Abstract
The extensively glycosylated lysosome-associated membrane proteins (LAMP)-2a, b, and c are derived from a single gene by alternative splicing that produces proteins with differences in the transmembrane and cytosolic domains. The lysosomal targeting signals reside in the cytosolic domain of these proteins. LAMPs are not restricted to lysosomes but can also be found in endosomes and at the cell surface. We investigated the subcellular distribution of chimeras comprised of the lumenal domain of avian LAMP-1 and the alternatively spliced domains of avian LAMP-2. Chimeras with the LAMP-2c cytosolic domain showed predominantly lysosomal distribution, while higher levels of chimeras with the LAMP-2a or b cytosolic domain were present at the cell surface. The increase in cell surface expression was due to differences in the recognition of the targeting signals and not saturation of intracellular trafficking machinery. Site-directed mutagenesis defined the COOH-terminal residue of the cytosolic tail as critical in governing the distributions of LAMP-2a, b, and c between intracellular compartments and the cell surface.
Lysosome-associated membrane proteins (LAMPs)1 are major components of the lysosomal membrane. Two structurally related classes of LAMPs, LAMP-1 and -2, occur in birds and mammals, encoded by separate but evolutionally related genes. Both classes of LAMPs have a large, extensively glycosylated lumenal/extracellular domain, a single transmembrane domain, and a short cytosolic tail (for reviews see Fukuda, 1991; Peters and von Figura, 1994). Recently, multiple mRNAs have been identified for the LAMP-2 class (Hatem et al., 1995; Konecki et al., 1995). These mRNAs arise by alternative splicing of a single transcript and encode LAMP-2 molecules with different transmembrane and cytosolic domains (Gough et al., 1995).
The consensus targeting sequence, G-Y-X-X-hydrophobe, for delivery of the LAMPs to lysosomes resides in the cytosolic tail (Williams and Fukuda, 1990; Mathews, et al., 1992; Guarnier et al., 1993; Höning and Hunziker, 1995; Höning et al., 1996). LAMPs can reach the lysosome via two intracellular routes: a “direct” pathway from the trans-Golgi network either to the late endosomes or directly to lysosomes (Green et al., 1987; Harter and Mellman, 1992; Hunziker and Geuze, 1996), or an “indirect” pathway, which involves transport to early endosomes and the cell surface, where rounds of exocytosis and endocytosis may occur before delivery to lysosomes (LippincottSchwartz and Fambrough, 1986, 1987; Nabi et al., 1991; Carlsson and Fukuda, 1992; Mathews, et al., 1992; Akasaki et al., 1995). The signals for both direct and indirect delivery are contained within the targeting sequence.
The relative amounts of LAMPs expressed at the cell surface in the steady state appear to vary with cell type and physiological state. Many cells have very low levels of LAMPs at the cell surface; however, highly metastatic tumor cells (Saitoh et al., 1992), activated macrophages (Ho and Springer, 1983), strongly stimulated platelets (Febbraio and Silverstein, 1990; Silverstein and Febbraio, 1992), and retinoic acid-induced embryonal carcinoma cells (Amos and Lotan, 1990) show increased levels of LAMPs at the cell surface. LAMPs expressed at the cell surface carry complex oligosaccharide chains that bind to E-selectin (Sawada et al., 1994) and galaptin (Inohara and Raz, 1994; Woynarowska et al., 1994) and are substrates for cell surface β-1,4-galactosyltransferase (Maillet and Shur, 1993). Thus, cell surface LAMPs may be important mediators of cellular adhesion through interactions of their carbohydrate chains with carbohydrate receptors. Regulation of the subcellular distribution of LAMPs between the plasma membrane and the endocytic pathway may therefore be crucial in control of LAMP-mediated cell–cell interactions. Perhaps this is why two targeting routes are available to LAMPs. In the study reported here, we explored the relationship between LAMP targeting signals and subcellular distribution.
The LAMP-2 splice variants have different transmembrane domains and cytosolic tails. These differences include different amino acids within the COOH-terminal G-Y-X-X-hydrophobe sequence that participates in protein targeting. Previously we showed that the lysosomal targeting signal in the cytosolic tail of each of the LAMP-2 variants is competent for the delivery of chimeric LAMP proteins to lysosomes (Hatem et al., 1995). In this study we tested the hypothesis that the differences in LAMP-2 targeting signals affect the steady-state distribution of the LAMP-2 variants in cells. We report that the steady state levels of the LAMP-1/LAMP-2 chimeras at the cell surface are different and that these differences are correlated with differences in the LAMP-2 COOH-terminal targeting signals.
Materials and Methods
Construction of Chimeras
Plasmids encoding the LAMP chimeras were created in the pCB6 vector, which contains the human cytomegalovirus promoter for expression in mammalian cells and the G418 resistance gene for selection in G418 medium. Table I shows the regions of amino acid sequence difference among the various chimeras, along with a schematic of the structure of the chimeras. All of the chimeras contain the lumenal domain of LAMP-1 up to the position defined by the boundary between exons 8 and 9 in the LAMP genes. The numbering of the amino acids in the chimeras starts with the first amino acid after the cleaved signal sequence of LAMP-1. Each of the first three chimeras in Table I has the transmembrane domain and the cytosolic tail from a single LAMP-2 variant. To dissect which region of the molecule is responsible for the differences in cellular distribution, the cytosolic tails were switched on the L/2b/2b and L/2c/2c chimeras creating L/2b/2c and L/2c/2b (Table I, chimeras 4 and 5). Four point mutations were created in the LAMP-2b cytosolic tail in the context of the L/2b/2b chimera to make the sequence more similar to the LAMP-2c tail (Table I, chimeras 6–9). The four point mutants include R391 to Y, S396 to T, V397 to L, and a combination mutant containing S396 to T and V397 to L. Finally, the reciprocal point mutation at the COOH-terminal residue of L396 to V was made in the L/2c/2c chimera (Table I, chimera 10).
Table I.
Primary Structure of the LAMP Chimeras
| Chimera | Amino acids from LAMP-1 | Amino acids from LAMP-2 | ||||||
|---|---|---|---|---|---|---|---|---|
| L/2a/2a | F1-V345 | NKFSIAEDCSPEVDY.FIVP | IAVGAALGGLVVLVIMAYFLG | HKKHHNTGYEQF | ||||
| L/2b/2b | F1-V345 | NKFSIAEECFADSDLNFLIP | VAVGMALGFLIILVFISYIIG | .RRKSRTGYQSV | ||||
| L/2c/2c | F1-V345 | NKFSIAQECSLDDD.TILIP | IVVGAALAGLIVIIVIAYIIG | .RRKSYAGYQTL | ||||
| L/2c/2b | F1-V345 | NKFSIAQECSLDDD.TILIP | IVVGAALAGLIVIIVIAYIIG | .RRKSRTGYQSV | ||||
| L/2b/2c | F1-V345 | NKFSIAEECFADSDLNFLIP | VAVGMALGFLIILVFISYIIG | .RRKSYAGYQTL | ||||
| L/2b/2b R391Y | F1-V345 | NKFSIAEECFADSDLNFLIP | VAVGMALGFLIILVFISYIIG | .RRKSYTGYQSV | ||||
| L/2b/2b S396T | F1-V345 | NKFSIAEECFADSDLNFLIP | VAVGMALGFLIILVFISYIIG | .RRKSRTGYQTV | ||||
| L/2b/2b V397L | F1-V345 | NKFSIAEECFADSDLNFLIP | VAVGMALGFLIILVFISYIIG | .RRKSRTGYQSL | ||||
| L/2b/2b S396T, V397L | F1-V345 | NKFSIAEECFADSDLNFLIP | VAVGMALGFLIILVFISYIIG | .RRKSRTGYQTL | ||||
| L/2c/2c L396V | F1-V345 | NKFSIAQECSLDDD.TILIP | IVVGAALAGLIVIIVIAYIIG | .RRKSYAGYQTL | ||||
| Structure | Lumenal/extracellular domain | Transmembrane domain | Cytoplasmic tail | |||||
Point mutations were created using the Quick Change kit (Stratagene, La Jolla, CA), and the nucleotide sequences encoding the complete transmembrane and cytosolic tail of each chimera were confirmed by dideoxy sequencing with the Sequenase kit (United States Biochemical, Cleveland, OH) or dye terminator automated sequencing with the Genetic Analyzer (Prism 310; ABI Adv. Biotechnologies, Columbia, MD). The “tail switch” chimeras were constructed by generating a PCR product containing the desired sequence and splicing this sequence into the appropriate plasmid between unique HpaI and XhoI restriction enzyme sites. The nucleotide sequences of all subcloned PCR fragments were determined to confirm the fidelity of each PCR product. All primers were synthesized in the trityl-on mode with the DNA synthesizer (PCR-MATE; Applied Biosystems, Foster City, CA) and purified through oligo purification cartridges.
Cell Lines and Culture
Plasmids were introduced into mouse L cells using Lipofectin (Life Technologies, Inc., Gaithersburg, MD) with Optimem medium (5 μg DNA/ 60-mm dish of 60% confluent cells). Stable cell lines were selected and maintained in Dulbecco's minimal essential medium (DMEM) with 10% fetal calf serum and 1% gentamycin, supplemented with 400 μg/ml G418. Expression of chimeric LAMPs was induced by treatment of the transfected cells with 1 mM butyrate for 48 ± 2 h, and the subcellular distribution of LAMPs in individual cell lines (designated with a number) was characterized. To minimize the possibility that observed differences in LAMP distribution were due to peculiarities of individual cell lines, entire dishes of nonclonal transfected L cells (designated “pool”) were also assayed as a single sample. Individual cell lines were heterogeneous in terms of expression level per cell and the number of positive cells, as judged by immunofluorescence microscopy.
Chimera Detection and Quantification
The distribution of the LAMP chimeras was determined using a monoclonal antibody (mAb-CV24) against the lumenal domain of avian LAMP-1, formerly called LEP100 (Lippincott-Schwartz and Fambrough, 1987). This antibody recognizes the lumenal/extracellular domain and will bind to LAMP chimeras expressed on the surface of intact cells. Also, this antibody can be radiolabeled with 125I without loss of activity, permitting direct detection and quantification of the amount of the chimera expressed by the transfected cells.
Immunofluorescent labeling of permeabilized cells was performed, after fixation in 1% formaldehyde, with 2 μg/ml mAb-CV24 in Hanks' balanced salt solution, 20 mM Tris-Cl, pH 7.5, and 2% horse serum (H/T/HS) supplemented with 0.1% saponin. The primary antibody was detected with FITC-conjugated goat anti–mouse secondary antibody (Kirkegaard & Perry, Gaithersburg, MD). Immunofluorescent labeling of intact cells was performed, after fixation in 1% formaldehyde, with 2 μg/ml FITC-conjugated mAb-CV24 in H/T/HS without saponin.
Iodinated antibody binding was performed on permeabilized and intact fixed cells. mAb-CV24 (100 μg) was iodinated by the Iodogen method (Salicinski et al., 1981). Binding was performed, after fixation in 1% formaldehyde, with 2 μg/ml 125I–mAb-CV24 in H/T/HS with or without 0.1% saponin. Nonspecific binding was measured in the presence of 25-fold excess cold mAb-CV24 in the presence and absence of saponin. Each sample was assayed in triplicate. Specific binding was calculated by subtracting the nonspecific counts per minute from total counts per minute for both permeabilized and intact conditions. The fraction of LAMP molecules present at the cell surface was calculated by dividing the specific counts per minute of 125I–mAb-CV24 bound in the absence of saponin by the specific counts per minute bound in the presence of saponin.
Antibody Internalization Assay
The internalization of LAMP molecules expressed on the cell surface was estimated by measuring the rate at which 125I-labeled mAb-CV24 bound to LAMP molecules at the cell surface became resistant to removal from the surface by acid stripping. Stable cell lines were plated in 6-well plates at 5 × 105 cells/well and induced with butyrate for 48 h. Cells were cooled on wet ice, washed once with cold H/T/HS, and incubated for 4 h with 2 μg/ml 125I–mAb-CV24 in H/T/HS. The cells were washed in 2 baths of ice cold H/T/HS for 15 min each to remove unbound antibody. Cells were either kept on wet ice or incubated at 37°C in prewarmed Hepes-buffered growth medium for the indicated times. The medium was collected. The surface antibody was stripped with ice cold 0.1 M acetic acid/1.5 M NaCl for 30 min on wet ice and collected. Finally, the cells were extracted in 1 N NaOH at room temperature to determine cell-associated antibody. The media, acetic acid solutions, and NaOH extracts were counted in a γ counter. Nonspecific binding was determined from cultures handled identically, except that a 25-fold excess of unlabeled mAb-CV24 was included in the 125I–mAb-CV24 binding solution. After corrections for nonspecific binding, the percent of bound antibody that was internalized was calculated by dividing the NaOH-extracted counts by the total counts present in the medium, acetic acid, and NaOH extract.
Quantification of Endogenous Mouse LAMP-1
Endogenous mouse LAMP-1 was detected with the monoclonal antibody 1D4B, a gift from Dr. J.T. August (The Johns Hopkins University School of Medicine, Baltimore, MD). For immunofluorescence microscopy and iodinated antibody binding, the cells were processed as described above for detection and quantification of avian LAMP chimeras. The fraction of endogenous mouse LAMP-1 expressed at the cell surface was determined from binding experiments with 125I-labeled mAb-1D4B; the binding experiments and calculations were analogous to those described above for quantifying the distribution of the avian LAMP chimeras. mAb-1D4B was used at a concentration of 10 μg/ml for both immunofluorescence microscopy and iodinated antibody binding assays. The binding assays were performed in two independent experiments with duplicate samples for total and nonspecific binding in the presence and absence of 0.1% saponin.
Results
Chimeric LAMPs Have Different Levels of Surface Expression
To determine whether the variable domains of the LAMP-2s could affect the levels of LAMP-2 expressed at the cell surface, chimeras between LAMP-1 and the LAMP-2 variants were made (Table I, and Hatem et al., 1995). The nomenclature for the chimeras is given in Table I. The first letter, L, represents the lumenal/extracellular domain from avian LAMP-1; the second characters, 2a, b, or c, represent the transmembrane domain from one of the avian LAMP-2 variants; and the last characters indicate the cytosolic tail domain of a LAMP-2 variant. For example, chimera L/2a/2a has the lumenal domain of LAMP-1, the transmembrane domain of LAMP-2a, and the cytosolic tail of LAMP-2a. Point mutations in the cytosolic tail are indicated by conventional notation. Chimeras in which the lumenal/extracellular domain of LAMP-1 was attached to the transmembrane and cytosolic domains of each of the three LAMP-2 variants, a, b, and c were expressed in stably transfected mouse L cells, and 1 mM butyrate was used to induce expression.
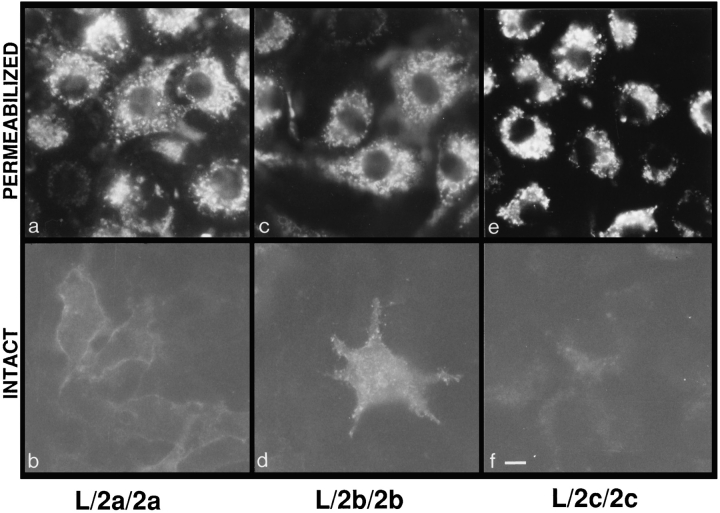
Although each of the LAMP-1/LAMP-2 chimeras was capable of being targeted to lysosomes (Hatem et al., 1995), differences in their levels of expression at the cell surface were readily apparent in direct immunofluorescent labeling of intact cells (Fig. 1). Cells expressing the L/2c/2c chimera showed negligible levels of surface immunofluorescent labeling, to the extent that it was difficult to identify positive expressing cells by direct immunofluorescence labeling without permeabilization (Fig. 1 f). Cells expressing the L/2a/2a chimeras were more variable in surface expression; some cell lines contained easily identifiable, positive cells (lines 19 and 20) while in other lines the cells were considerably fainter in their immunofluorescence (line 10) without permeabilization. Cells expressing the L/2b/2b chimera showed the highest levels of surface expression (Fig. 1 d).
Figure 1.
Immunofluorescent labeling of intact and permeabilized L cells expressing the L/2a/2a, L/2b/2b, or L/2c/2c chimera. Stable lines of L cells expressing the indicated chimera were induced for 48 h. a and b are L/2a/2a clone 19. c and d are L/2b/2b clone 20. e and f are L/2c/2c clone 2–12. Intact cells (b, d, and f) were fixed and incubated with directly conjugated fluorescein–mAbCV24. Permeabilized cells (a, c, and e) were fixed and indirectly labeled with mAb-CV24 in the presence of saponin using fluorescein-conjugated goat anti–mouse secondary antibody. Bar, 10 μm.
The ability to visualize the chimeras at the cell surface is dependent on both the level of expression and the percentage of molecules at the cell surface. Therefore, the amount of LAMP-1/LAMP-2 chimera at the cell surface was quantified by 125I–mAb-CV24 binding to intact and permeabilized cells. Several clonal cell lines and also nonclonal pools expressing each chimera were analyzed (Table II). When the data from the cell lines expressing each chimera are pooled and compared, differences in levels of expression of the chimeras at the cell surface are apparent (Fig. 2). Cells expressing L/2a/2a show 3-fold higher levels of surface expression than cells expressing L/2c/2c; while cells expressing L/2b/2b show 4-fold higher levels of surface expression than L/2c/2c.
Table II.
Percent of LAMP-1/LAMP-2 Chimera Expressed at the Cell Surface
| Chimera | Cell line | n* | Average surface expression (%)‡ | |||
|---|---|---|---|---|---|---|
| pool | 3 | 7 ± 1 | ||||
| L/2c/2c | 1-23 | 3 | 10 ± 2 | |||
| 4 | 2 | 8 | ||||
| 5 | 2 | 4 | ||||
| 2-12 | 5 | 4 ± 1 | ||||
| 2-5 | 3 | 3 ± 2 | ||||
| L/2a/2a | pool | 2 | 21 | |||
| 6 | 3 | 14 ± 4 | ||||
| 8 | 3 | 14 ± 1 | ||||
| 10 | 3 | 9 ± 2 | ||||
| 19 | 4 | 26 ± 4 | ||||
| 20 | 3 | 21 ± 2 | ||||
| 23 | 3 | 17 ± 5 | ||||
| L/2a/2a | pool | 3 | 26 ± 7 | |||
| 6 | 3 | 13 ± 5 | ||||
| 9 | 3 | 17 ± 2 | ||||
| 11 | 3 | 25 ± 7 | ||||
| 14 | 3 | 28 ± 7 | ||||
| 20 | 5 | 39 ± 15 | ||||
| 24 | 4 | 27 ± 7 |
n represents the number of independent experiments in which each sample was assayed in triplicate.
average per cell line ± standard deviation; calculated from 125I-labeled mAb-CV24 binding to intact and permeabilized cells as described in Materials and Methods.
Figure 2.
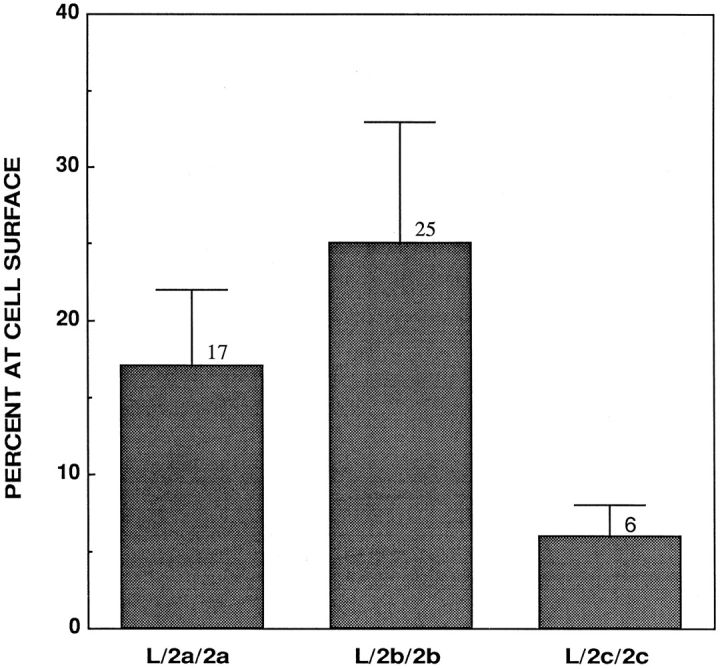
Quantification of the percent of L/2a/2a, L/2b/2b, and L/2c/2c at the cell surface. Individual clones and pools of transfected L cells were induced for 48 h. Percent of each chimera detected at the cell surface with 125I-labeled mAb-CV24 was calculated as described in Materials and Methods. Data represent the average and standard error for all individual clones and pools of cells combined.
Endogenous Mouse LAMP-1 Retains Its Intracellular Distribution in Cell Lines Expressing LAMP Chimeras
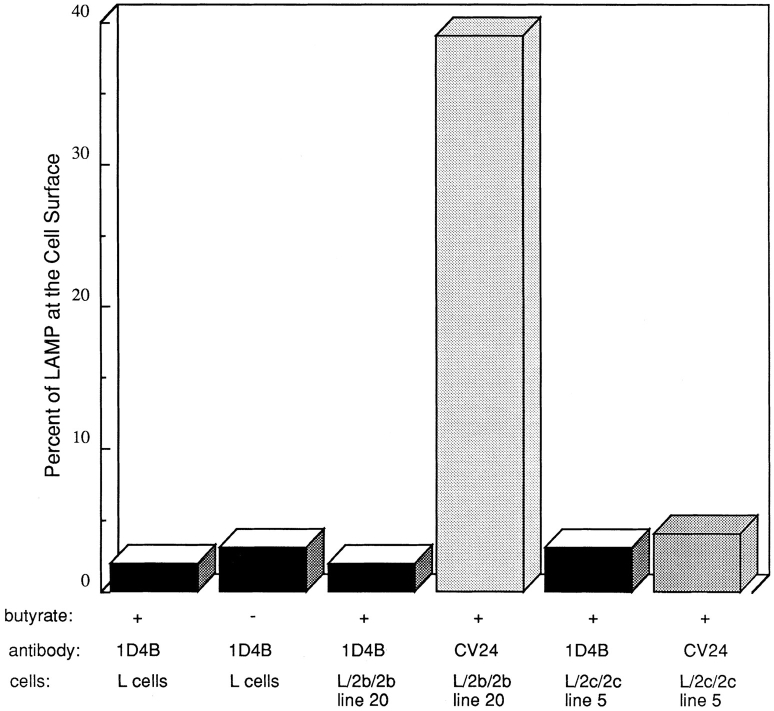
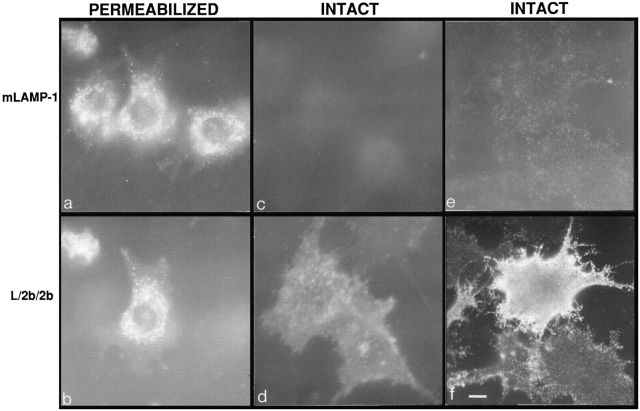
It has been shown in previous studies that overexpression of LAMP molecules can appear to saturate the lysosomal targeting mechanism and result in increased expression of LAMPs, including endogenous LAMPs, at the cell surface (Harter and Mellman, 1992; Uthayakumar and Granger, 1995; Marks et al., 1996). Therefore, it was important to ascertain whether levels of expression achieved in the present studies were sufficiently high to cause the misdirection of endogenous LAMPs to the cell surface. As shown in Fig. 3, the amount of endogenous mouse LAMP-1 at the cell surface in mouse L cells is very low in nontransfected and also transfected cells both in the presence and absence of 1 mM butyrate. The amount of mouse LAMP-1 at the cell surface in the cells under every condition (transfected, nontransfected, treated with butyrate, or untreated) was 2–4%, as quantified by 125I-labeled mAb-1D4B binding. In cells induced to express L/2b/2b (line 20) or L/2c/2c (line 5) with 39 and 5% of chimera molecules at the surface, respectively (Table II), the endogenous mouse LAMP-1 remains predominantly intracellular with only 3–4% of mouse LAMP-1 at the cell surface. This result indicates that expression of the exogenous LAMP chimeras is not so high as to perturb the targeting of endogenous LAMP-1 molecules. Cells induced to express L/2b/2b (line 20) and then double labeled for surface expression of L/2b/2b with mAb-CV24 and mouse LAMP-1 with mAb-1D4B show that the L/2b/2b (Fig. 4, d and f) is much more abundant than the mouse LAMP-1 at the cell surface (Fig. 4, c and e), indicating that the molecules are distributed differently within the L cells. L/2b/2b and mouse LAMP-1 colocalize in lysosomes in double-labeled permeabilized cells (Fig. 4, a and b).
Figure 3.
Quantification of endogenous LAMP-1 and transfected chimera at the cell surface. The distribution of mouse LAMP-1 was determined by 125I-labeled mAb-1D4B binding as described in Materials and Methods. The distribution of L/2b/2b or L/2c/2c was determined by iodinated mAb-CV24 binding as described. Nontransfected L cells were tested with and without 48-h treatment in 1 mM butyrate. Each condition was tested in at least two independent experiments.
Figure 4.
The distribution of mouse LAMP-1 and L/2b/2b in transfected mouse L cells. Induced L/2b/2b (clone 20) cells were fixed and immunolabeled, with or without permeabilization, with mAb1D4B against endogenous mouse LAMP-1 and mAbCV24 against L/2b/2b, as indicated in the figure. Cells in a and c and b and d were labeled with directly conjugated rhodamine–mAb-1D4B and fluorescein–mAb-CV24, respectively. Permeabilized cells (a and b) were fixed, and antibodies were incubated in the presence of saponin. Cells in e and f were indirectly labeled with mAb1D4B followed by fluorescein-conjugated goat anti–rat secondary antibody and then labeled with mAb-CV24 followed by rhodamine-conjugated goat anti–mouse secondary antibody. a, c, and f were photographed with rhodamine epifluorescence. b, d, and e were photographed with fluorescein epifluorescence. Bar, 10 μm.
As an additional line of evidence that saturation of a targeting mechanism did not occur, there was no correlation between the level of expression of a chimera and the fraction of molecules at the cell sufrace. In most of the cell lines, the average number of chimera molecules per expressing cell varied from ∼50,000 to ∼400,000. In one extreme case, a line expressing ∼1 × 106 molecule/cell of a chimera with the LAMP-2c cytosolic tail had only 2% of the molecules at the cell surface. Thus, overexpression resulting in mistargeting of molecules to the plasma membrane was not a complication in our study.
The Cytosolic Tail Determines Subcellular Distribution
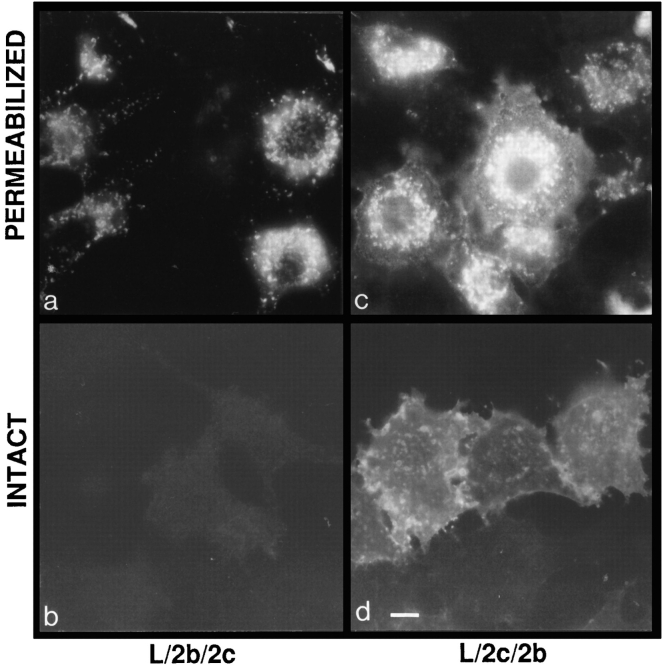
To determine whether the differences in subcellular distribution of the various LAMP chimeras were due to differences in the transmembrane domain or in the cytosolic tail, “tail switch” chimeras (L/2b/2c and L/2c/2b) were constructed, and their subcellular distributions in transfected mouse L cells were determined. As shown in Fig. 5, cells expressing chimeras with the LAMP-2c cytosolic tail showed a very low level immunofluorescent labeling at the cell surface of intact cells while showing intense labeling after permeabilization (Fig. 5, a and b). Cells induced to express the chimeras with the LAMP-2b cytosolic tail showed high levels of surface expression, evidenced by immunofluorescent labeling of intact cells (Fig. 5, c and d). Quantitatively, the L/2c/2b chimeras were expressed at 3.3-fold higher levels at the cell surface than the L/2b/2c chimeras (Table III).
Figure 5.
Immunofluorescent labeling of mouse L cells expressing the tail switch chimeras. Intact (b and d) cells were fixed and incubated with directly conjugated fluorescein–mAb-CV24. Permeabilized cells (a and c) were fixed and incubated in the presence of saponin with mAb-CV24 followed by fluorescein-conjugated goat anti–mouse secondary antibody. a and b are L/2b/2c clone 12. c and d are L/2c/2b clone 10. Bar, 10 μm.
Table III.
Percent of the Tail Switch Chimera Expressed at the Cell Surface
| Chimera | Cell line | n * | Average surface expression (%)‡ | |||
|---|---|---|---|---|---|---|
| L/2c/2b | pool | 2 | 40 | |||
| 10 | 3 | 24 ± 3 | ||||
| 2-10 | 3 | 36 ± 4 | ||||
| L/2b/2c | 11 | 2 | 9 | |||
| 12 | 2 | 15 | ||||
| 16 | 2 | 6 | ||||
| 17 | 2 | 15 | ||||
| 18 | 2 | 12 | ||||
| 19 | 2 | 10 | ||||
| 21 | 2 | 6 |
n represents the number of independent experiments in which each sample was assayed in triplicate.
average per cell line ± standard deviation; calculated from 125I-labeled mAb-CV24 binding to intact and permeabilized cells as described in Materials and Methods.
Levels of cell surface expression of these tail switch chimeras (Table III) as well as the parent chimeras (Table II) correlated well with the identity of the cytosolic tail. That is, chimeras with the LAMP-2b cytosolic tail showed the highest levels of cell surface expression, while those with the LAMP-2c cytosolic tail showed much lower levels of cell surface expression. The differences in cell surface expression between L/2b/2c and L/2c/2b are consistent with the targeting information being part of the cytosolic tail and may be attributable to differences in the G-Y-X-X- hydrophobe motif of the COOH-terminal five amino acids of LAMP-2b and c. The cytosolic tails of LAMP-2b and c differ in only 4 of the 12 residues (Table I). Two of these differences are in the G-Y-X-X-hydrophobe sequence.
The COOH-terminal Residue Influences LAMP Subcellular Distribution
To assess which amino acids within the cytosolic tails of the LAMP-2 molecules are critical in conferring high or low levels of surface expression, point mutations were created in the cytosolic tails of the L/2b/2b and L/2c/2c chimeras. Amino acids in the cytosolic tail of LAMP-2b were replaced with amino acids present in the LAMP-2c tail and vice versa. L cells expressing the chimeras containing the point mutations were analyzed by immunofluorescence microscopy (Fig. 6) and quantitative antibody binding (Table IV). The first mutation L/2b/2b R391Y was outside the G-Y-X-X-hydrophobe targeting sequence. This mutant showed high levels of surface expression similar to its parent chimera L/2b/2b, suggesting that this residue was not part of the signal for efficient lysosomal targeting of LAMP-2c (Fig. 6 b, and Table IV). Two mutations were created in the G-Y-X-X-hydrophobe sequence of LAMP-2b S396T and V397L. The L/2b/2b S396T mutant showed high levels of surface expression, indicating that this residue was not sufficient to confer more efficient lysosomal targeting information to the LAMP-2b cytosolic tail (Fig. 6 d, and Table IV). However, the L/2b/2b V397L mutant showed remarkably lower surface expression, similar to that seen with the L/2c/2c chimera, suggesting that the COOH-terminal residue is important for the high efficiency of lysosomal targeting of LAMP-2c (Fig. 6 f, and Table IV). The double mutant containing the S396T and V397L mutations behaved as the V397L mutant with low expression at the cell surface (Table IV). To confirm that the COOH-terminal residue could dictate the relative levels of surface expression of the LAMP-2 variants, one additional chimera (L/2c/2c L396V) was generated which changes the COOH-terminal leucine of LAMP-2c to a valine, the COOH-terminal residue of LAMP-2b. (Note that LAMP-2c is shorter by one amino acyl residue than LAMP-2b; position 396 is the penultimate residue in LAMP-2b but the COOH-terminal residue in LAMP-2c.) This chimera was expressed at high levels at the cell surface (Fig. 6 h, and Table IV), consistent with the COOHterminal residue being the essential component of the signal for dictating relative surface expression of the LAMP2 variants in the steady state.
Figure 6.
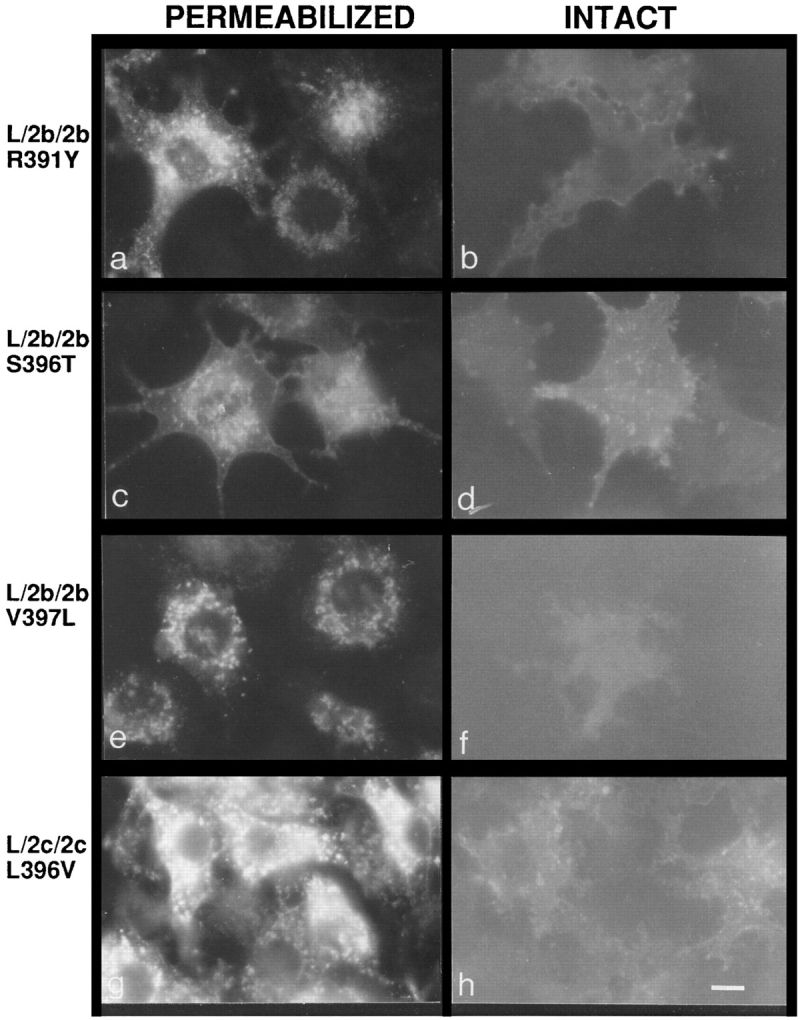
Immunofluorescent labeling of mouse L cells expressing point mutants of L/2b/2b or L/2c/2c. Intact (b, d, f, and h) cells were fixed and incubated with directly conjugated fluorescein– mAb-CV24. Permeabilized cells (a, c, e, and g) were fixed and incubated in the presence of saponin with mAb-CV24 followed by fluorescein-conjugated goat anti–mouse secondary antibody. a and b are L/2b/2b R391Y clone 5. c and d are L/2b/2b S396T clone 6. e and f are L/2b/2b V397L clone 13. g and h are L/2c/2c L396V clone 3. Bar, 10 μm.
Table IV.
Percent of LAMP-1/LAMP-2 Point Mutant Chimera Expressed at the Cell Surface
| Chimera | Cell line | n* | Average surface expression (%)‡ | |||
|---|---|---|---|---|---|---|
| L/2b/2b S396T | pool | 2 | 40 | |||
| 3 | 3 | 44 ± 9 | ||||
| 6 | 3 | 45 ± 9 | ||||
| L/2b/2b R391Y | 4 | 2 | 40 | |||
| 5 | 2 | 31 | ||||
| 6 | 2 | 28 | ||||
| 8 | 2 | 36 | ||||
| 14 | 2 | 24 | ||||
| 17 | 2 | 32 | ||||
| 19 | 2 | 29 | ||||
| 23 | 2 | 44 | ||||
| L/2b/2b V397L | pool | 3 | 8 ± 2 | |||
| 7 | 3 | 4 ± 1 | ||||
| 13 | 3 | 6 ± 1 | ||||
| L2b/2b S396T, V397L | pool | 6 | 8 ± 2 | |||
| 6 | 3 | 5 ± 1 | ||||
| 19 | 2 | 6 | ||||
| 21 | 2 | 6 | ||||
| 24 | 3 | 5 ± 2 | ||||
| L/2c/2c L396V | pool | 5 | 22 ± 2 | |||
| 3 | 2 | 21 | ||||
| 12 | 2 | 28 |
n represents the number of independent experiments in which each sample was assayed in triplicate.
average per cell line ± standard deviation; calculated from 125I-labeled mAb-CV24 binding to intact and permeabilized cells as described in Materials and Methods.
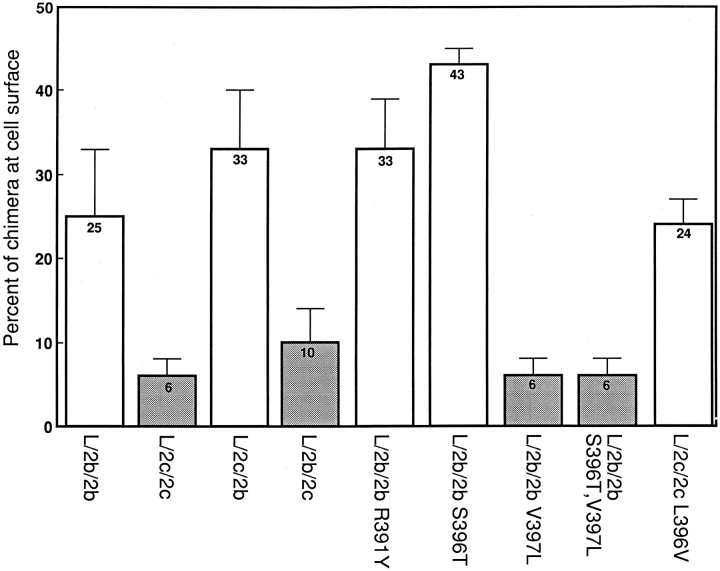
The average cell surface expression levels of all the cell lines and populations expressing the LAMP-2b and c chimeras and point mutants are compared in Fig. 7. The chimeric molecules clearly segregate into two populations based on their COOH-terminal residue: one with high levels of surface LAMP in the steady state and one with low levels of surface LAMP in the steady state. Cells expressing chimeras with a COOH-terminal leucine residue (L/2c/ 2c; L/2b/2c; L/2b/2b V397L; L/2b/2b S396T, V397L) showed 4.6-fold lower levels of surface expression than cells expressing the chimeras with COOH-terminal valine (L/2b/ 2b; L/2c/2b; L/2b/2b S396T; L/2b/2b R391Y; L/2c/2c L396V), demonstrating that the COOH-terminal amino acid greatly influences the subcellular distribution of LAMP-2 molecules.
Figure 7.
Quantification of the L/2b/2b and L/2c/2c based chimeras at the cell surface. Individual clones and pools of transfected L cells were induced with 1 mM butyrate for 48 h. Percent of each chimera detected at the cell surface with 125I-labeled mAb-CV24 was calculated as described in Materials and Methods. Data represent the average and standard error for each individual clone and pool of cells combined. The open bars represent those chimeras with a valine at their COOHterminal position. The shaded bars represent those chimeras with a leucine at their COOH-terminal position.
Chimeras with Increased Surface Expression Show Decreased Rates of Internalization from the Cell Surface
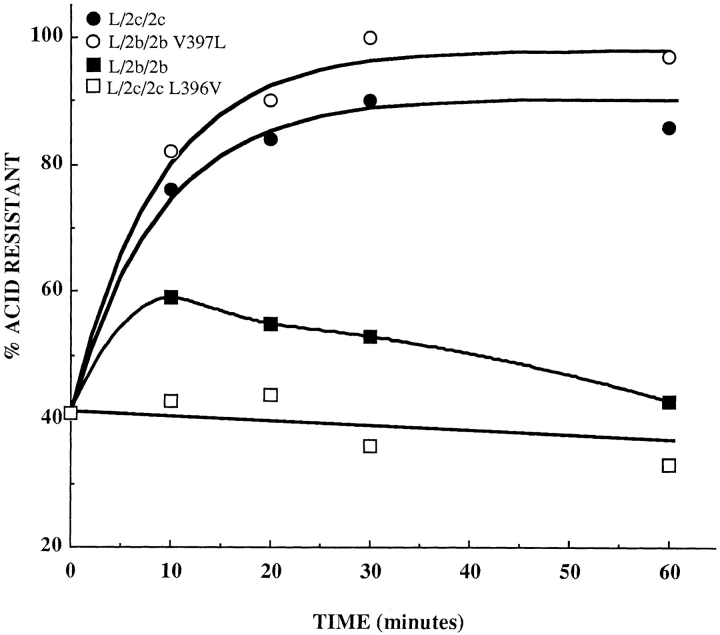
LAMPs can be internalized from the cell surface via clathrin-mediated endocytosis. To determine whether differences in the rates of internalization could contribute to the increased surface expression of those chimeras containing a COOH-terminal valine, the internalization rates of four of the chimeras (L/2b/2b, L/2c/2c, L/2b/2b V397L, and L/2c/2c L396V) were determined (Fig. 8). Cells expressing the L/2c/2c chimera or the L/2b/2b V397L mutant chimera internalized iodinated mAb-CV24 with a half-time of ∼6 min, indicating that the cytosolic tails of these two chimeras can interact with the molecular machinery that mediates endocytosis. These cells essentially cleared the surface of the chimera during the 60 min incubation at 37°C, suggesting either that the endosomal pool of chimera molecules was much larger than the surface pool or that most of the endocytosed molecules were not being recycled back to the cell surface. Cells expressing chimera L/2b/2b or L/2c/2c L396V did not internalize iodinated mAb-CV24 efficiently, indicating that valine in the COOH-terminal position impaired the ability of the cytosolic tail to be recognized by the endocytic machinery. We suggest that the relatively high levels of surface expression of the L/2b/2b and L/2c/2c L396V chimeras are attributable, at least in part, to drastically decreased rates of endocytosis.
Figure 8.
Endocytosis of chimeras from the cell surface. Individual clones of mouse L cells expressing chimeras L/2b/2b and L/2c/ 2c and chimeras with COOH-terminal mutations L/2b/2b V397L and L/2c/2c L396V were induced with 1 mM butyrate for 48 h. The amount of internalization was calculated from the amount of 125I-labeled mAb-CV24 (initially bound to the cell surface at 4°C) that became resistant to stripping by low pH upon incubation at 37°C. Each point is the average of two independent experiments performed in triplicate except the zero time point, which represents the average value from all experiments. Clonal cell lines tested were L/2b/2b 20, L/2c/2c 4, L/2b/2b V397L 7, and L/2c/2c L396V 3. Curves for chimeras L/2c/2c and L/2b/2b V397L are fit by first order exponentials with half-times of 6 min.
Discussion
LAMPs are not restricted to lysosomes but can also be found within the endosomal system and at the plasma membrane. Regulation of LAMP distribution among these compartments has been noted for several cell types. For example, macrophages (Ho and Springer, 1983) and platelets (Febbraio and Silverstein, 1990; Silverstein and Febbraio, 1992) redistribute their LAMP-1 and -2 molecules to the cell surface in high amounts upon stimulation. Additionally, the distribution of LAMPs is altered during the formation of a confluent monolayer of MDCK cells (Nabi and Rodriguez-Boulan, 1993), upon retinoic acid-induced differentiation of embryonal carcinoma cells (Amos and Lotan, 1990), and in highly metastatic tumor cells (Saitoh et al., 1992). Frequently, the changes in LAMP distribution are accompanied by alterations in the glycosylation pattern of the LAMPs, such as is seen in the metastatic tumor cells (Saitoh et al., 1992) and the MDCK cells (Nabi and Rodriguez-Boulan, 1993). The appearance of LAMPs at the cell surface may result in changes in the adhesion properties of cells, since LAMPs can interact with E-selectin (Sawada et al., 1994) and galaptin (Inohara and Raz, 1994; Woynarowska et al., 1994). Thus, regulation of the abundance of LAMP molecules at the cell surface may play an important role in regulation of cellular adhesion. The abundance of LAMPs at the cell surface may be governed not only by extracellular stimuli but also by which isoform (LAMP-2a, b, or c) is being expressed.
As the results of this study demonstrate, LAMP-1/ LAMP-2 chimeras show differences in steady state distribution that are attributable to amino acid differences in the cytosolic tail domains of the splice variants of LAMP-2. Cells expressing LAMP-1/LAMP-2c showed very low levels of surface expression similar to the levels of LAMP-1, as described for primary cultures of chicken embryo fibroblasts (Lippincott-Schwartz and Fambrough, 1986) and L cells (Mathews et al., 1992) and MDCK cells transfected with chicken LAMP-1 (Nabi et al., 1991). Cells expressing LAMP-1/LAMP-2a or LAMP-1/LAMP-2b showed much higher levels of surface expression, suggesting that the steady state distribution may be influenced by the different cytosolic tail sequences. Chimeras in which the cytosolic tails of LAMP-2b and c were switched and chimeras with point mutations in the cytosolic tails of LAMP-2b and c showed different levels of surface expression, demonstrating that differences in subcellular distribution of LAMP-2 splice variants can be attributed to differences in their cytosolic tails. These results suggest that the LAMP-2 splice variants may have quantitatively different subcellular distributions in vivo and that the function of LAMP-2 molecules may be related, at least in part, to their expression at the plasma membrane.
It is possible that each LAMP has multiple functions and that different functions involve different compartments of the cell. Additionally, it is possible that each LAMP variant plays a unique role in cells that express it. Within the lysosome, the extensively glycosylated LAMPs that accumulate in high abundance, such as LAMP-1 and -2c, may provide a barrier to prevent degradation of the lysosomal membrane lipid bilayer and the lysosomal membrane proteins that are important for lysosomal function. On the other hand, the expression of LAMP molecules at high levels in the plasma membrane, such as occurs with LAMP-2a and b, as well as the redistribution of LAMPs to the cell surface in certain situations, may provide opportunities for interactions between LAMPs and receptors on other cells or in the extracellular matrix. Such interactions could influence cellular adhesion or participate in other intercellular events. Less likely, but not excluded by present information, LAMPs could be binding to extracellular components which are then endocytosed together with the LAMP molecules, the LAMP molecules essentially serving as receptors in receptor-mediated endocytosis.
Mechanistically, the differences in subcellular distribution may reflect differences in the abilities of the cytosolic tail targeting sequences to be recognized by intracellular trafficking machinery. The G-Y-X-X-hydrophobe sequence is believed to be recognized by adaptin complexes, AP-1 at the trans-Golgi network and AP-2 at the plasma membrane (Ohno et al., 1995; Höning et al., 1996). Differences within this G-Y-X-X-hydrophobe consensus sequence, such as those seen among the LAMP-2 variants, may result in different affinities for the adaptin complexes at various steps in the biosynthetic/targeting pathway. Consistent with this possibility, the chimeras with high surface expression, such as L/2b/2b and L/2c/2c L396V, were found to be deficient in their ability to be endocytosed from the cell surface, suggesting that the AP-2 complex may not efficiently recognize LAMP cytosolic tails that have a valine in the COOH-terminal position. Such an inference is congruent with observations of Ohno et al. (1995) that when the targeting sequence of TGN38 (SDYQRL) was made COOH terminal, replacing the COOH-terminal leucine with valine greatly decreased its interaction with the AP-2 m subunit and to a lesser degree decreased its interaction with the AP-1 m subunit, assessed in yeast two hybrid assays. Differences in recognition of LAMP cytosolic tails by intracellular trafficking machinery at the level of the trans-Golgi network, the plasma membrane, and the endosomal compartment may also contribute to the differences in subcellular distribution of LAMPs. Whatever the critical sorting sites and molecular mechanisms, the identity of the COOH-terminal residue provides an important signal to determine the subcellular distribution of the LAMP-2 variants.
Acknowledgments
We would like to thank Christine L. Hatem for technical assistance and critical review of the manuscript, Mark Zweifel for technical assistance especially in cell culture, and Mitch Kostich for the preparation of purified anti–mouse LAMP-1 monoclonal antibody (all at The Johns Hopkins University, Baltimore, MD).
This work was supported by National Institutes of Health grant NS23241 awarded to Dr. D.M. Fambrough.
Footnotes
1. Abbreviation used in this paper: LAMP, lysosome-associated membrane protein.
Please address all correspondence to Douglas M. Fambrough, Department of Biology, The Johns Hopkins University, Baltimore, MD 21218. Tel.: (410) 516-8417; Fax: (410) 516-5213.
References
- Akasaki K, Michihara A, Mibuka K, Fujiwara Y, Tsuji H. Biosynthetic transport of a major lysosomal membrane glycoprotein, Lamp-1: convergence of biosynthetic and endocytic pathways occurs at three distinctive points. Exp Cell Res. 1995;220:464–473. doi: 10.1006/excr.1995.1338. [DOI] [PubMed] [Google Scholar]
- Amos B, Lotan R. Modulation of lysosomal-associated membrane glycoproteins during retinoic acid-induced embryonal carcinoma cell differentiation. J Biol Chem. 1990;265:19192–19198. [PubMed] [Google Scholar]
- Carlsson SR, Fukuda M. The lysosomal membrane glycoprotein Lamp-1 is transported to lysosomes by two alternative pathways. Arch Biochem Biophys. 1992;296:630–639. doi: 10.1016/0003-9861(92)90619-8. [DOI] [PubMed] [Google Scholar]
- Febbraio M, Silverstein RL. Identification and characterization of LAMP-1 as an activation-dependent platelet surface glycoprotein. J Biol Chem. 1990;265:18531–18537. [PubMed] [Google Scholar]
- Fukuda M. Lysosomal membrane glycoproteins. J Biol Chem. 1991;266:21327–21330. [PubMed] [Google Scholar]
- Gough NR, Hatem CL, Fambrough DM. The family of LAMP-2 proteins arise by alternative splicing from a single gene. DNA Cell Biol. 1995;14:836–867. doi: 10.1089/dna.1995.14.863. [DOI] [PubMed] [Google Scholar]
- Green SA, Zimmer K-P, Griffiths G, Mellman I. Kinetics of intracellular transport and sorting of lysosomal membrane and plasma membrane proteins. J Cell Biol. 1987;105:1227–1240. doi: 10.1083/jcb.105.3.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnieri FG, Arterburn LM, Penno MB, Cha Y, August JT. The motif tyr-X-X-hydrophobic residue mediates lysosomal membrane targeting of lysosome-associated membrane protein 1. J Biol Chem. 1993;268:1941–1946. [PubMed] [Google Scholar]
- Harter C, Mellman I. Transport of the lysosomal membrane glycoprotein lgp120 (lgp-A) does not require appearance on the plasma membrane. J Cell Biol. 1992;117:311–325. doi: 10.1083/jcb.117.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatem CL, Gough NR, Fambrough DM. Multiple mRNAs encode the avian lysosomal membrane protein LAMP-2, resulting in alternative transmembrane and cytoplasmic domains. J Cell Sci. 1995;108:2093–2100. doi: 10.1242/jcs.108.5.2093. [DOI] [PubMed] [Google Scholar]
- Ho M-K, Springer TA. Tissue distribution, structural characterization, and biosynthesis of Mac-3, a macrophage surface glycoprotein exhibiting molecular weight heterogeneity. J Biol Chem. 1983;258:636–642. [PubMed] [Google Scholar]
- Höning S, Hunziker W. Cytoplasmic determinants involved in direct lysosomal sorting, endocytosis, and basolateral targeting of rat Igp120 (lamp-1) in MDCK cells. J Cell Biol. 1995;128:321–332. doi: 10.1083/jcb.128.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höning S, Griffith J, Geuze HJ, Hunziker W. The tyrosine-based lysosomal targeting signal in lamp-1 mediates sorting into Golgi-derived clathrin-coated vesicles. EMBO (Eur Mol Biol Organ) J. 1996;15:5230–5239. [PMC free article] [PubMed] [Google Scholar]
- Hunziker W, Geuze HJ. Intracellular trafficking of lysosomal membrane proteins. Bioessays. 1996;18:379–389. doi: 10.1002/bies.950180508. [DOI] [PubMed] [Google Scholar]
- Inohara H, Raz A. Identification of human melanoma cellular and secreted ligands for galectin-3. Biochem Biophys Res Commun. 1994;201:1366–1375. doi: 10.1006/bbrc.1994.1854. [DOI] [PubMed] [Google Scholar]
- Konecki DS, Foetisch K, Zimmer K-P, Schlotter M, Lichter U - Konecki. An alternatively spliced form of the human lysosome-associated membrane protein-2 gene is expressed in a tissue specific manner. Biochem Biophys Res Commun. 1995;215:757–767. doi: 10.1006/bbrc.1995.2528. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Fambrough DM. Lysosomal membrane dynamics: structure and interorganellar movement of a major lysosomal membrane glycoprotein. J Cell Biol. 1986;102:1593–1605. doi: 10.1083/jcb.102.5.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Fambrough DM. Cycling of the integral membrane glycoprotein LEP100, between plasma membrane and lysosomes: kinetic and morphological analysis. Cell. 1987;49:669–677. doi: 10.1016/0092-8674(87)90543-5. [DOI] [PubMed] [Google Scholar]
- Maillet CM, Shur B. Uvomorulin, LAMP-1, and laminin are substrates for cell surface β-1,3-galactosyltransferase on F9 embryonal carcinoma cells: comparisons between wild-type and mutant 5.51 att−cells. Exp Cell Res. 1993;208:282–295. doi: 10.1006/excr.1993.1248. [DOI] [PubMed] [Google Scholar]
- Marks MS, Woodruff L, Ohno H, Bonifacino JS. Protein targeting by tyrosine- and di-leucine-based signals: evidence for distinct saturable components. J Cell Biol. 1996;135:341–354. doi: 10.1083/jcb.135.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews PM, Martinie JB, Fambrough DM. The pathway and targeting signal for delivery of the integral membrane glycoprotein LEP100 to lysosomes. J Cell Biol. 1992;118:1027–1040. doi: 10.1083/jcb.118.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi IR, Rodriguez-Boulan E. Increased LAMP-2 polylactosamine glycosylation is associated with its slower Golgi transit during establishment of a polarized MDCK epithelial monolayer. Mol Biol Cell. 1993;4:627–635. doi: 10.1091/mbc.4.6.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi IR, Le Bivic A, Fambrough DM, Rodriguez-Boulan E. An endogenous MDCK lysosomal membrane glycoprotein is targeted basolaterally before delivery to lysosomes. J Cell Biol. 1991;115:1573–1584. doi: 10.1083/jcb.115.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H, Stewart J, Fournier M-C, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino J. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science (Wash DC) 1995;269:1872–1874. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Peters C, von Figura K. Biogenesis of lysosomal membranes. FEBS (Fed Eur Biochem Soc) Lett. 1994;346:108–114. doi: 10.1016/0014-5793(94)00499-4. [DOI] [PubMed] [Google Scholar]
- Saitoh O, Wang W-C, Lotan R, Fukuda M. Differential glycosylation and cell surface expression of lysosomal membrane glycoproteins in sublines of a human colon cancer exhibiting distinct metastatic potentials. J Biol Chem. 1992;267:5700–5711. [PubMed] [Google Scholar]
- Salicinski PRP, McLean C, Sykes JE, Clement-Jones VV, Lowry PJ. Iodination of proteins, glycoproteins, and peptides using solid phase oxidizing agent, 1,3,4,6-tetrachloro-3,6-diphenylglycoluril (Iodogen) Anal Biochem. 1981;117:136–146. doi: 10.1016/0003-2697(81)90703-x. [DOI] [PubMed] [Google Scholar]
- Sawada R, Tsubo S, Fukuda M. Differential E-selectin-dependent adhesion efficiency in sublines of a human colon cancer exhibiting distinct metastatic potentials. J Biol Chem. 1994;269:1425–1431. [PubMed] [Google Scholar]
- Silverstein RL, Febbraio M. Identification of lysosome-associated membrane protein-2 as an activation-dependent platelet surface glycoprotein. Blood. 1992;80:1470–1475. [PubMed] [Google Scholar]
- Uthayakumar S, Granger BL. Cell surface accumulation of overexpressed hamster lysosomal membrane glycoproteins. Cell Mol Biol Res. 1995;41:405–420. [PubMed] [Google Scholar]
- Williams MA, Fukuda M. Accumulation of membrane glycoproteins in lysosomes requires a tyrosine residue at a particular position in the cytoplasmic tail. J Cell Biol. 1990;111:955–966. doi: 10.1083/jcb.111.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woynarowska B, Skrincosky DM, Haag A, Sharma M, Matta K, Bernacki RJ. Inhibition of lectin-mediated ovarian tumor cell adhesion by sugar analogs. J Biol Chem. 1994;269:22797–22803. [PubMed] [Google Scholar]