion by activating a basolateral K+ channel in mouse jejunum. Pflügers Arch. 1999;439:158–166. doi: 10.1007/s004249900137. [DOI] [PubMed] [Google Scholar]
- 15.Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishikawa T, Jiang C, Stutts MJ, Marunaka Y, Rotin D. Regulation of the epithelial Na+ channel (ENaC) by cytosolic ATP. J Biol Chem. 2003;278:38276–38286. doi: 10.1074/jbc.M307216200. [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa T, Marunaka Y, Rotin D. Electrophysiological characterization of the rat epithelial Na+ channel (rENaC) expressed in MDCK cells. Effects of Na+ and Ca2+ J Gen Physiol. 1998;111:825–846. doi: 10.1085/jgp.111.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacob R. Agonist-stimulated divalent cation entry into single cultured human umbilical vein endothelial cells. J Physiol. 1990;421:55–77. doi: 10.1113/jphysiol.1990.sp017933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- 20.Khanna R, Chang MC, Joiner WJ, Kaczmarek LK, Schlichter LC. hSK4/hIK1, a calmodulin-binding KCa channel in human T lymphocytes. Roles in proliferation and volume regulation. J Biol Chem. 1999;274:14838–14849. doi: 10.1074/jbc.274.21.14838. [DOI] [PubMed] [Google Scholar]
- 21.Ko WH, Law VW, Wong HY, Wilson SM. The simultaneous measurement of epithelial ion transport and intracellular free Ca2+ incultured equine sweat gland secretory epithelium. J Membr Biol. 1999;170:205–211. doi: 10.1007/s002329900550. [DOI] [PubMed] [Google Scholar]
- 22.Kulaksiz H, Schmid A, Hönscheid M, Ramaswamy A, Cetin Y. Clara cell impact in air side activation of CFTR in small pulmonary airways. Proc Natl Acad Sci USA. 2002;99:6796–6801. doi: 10.1073/pnas.102171199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lazarowski ER, Paradiso AM, Watt WC, Harden TK, Boucher RC. UDP activates a mucosal-restricted receptor on human nasal epithelial cells that is distinct from the P2Y2 receptor. Proc Natl Acad Sci USA. 1997;94:2599–2603. doi: 10.1073/pnas.94.6.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazrak A, Matalon S. cAMP-induced changes in apical membrane potentials of confluent H441 monolayers. Am J Physiol Lung Cell Mol Physiol. 2003;285:L443–L450. doi: 10.1152/ajplung.00412.2002. [DOI] [PubMed] [Google Scholar]
- 25.Ludens JH. Studies on the inhibition of Na+ transport in toad bladder by the ionophore A23187. J Pharmacol Exp Ther. 1978;206:414–422. [PubMed] [Google Scholar]
- 26.Mall M, Grubb BR, Harkema JR, O’Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10:487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 27.Palmer LG, Frindt G. Effects of cell Ca and pH on Na channels from rat cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol. 1987;253:F333–F339. doi: 10.1152/ajprenal.1987.253.2.F333. [DOI] [PubMed] [Google Scholar]
- 28.Pedersen KA, Shrøder RL, Skaaning-Jensen B, Strøbæk D, Olesen SR, Chrosophersen P. Activation of the human intermediate-conductance Ca2+-activated K+ channel by 1-ethyl-2-benzimidazolinone is strongly Ca2+-dependent. Biochim Biophys Acta. 1999;1420:231–240. doi: 10.1016/s0005-2736(99)00110-8. [DOI] [PubMed] [Google Scholar]
- 29.Ramminger SJ, Collett A, Baines DL, Murphie H, McAlroy HL, Olver RE, Inglis SK, Wilson SM. P2Y2 receptor-mediated inhibition of ion transport in distal lung epithelial cells. Br J Pharmacol. 1999;128:293–300. doi: 10.1038/sj.bjp.0702767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramminger SJ, Richard K, Inglis SK, Land SC, Olver RE, Wilson SM. A regulated apical Na+ conductance in dexamethasonetreated H441 airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L411–L419. doi: 10.1152/ajplung.00407.2003. [DOI] [PubMed] [Google Scholar]
- 31.Richard K, Ramminger SJ, Forsyth L, Burchell A, Wilson SM. Thyroid hormone potentiates glucocorticoid-evoked airway Na+ transport without affecting α-ENaC transcription. FEBS Lett. 2004;576:339–342. doi: 10.1016/j.febslet.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 32.Sayegh R, Auerbach SD, Li X, Loftus RW, Husted RF, Stokes JB, Thomas CP. Glucocorticoid induction of epithelial sodium channel expression in lung and renal epithelia occurs via trans-activation of a hormone response element in the 5′-flanking region of the human epithelial sodium channel alpha subunit gene. J Biol Chem. 1999;274:12431–12437. doi: 10.1074/jbc.274.18.12431. [DOI] [PubMed] [Google Scholar]
- 33.Shlyonsky V, Goolaerts A, Van Beneden R, Sariban-Sohraby S. Differentiation of epithelial Na+ channel function: an in vitro model. J Biol Chem. 2005;280:24181–24187. doi: 10.1074/jbc.M413823200. [DOI] [PubMed] [Google Scholar]
- 34.Singh S, Syme CA, Singh AK, Devor DC, Bridges RJ. Benzimidazolone activators of chloride secretion: potential therapeutics for cystic fibrosis and chronic obstructive pulmonary disease. J Pharmacol Exp Ther. 2001;296:600–611. [PubMed] [Google Scholar]
- 35.Syme CA, Gerlach AC, Singh AK, Devor DC. Pharmacological activation of cloned intermediate- and small-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol. 2000;278:C570–C581. doi: 10.1152/ajpcell.2000.278.3.C570. [DOI] [PubMed] [Google Scholar]
- 36.Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc Natl Acad Sci USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ussing HH, Zerahn K. Active transport of sodium as the source of electric current in the short circuited isolated frog skin. Acta Physiol Scand. 1951;23:110–127. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]
- 38.Venglarik CJ, Dawson DC. Cholinergic regulation of Na absorption by turtle colon: role of basolateral K conductance. Am J Physiol Cell Physiol. 1986;251:C563–C570. doi: 10.1152/ajpcell.1986.251.4.C563. [DOI] [PubMed] [Google Scholar]
- 39.Warth R, Bleich M. K+ channels and colonic function. Rev Physiol Biochem Pharmacol. 2000;140:1–62. doi: 10.1007/BFb0035550. [DOI] [PubMed] [Google Scholar]
- 40.Webb KF, Merriman-Smith BR, Stobie JK, Kistler J, Donaldson PJ. Cl- influx into rat cortical lens fiber cells is mediated by a Cl- conductance that is not ClC-2 or -3. Invest Opthalmol Vis Sci. 2004;45:4400–4408. doi: 10.1167/iovs.04-0205. [DOI] [PubMed] [Google Scholar]


