Abstract
As a rule, hepatocyte growth factor/scatter factor (HGF/SF) is produced by mesenchymal cells, while its receptor, the tyrosine kinase encoded by the met proto-oncogene, is expressed by the neighboring epithelial cells in a canonical paracrine fashion. In the present work we show that both HGF/SF and met are coexpressed by undifferentiated C2 mouse myoblasts. In growing cells, the autocrine loop is active as the receptor exhibits a constitutive phosphorylation on tyrosine that can be abrogated by exogenously added anti-HGF/SF neutralizing antibodies. The transcription of HGF/SF and met genes is downregulated when myoblasts stop proliferating and differentiate. The coexpression of HGF/SF and met genes is not exclusive to C2 cells since it has been assessed also in other myogenic cell lines and in mouse primary satellite cells, suggesting that HGF/SF could play a role in muscle development through an autocrine way.
To analyze the biological effects of HGF/SF receptor activation, we stably expressed the constitutively activated receptor catalytic domain (p65tpr-met) in C2 cells. This active kinase determined profound changes in cell shape and inhibited myogenesis at both morphological and biochemical levels. Notably, a complete absence of muscle regulatory markers such as MyoD and myogenin was observed in p65tpr-met highly expressing C2 clones. We also studied the effects of the ectopic expression of human isoforms of met receptor (h-met) and of HGF/SF (h-HGF/SF) in stable transfected C2 cells. Single constitutive expression of h-met or h-HGF/SF does not alter substantially the growth and differentiation properties of the myoblast cells, probably because of a species-specific ligand–receptor interaction. A C2 clone expressing simultaneously both h-met and h-HGF/SF is able to grow in soft agar and shows a decrease in myogenic potential comparable to that promoted by p65tpr-met kinase. These data indicate that a met kinase signal released from differentiation-dependent control provides a negative stimulus for the onset of myogenic differentiation.
Considerable evidence has been accumulated indicating the importance of hepatocyte growth factor (HGF)1 in liver physiology (for review see Michalopoulos and Zarnegar, 1992). This protein is a potent mitogen for mature hepatocytes in primary culture (Miyazawa et al., 1989; Nakamura et al., 1989) and functions in vivo as a hepatotrophic factor during the regenerative events in the liver injured by a partial hepatectomy or by hepatotoxin treatment (Ishiki et al., 1992). In addition, mice lacking HGF exhibit severe impairment in liver development and die in utero (Schmidt et al., 1995). Although its role in maintaining liver homeostasis is well accepted, other studies have shown that HGF is a multifunctional cytokine possessing a wide spectrum of biological activities besides the hepato-specific ones by which it was first identified (for review see Goldberg and Rosen, 1993). HGF has been reported to stimulate proliferation of endothelial cells and various epithelial cells, including melanocytes and keratinocytes (Igawa et al., 1991; Kan et al., 1991; Matsumoto et al., 1991; Rubin et al., 1991; Bussolino et al., 1992). The mitogenicity exerted on renal tubular and on pulmonar cells reflects the active role of HGF in promoting regeneration in kidney (Nagaike et al., 1991; Kawaida et al., 1994) and lung (Yanagita et al., 1993) upon tissue damage.
Moreover, HGF has been shown to be identical to scatter factor (SF), a fibroblast-derived soluble polypeptide that disperses cohesive epithelial colonies, increasing cell motility and invasiveness (Stoker et al., 1987; Gherardi et al., 1989; Weidner et al., 1990, 1991; Naldini et al., 1991b ). HGF/SF has been also qualified as a morphogen for its ability to induce the organization of cells into ordered tubule-like structures (Montesano et al., 1991; Tsarfaty et al., 1992); on endothelial cells, the morphogenic stimulus assumes the character of angiogenesis with formation of blood vessels in vivo (Bussolino et al., 1992; Grant et al., 1993).
In virtue of its mitogenic, motogenic, and morphogenic properties, it is believed that HGF/SF could be involved in many processes where both cell growth and migration are required, such as embryonal development, tissue repair, and organ regeneration. This notion is further strengthened by the observation that HGF/SF mRNA is detected in several organs (Tashiro et al., 1990; Zarnegar et al., 1990; Sonnenberg et al., 1993), and that HGF/SF protein is still broadly localized by immunohistochemical means (Wolf et al., 1991; DeFrances et al., 1992). Finally, there is evidence of the involvement of HGF/SF in tumorigenesis and in tumor progression toward a more aggressive phenotype (Weidner et al., 1990; Rong et al., 1992, 1994; Bellusci et al., 1994).
HGF/SF shares structural homologies with plasminogen (38% amino acid sequence identity) and other blood coagulation serine proteases, although it has no protease activity (Miyazawa et al., 1989; Nakamura et al., 1989). Similarly to the biosynthetic pathway of the serine proteases, HGF/ SF is secreted by cells of mesodermal origin as a singlechain precursor that is then processed to yield a disulfidelinked complex composed of a heavy and a light chain (Miyazawa et al., 1989; Nakamura et al., 1989; Weidner et al., 1990). The proteolytic maturation is serum dependent and is necessary for the acquisition of biological activity (Hartmann et al., 1992; Lokker et al., 1992; Naka et al., 1992; Naldini et al., 1992, 1995).
The pleiotropic action of HGF/SF is transduced by a single transmembrane receptor encoded by the met protooncogene (Bottaro et al., 1991; Naldini et al., 1991a ,b; Weidner et al., 1993). The met protein has a heterodimeric structure consisting of an extracellular α subunit (50 kD) and a membrane-spanning β subunit (145 kD) endowed with a tyrosine kinase domain in its cytoplasmic region. Both subunits originate from glycosylation and from processing of a common precursor of 170 kD (Giordano et al., 1989a ,b).
While its ligand is produced prevalently by stromal cells, met receptor exhibits a complementary pattern of distribution, with the highest expression levels observed primarily in epithelial cells (Chan et al., 1988; Iyer et al., 1990; Di Renzo et al., 1991; Prat et al., 1991). Since HGF/SFproducing and -responding (met-positive) cells belong to adjacent tissue compartments, HGF/SF is actually considered a major paracrine mediator of mesenchyme–epithelium interactions (Sonnenberg et al., 1993; Comoglio and Boccaccio, 1996).
HGF/SF binding triggers met kinase activation and its autophosphorylation on specific tyrosine residues that will represent docking sites for multiple transductional proteins (Ponzetto et al., 1994). It is thought that the particular cellular response (cell growth vs cell locomotion or morphogenesis) may be ensured by the integration of distinct signaling pathways into different cell types. Recent studies have shown an active role of HGF/SF in the control of muscle development (Bladt et al., 1995; Maina et al., 1996; Takayama et al., 1996; Yang et al., 1996).
In this study we have examined the expression of HGF/ SF and its receptor in in vitro mouse muscle cells so as to determine whether HGF/SF might be implicated in myogenesis. In C2 myoblasts, we have detected the presence of transcripts specific for both the receptor and its ligand. The synthesis of the corresponding protein products has also been demonstrated. The hypothesis that HGF/SF could be an autocrine factor for C2 myoblasts has been confirmed by the observation that its cognate receptor is highly tyrosine phosphorylated and that this phosphorylation is inhibited by an anti-HGF/SF neutralizing antibody. Moreover, the finding that other myogenic cell lines and primary satellite cell cultures coexpress both HGF/SF and met receptor supports a broader physiological relevance of this growth factor in muscle development.
The expression of both murine met and HGF/SF genes was found to be downregulated in concomitance with C2 myogenic differentiation. In fact, we observed a coordinate transcriptional repression during the transition from proliferating myoblasts to differentiated myotubes. Such interesting behavior of endogenous genes prompted us to investigate the consequences of a constitutive met activation.
Here we show that the expression of p65tpr-met kinase (Cooper et al., 1984; Park et al., 1986; Gonzatti-Haces et al., 1988; Rodriguez and Park, 1993) results in substantial alterations of cell growth and inhibition of myogenesis, as does the constitutive activation of the human isoform of met receptor (h-met) through a species-specific autocrine stimulation by human HGF/SF (h-HGF/SF).
Materials and Methods
Cell Cultures
Cells of the mouse myogenic C2 cell line (Yaffe and Saxel, 1977) clone 7 and of the mouse teratocarcinoma-derived myogenic PCD2 cell line (Boon et al., 1974) were maintained as undifferentiated myoblasts in DME supplemented with 20% FCS in a 10% CO2 atmosphere. To induce cell differentiation, myoblasts were grown to confluence, and then shifted to DME supplemented with 0.5–2% FCS or 10% horse serum (HS). Extensive morphological and biochemical differentiation was obtained after 24–48 h. Mouse primary satellite cells (kindly provided by Dr. Giulio Cossu, Institute of Histology, University of Rome “La Sapienza,” Roma, Italy) were derived from limbs of 14-d-old mice, purified according to Salvatori et al. (1993) and amplified once.
C3H-10T1/2 mouse embryonic fibroblasts and HepG2 human hepatocarcinoma cells (used as positive control for the expression of met receptor in Northern and Western blot experiments) were cultured in 10% FCS-containing DME. C3H-10T1/2-MyoD is a C3H-10T1/2 subclone stably expressing the myogenic determinant MyoD (Davis et al., 1987). The epithelial cell lines (MDCK and mouse liver progenitor) used in the scatter assay are described below.
Agar selection was carried out by seeding 103 or 104 cells per 60-mmdiam dish in DME containing 5% FCS and 0.35% Bacto-Agar (Difco Laboratories, Detroit, MI) and layering this suspension in quadruplicate onto a base of 0.7% agar.
Indirect Immunofluorescence Staining
Cells grown on glass coverslips were fixed by immersion in methanolacetone (3:7 vol/vol) for 15 min at −20°C and then air dried. Coverslips were then incubated at 37°C in a humidified atmosphere with the primary antibody, in some cases diluted in PBS containing 1% BSA. After three washes with PBS, the coverslips were incubated with the secondary fluorochrome-conjugated antibody diluted in PBS plus 1% BSA, washed repeatedly with PBS, and mounted with 70% glycerol in PBS.
To detect the embryonic myosin heavy chain (MHCe), we used the mouse mAb MF20 (Bader et al., 1982) as undiluted hybridoma supernatant. As secondary antibody, we used a goat anti–mouse IgG rhodamineconjugated IgG fraction (Cappel Immunochemical Products, Malvern, PA) diluted 1:100.
Plasmids and Probes
The expression vectors used in the transfection assay were: (a) pRK5-met, in which the entire coding sequence of human met receptor is cloned under the control of the cytomegalovirus promoter; (b) pPEB-HGF/SF, containing the full-length human HGF/SF cDNA cloned downstream of the murine sarcoma virus LTR; and (c) pMT2-tpr-met, in which the human tpr-met cDNA is cloned downstream of the adenovirus late promoter (Ponzetto et al., 1994). As selectable markers for stably transfected cells, we used plasmids: (a) pRSV-neo, containing the neomycin resistance gene under the control of the Rous Sarcoma virus long terminal repeat (LTR) (Mulligan and Berg, 1980); (b) pSV2-hygro, containing the hygromycin resistance gene under the control of the SV-40 promoter-enhancer region (Maione et al., 1992); and (c) pBABE-puro, containing the puromycin resistance gene under the control of Moloney murine leukemia virus LTR (Morgenstern and Land, 1990).
For Northern blot hybridization experiments, probes were labeled with α[32P]dATP by the random priming method. To detect specific transcripts, the following probes were used: (a) the human full-length met cDNA (Ponzetto et al., 1991); (b) the human full-length HGF/SF cDNA (Naldini et al., 1991); (c) the plasmid pMHC 2.2, specific for MHCe transcript (Weydert et al., 1985); (d) the EcoRI fragment from plasmid pEMC11s specific for MyoD transcript (Davis et al., 1987); and (e) the human cDNA myf4 (Braun et al., 1989) highly homologous to the mouse myogenin transcript, provided by Dr. Hans Henning Arnold (University of Hamburg Medical School, Hamburg, Germany).
Cell Transfections
Cells were transfected by the calcium phosphate precipitation method (Wigler et al., 1977), with ∼105 cells per 60-mm-diam dish. Single stable transfectants were obtained by cotransfecting each dish with the following amounts of DNA: 5 μg of the expression vector and 0.5 μg of the selection plasmid. For h-met/h-HGF/SF double transfections, 2.5 μg of pRK5-met plasmid, 2.5 μg of pPEB-HGF/SF plasmid, and 0.5 μg of pRSV-neo plasmid were used.
2 d after transfection, cells were split 1:7 in selective medium. As required, 400 μg/ml G418, 200 μg/ml hygromycin, or 1 μg/ml puromycin was used. The medium was changed every 2–3 d and, after 10–15 d, the surviving colonies were isolated and separately amplified.
RNA Extraction and Northern Analysis
Total RNA was prepared according to the acid guanidinium thiocyanatephenol-chloroform single step method as described by Chomczynski and Sacchi (1987).
For Northern blot analysis, 10–15 μg of each RNA sample was fractionated by electrophoresis through 0.8% agarose gel containing 0.66 M formaldehyde and 1× MOPS buffer (20 mM MOPS, 5 mM NaAc, 1 mM EDTA, pH 7.0). After running, gel was washed twice in 10× SSC (1.5 M NaCl, 0.15 M sodium citrate, pH 7.2) for 30 min at room temperature to remove formaldehyde, and RNAs were transferred to nylon filters (Hybond N; Amersham Intl., Little Chalfont, UK) by a capillarity fluid of the same buffer (Sambrook et al., 1989). Filters were fixed by Stratalinker (1,200 μJ × 100). Prehybridization and hybridization were carried out at 42°C using the commercial Hybrisol solution (Oncor, Gaithersburg, MD). α-[32P]dATP-labeled probes were used at a 2 × 106 counts per ml concentration. Hybridized filters were washed once at 42°C in a 2× SSPE (a 20× SSPE solution was 3 M NaCl, 200 mM NaH2PO4·H2O, 20 mM EDTA, pH 7.4), 0.1% SDS solution, and twice at 60°C in a 1× SSPE, 0.1% SDS solution. Finally, filters were exposed to an autoradiographic film (Fuji Photo Film Co., Tokyo, Japan) at −70°C with intensifying screens for variable periods.
Comparative quantitation of RNAs was estimated by staining the gel with ethidium bromide and rehybridizing the filters with a probe for a constitutively expressed gene (GAPDH) (glyceraldehyde-3-phosphatedehydrogenase) (Piechaczyk et al., 1984).
Immunoprecipitation and Western Blotting
Subconfluent cell cultures were washed three times in cold PBS and lysed for 30 min on ice in a buffer containing 50 mM Tris-Cl pH 7.5, 150 mM NaCl, 10% glycerol, 5 mM EGTA, pH 7.5, 50 mM NaF, pH 8, 1.5 mM MgCl2, 1% (vol/vol) Triton X-100, supplemented with various protease and phosphatase inhibitors (leupeptin, aprotinin, PMSF, sodium-orthovanadate). After clearing by centrifugation at 15,000 rpm for 20 min at 4°C, the protein content of samples was quantified by the Biorad method.
Equal amounts of cellular extracts were pretreated with 50 μl protein A–Sepharose (Pharmacia, Uppsala, Sweden) (50% vol/vol in PBS + 3% BSA) to remove nonspecific binding, and then incubated for 1 h at 4°C with anti-met antibodies. The following antibodies were used: (a) rabbit anti–h-met antiserum (C-12 antibody) and (b) rabbit anti–m-met antiserum (SP260 antibody), both purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
The immunocomplexes were collected by adding 50 μl protein A–Sepharose (incubation for 1 h at 4°C) and washed four times with NET-gel buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% NP-40, 1 mM EDTA, pH 8.0, 0.25% gelatin, 0.02% sodium azide). Finally, the immunocomplexes were solubilized in boiling Laemmli buffer containing 5% β-mercaptoethanol, electrophoresed on 8–12% SDS polyacrylamide gels, and transferred onto nitrocellulose filters (Hybond C; Amersham Intl.) by the semidry blot method.
Western blot analysis was carried out by using the anti-met polyclonal antibodies described above or an anti-phosphotyrosine mAb (Upstate Biotechnology Inc., Lake Placid, NY). The staining of the blots was performed by the enhanced chemiluminescence system (ECL; Amersham Intl.).
The autocrine mode of action of HGF/SF in C2 cells was investigated by measuring the degree of tyrosine phosphorylation of met receptor in different culture conditions. Briefly, C2 cells were treated for 5 min on ice with an acid solution (20 mM acetic acid, 150 mM NaCl, 0.25% BSA), and subsequently incubated with 0.5 M NaCl-containing DME for 20 min at 37°C to dissociate HGF/SF molecules from cell surface (Sturani et al., 1988; Naldini et al., 1992). Then cells were lysed immediately or after an additional incubation (30 min at 37°C) with fresh 10% FCS-containing medium, C2-conditioned medium, or C2-conditioned medium previously incubated with a neutralizing goat antibody directed against mouse HGF/SF (kindly supplied by Dr. Ermanno Gherardi, Imperial Cancer Research Fund, Cambridge, UK).
Scatter Assay
The production of HGF/SF by C2 cells and transfected clones was investigated by measuring the ability of the corresponding conditioned medium to dissociate epithelial MDCK cell colonies (Stoker et al., 1987). The assay was performed in 24-well Costar plates (Cambridge, MA). 4,000–6,000 cells per well were plated and exposed to serial dilutions of conditioned medium in 10% FCS-containing DME for 15–20 h at 37°C. After being fixed with 11% glutaraldehyde and colored with crystal violet, cell scattering was examined by a phase-contrast light microscope. Scatter activity was generally detected in the range of 1:30–1:300 dilutions.
To verify the extension of HGF/SF processing, the scatter assay was performed using mouse liver progenitor cells (Medico et al., 1996), which can be cultivated in low serum; in this case, the testing conditioned medium was diluted in 1% FCS-containing DME.
Results
Expression of Endogenous met and HGF/SF in Myogenic Cells
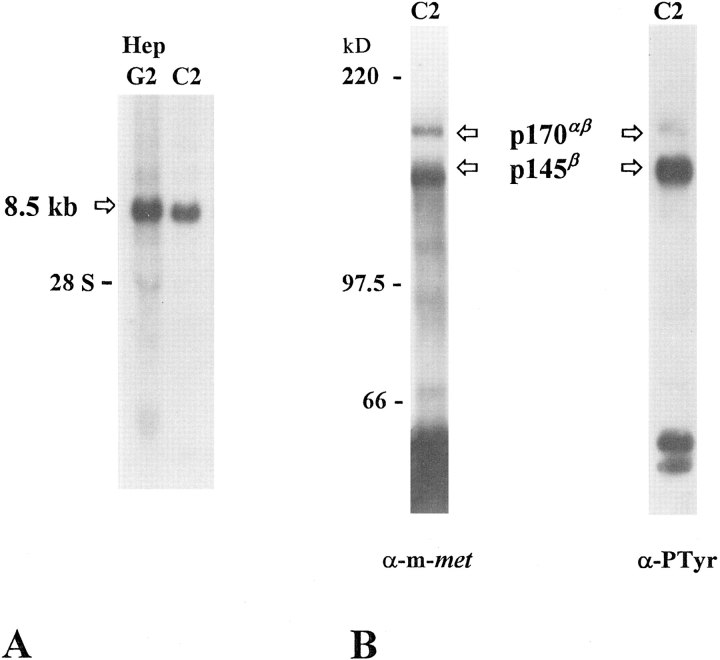
To determine whether C2 myoblasts could be a potential target for HGF/SF action, we analyzed the expression of HGF/SF receptor (met) in proliferating cells by Northern blotting of total RNA. Using the full-length human met cDNA as a probe, we have detected a transcript of ∼8.5 kb, which represents the major met mRNA expressed in murine tissues and in human hepatocarcinoma (Hep G2) cells (Fig. 1 A).
Figure 1.
Synthesis of met receptor by C2 myoblast cells. (A) Northern blot of total RNA extracted from human hepatocarcinoma HepG2 cells and C2 cells probed with human full-length met cDNA (Ponzetto et al., 1991). The position of 8.5-kb metrelated transcript and the 28S ribosomal RNA is indicated. (B) Activation state of met receptor expressed by C2 myoblasts. Protein extracts from proliferating C2 myoblasts were immunoprecipitated with anti–met rabbit SP260 antiserum (directed against the COOH-terminal portion of the murine protein), electrophoresed onto 8% reducing SDS-PAGE, and immunoblotted with SP260 antiserum (α-m-met). In these conditions, both the 145-kD β subunit of mature met receptor (p145β) and the 170-kD single-chain precursor (p170αβ) were detected. The same filter was stripped and reprobed with monoclonal anti-phosphotyrosine antibodies (α-PTyr) to provide evidence for the activation degree of p145β.
Met protein was visualized from C2 cell lysates by immunoprecipitation and Western blotting with an anti– murine met polyclonal antibody (Fig. 1 B). In this experiment both the 170-kD single chain precursor (p170αβ) and the 145-kD β subunit of met receptor (p145β) were clearly detected. Furthermore, to establish if met receptor was activated in C2 cells, a Western blot with an anti-phosphotyrosine mAb was performed on the immunoprecipitated protein. As shown in Fig. 1 B, p145β exhibits a high degree of phosphorylation on tyrosine residues, indicating that met receptor is activated in C2 myoblasts.
To determine if met activation was due to an autocrine stimulation, the production of HGF/SF by C2 cells was investigated, since muscle cells belong to the mesenchymal compartment that is known to be a preferential expression site of met ligand (Sonnenberg et al., 1993). Northern blot analysis has brought evidence of the presence of a 6-kb transcript (Fig. 2 A) equivalent in size to the principal HGF/SF mRNA species described by other authors (Nakamura et al., 1989; Tashiro et al., 1990). The presence of HGF/SF protein in the supernatant of C2 cells was tested by assaying its scatter activity on MDCK epithelial cells (Fig. 2 B), in comparison with the standard medium (Fig. 2 C). Titration by serial dilutions indicated the production of amounts comparable to that of HGF/SF-producing fibroblast cells (Stoker et al., 1987). The existence of a natural autocrine loop for HGF/SF in C2 myoblasts was confirmed by exposing C2 cell cultures to an acid treatment followed by an incubation with DME supplemented with 0.5 M NaCl. These conditions have been shown to effectively dissociate the ligand–receptor complexes and remove the HGF/SF molecules linked to extracellular matrix (Sturani et al., 1988; Naldini et al., 1992). Duplicate plates of C2 cells were treated in the same way and then further incubated for 30 min with C2-conditioned or fresh 10% FCS-containing medium, respectively. Results shown in Fig. 3 demonstrate that the treatment causes a net decrease of met tyrosine kinase phosphorylation and that only C2-conditioned medium restores phosphorylation. Moreover, C2-conditioned medium loses its capacity to stimulate met kinase if previously incubated with a neutralizing anti-HGF/SF antibody. These results demonstrate that met receptor expressed by C2 myoblasts is activated by the endogenously produced HGF/SF.
Figure 2.
Synthesis of HGF/ SF by C2 myoblast cells. (A) Northern blot analysis of total RNA extracted from human hepatocarcinoma cells (HepG2) and C2 cells to illustrate the production of a 6-kb HGF/SF mRNA. As previously reported in literature (Shiota et al., 1992), HepG2 cells are negative for the expression of HGF/SF gene. (B and C) Phase-contrast micrographs of a scatter assay carried out with MDCK epithelial cells incubated with C2 myoblast–conditioned medium (B) or standard medium (C).
Figure 3.
HGF/SF is an autocrine factor for C2 myoblasts. C2 proliferating cells exposed to culture conditions that remove HGF/SF molecules from cell surface (see Materials and Methods) were lysed, immunoprecipitated with anti–murine met antibodies, and analyzed for the tyrosine phosphorylation degree of HGF/SF receptor by Western blotting with anti-phosphotyrosine antibodies (α-PTyr). Control cells (lane 1); C2 cells acid treated and incubated with 0.5 M NaCl-containing DME (lane 2); cells treated as in lane 2 and further incubated with C2-conditioned medium (lane 3) or C2-conditioned medium pretreated with an antiHGF/SF neutralizing antibody (lane 4); cells as in lane 2 and further incubated with fresh 10% FCS-containing medium (lane 5).
The existence of a natural HGF/SF autocrine loop in C2 cells prompted us to assess if this autocrinia could reflect a common situation in cells belonging to the myogenic lineage and not represent a peculiarity of C2 myoblasts only. Therefore, we analyzed the expression of met and HGF/SF in mouse primary satellite cells and in the PCD2 teratocarcinoma-derived mouse cell line, which undergoes myogenic differentiation (Boon et al., 1974). By Northern blot analysis, we have detected specific signals for both the receptor and its ligand (Fig. 4). Here it should be noted that primary cultures were double precleared from fibroblasts to reduce as much as possible any contribution of these cells to the HGF/SF mRNA signal detected, since HGF/SF is a main fibroblast-secreted cytokine (Stoker et al., 1987; Gherardi et al., 1989; Weidner et al., 1990). The persistence of high expression levels of the muscle-specific determinant MyoD confirmed the identity of these cells. Note that PCD2 cells undergo differentiation in the absence of MyoD expression, whose function could be replaced by other members of the family of myogenic determinants (Weintraub, 1993).
Figure 4.
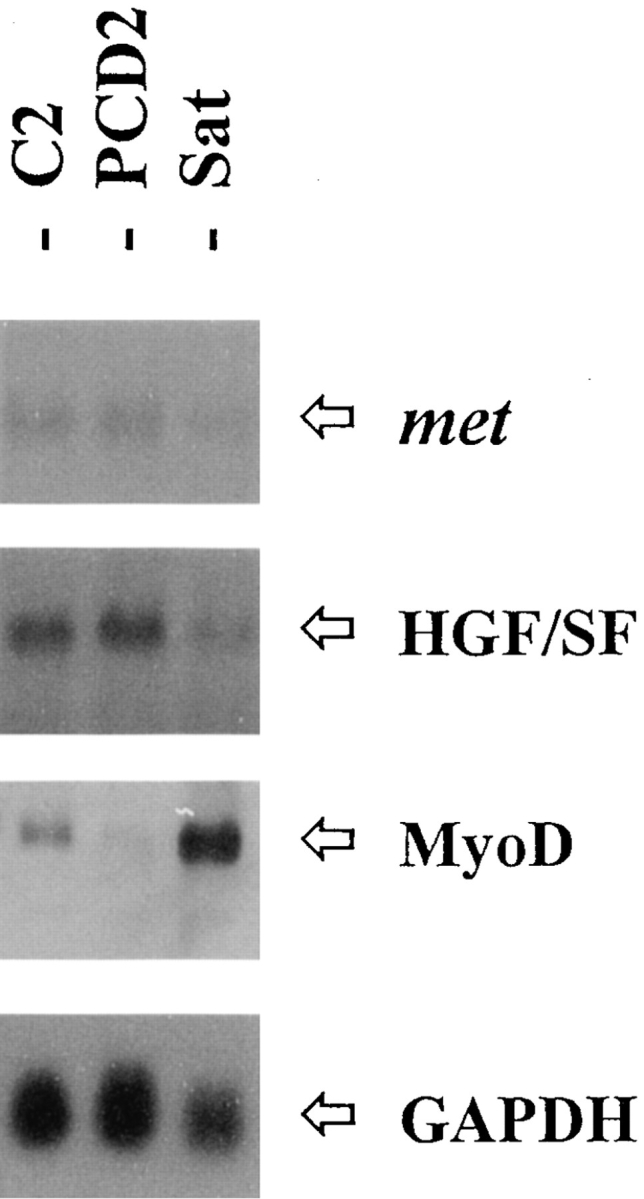
Coexpression of met and HGF/SF in other myogenic cells. Total RNA from C2 myoblasts, PCD2 mouse myoblasts, and mouse primary satellite (Sat) cell cultures was hybridized with the indicated probes.
Met and HGF/SF Genes Are Coordinately Downregulated during Myogenic Differentiation
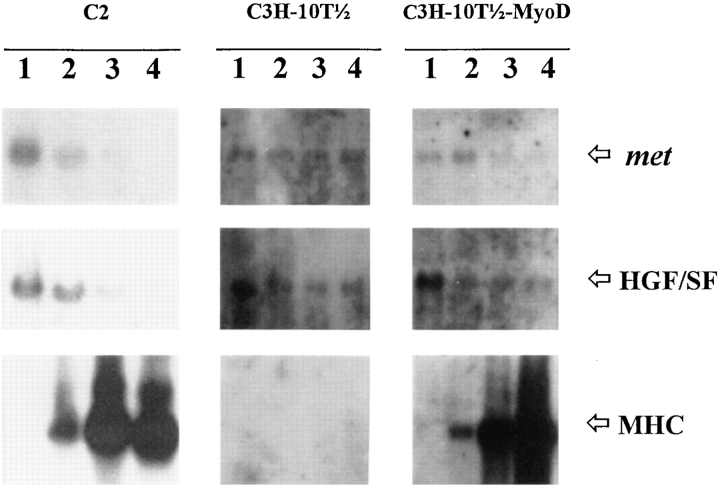
Previous works have shown that the expression of different genes encoding for growth factors and their cognate receptors is modulated during myogenesis (Ewton et al., 1988; Olwin and Hauschka, 1988; Tollefsen et al., 1989a ,b; Hu and Olson, 1990; Lafyatis et al., 1991; Moore et al., 1991). To learn whether the expression of met and HGF/SF genes was subjected to quantitative variations during muscle differentiation, we have performed a Northern blot analysis of total RNA prepared from undifferentiated and differentiating C2 cells. Results reported in Fig. 5 show that the highest level of met and HGF/SF mRNA was found in actively proliferating myoblasts, cultured in 20% FCS-containing medium. When the myoblasts were induced to differentiate by a shift to low mitogen–containing medium (2% FCS or 10% HS), an evident decrease for both transcripts was observed. The transcriptional repression is strictly coupled with the myogenic process since the hybridization signals are almost undetectable at day 3 postserum deprivation, when a consistent number of mature multinucleated myotubes are present. Muscle differentiation was also assessed by measuring the expression of myosin heavy chain (MHC) mRNA, whose gene is transcriptionally activated early in differentiation.
Figure 5.
The expression of met and HGF/SF genes is downregulated concomitantly with the onset of myogenic differentiation. C2, C3H-10T1/2, and C3H-10T1/2-MyoD cells were cultured in DME containing 20% FCS up to confluency, and then transferred to low serum–containing medium (2% FCS). Total RNA extracted before serum deprivation (lanes 1) and 1 (lanes 2), 2 (lanes 3), and 3 (lanes 4) d after serum deprivation was assayed for the expression of met, HGF/SF, and a muscle-specific marker, MHC, transcripts. Quantitative decrease of met and HGF/SF mRNA synthesis was observed only in the presence of muscle differentiation.
The observation that mRNA decline is differentiation dependent enables us to infer that the mechanisms responsible for cell growth–arrest and muscle-specific gene activation can also be involved in the control of both met and HGF/SF gene expression. To test this hypothesis, we have analyzed the expression of met and HGF/SF genes in C3H10T1/2 mouse fibroblasts, which can be converted to myogenic lineage by stable ectopic expression of MyoD (Davis et al., 1987). As reported in Fig. 5, C3H-10T1/2 cells synthesize both met and HGF/SF mRNAs that are downregulated in low serum only upon myogenic conversion.
Effect of tpr-met Expression in C2 cells
The observation that endogenous met and HGF/SF genes are coordinately downregulated during myogenic differentiation of C2 cells raises the question if HGF/SF-mediated signals could exert a negative constraint on muscle differentiation. To test this hypothesis, we have studied the consequences of the expression of p65tpr-met, a constitutively activated version of the met kinase (Cooper et al., 1984; Park et al., 1986; Gonzatti-Haces et al., 1988; Rodriguez and Park, 1993).
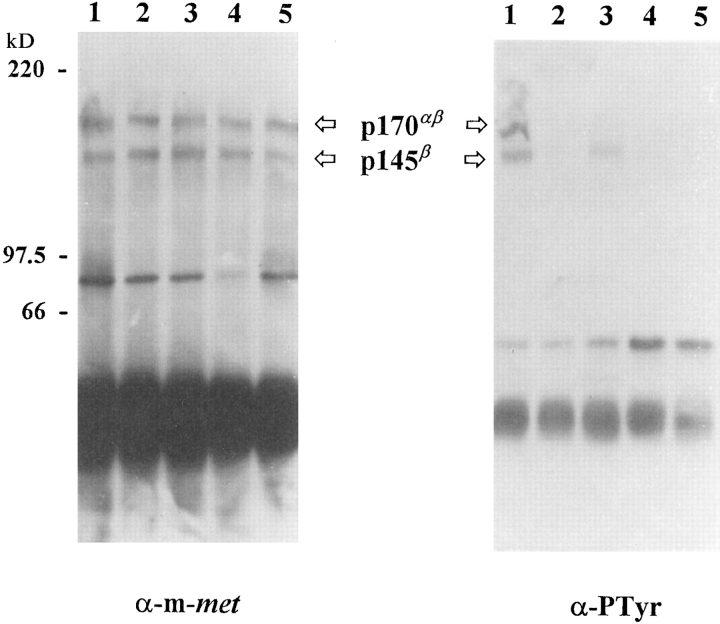
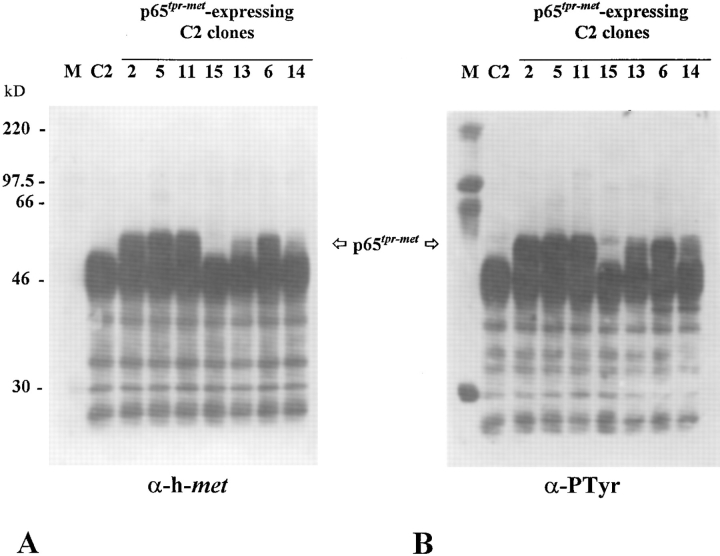
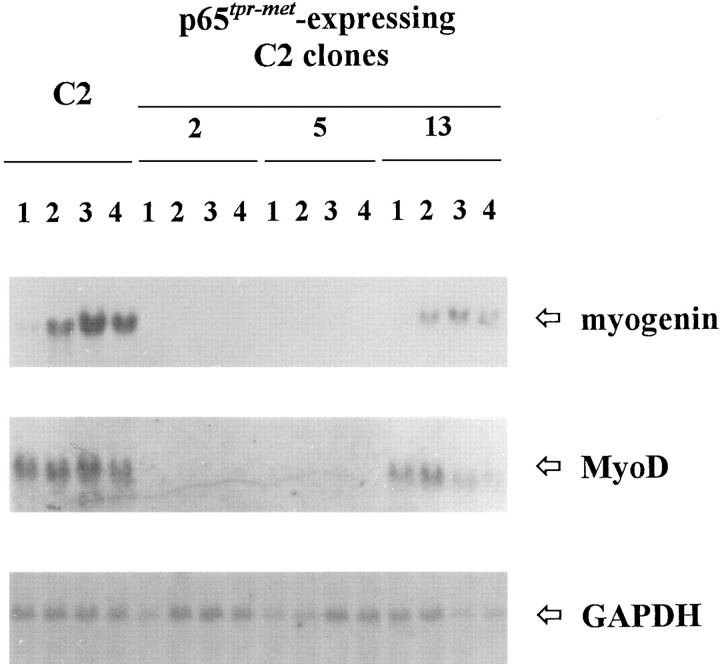
C2 cells were transfected with pMT2–tpr-met construct in which the human tpr-met cDNA is cloned downstream of the adenovirus late promoter. The construct pBABEPuro encoding for the puromycin resistance was used as a selection marker. 20 puromycin-resistant clones were isolated, individually amplified, and tested for the presence of p65tpr-met protein by Western blot analysis. All together, eight clones resulted to express variable levels of the heterologous protein and three of them were found to express high levels of p65tpr-met (clones 2, 5, and 11; Fig. 6 A). In all the positive clones, p65tpr-met was strongly evidenced by an anti-phosphotyrosine antibody in a Western blot performed on the immunoprecipitated protein (Fig. 6 B); this argues for a full activation of p65tpr-met kinase in transfected myoblast cells. Moreover, the degree of tyrosine phosphorylation parallels the protein content. As expected, kinase activity of p65tpr-met is retained also in cells grown at a low serum condition (data not shown).
Figure 6.
Extensive p65tpr-met kinase activation in p65tpr-met- expressing C2 clones. Total cell lysates were immunoprecipitated with rabbit anti–met C12 antiserum (directed against the COOHterminal portion of the human protein), electrophoresed onto 12% reducing SDS-PAGE, and immunoblotted with the same antibody (A) or anti-phosphotyrosine antibodies (B). A good correlation was found between the p65tpr-met protein levels and its degree of tyrosine phosphorylation. Clone 15 was negative for the expression of p65tpr-met. Size in kD was estimated using a molecular mass standard (M).
The p65tpr-met highly expressing clones exhibit a singular cell morphology that is shown in Fig. 7. In detail, cells are very spindle-shaped and little adherent to the substrate, implying a profound reorganization of the cytoskeletal apparatus. This scattered appearance is identical to the motogenic response observed when epithelial cells are stimulated by HGF/SF (see Fig. 2 B). The increase of cell motility in p65tpr-met highly expressing clones was confirmed by the Boyden chamber assay (data not shown). The morphology of the clones that express low quantities of p65tpr-met is comparable to that of C2 parental myoblasts (not shown).
Figure 7.

Phenotypic appearance and differentiating properties of p65tpr-met-expressing C2 clones. Micrographs of p65tpr-met highly expressing cells (B) and C2 parental cells (A) cultured either in growth or differentiation medium. The myogenic potential was assessed by detecting myotube formation and by immunofluorescence staining for MHC in 48-h differentiation medium–exposed cell cultures.
It has been shown that p65tpr-met promotes cell proliferation and tumorigenesis (Cooper et al., 1984; Park et al., 1986); nevertheless, the growth rate of p65tpr-met-expressing clones, independent from the protein expression levels, is not significantly modified in comparison with the C2 parental cell line (data not shown).
The motogenic properties of p65tpr-met highly expressing clones are accompanied by a marked inability to differentiate when confluent cultures are deprived of mitogens. Immunofluorescence staining of MHC protein synthesized after 48 h of incubation in differentiating medium shows few MHC-positive cells, which appear as single isolated and fusiform cells and very sporadically as multinucleated cells (Fig. 7).
To characterize at a biochemical level the decrease of myogenic potential of p65tpr-met expressing clones, we investigated the expression of the muscle-specific regulatory genes MyoD and myogenin (Weintraub, 1993). In C2 parental cells, the gene encoding for myogenin is transcriptionally activated in concomitance with the beginning of muscle differentiation; on the contrary, MyoD gene is expressed also at the myoblast stage although the protein product becomes functional only in differentiating conditions. By Northern blot analysis, we have found a complete absence of MyoD and myogenin transcripts in p65tpr-met highly expressing cells (Fig. 8).
Figure 8.
Decreased expression of muscle-regulatory genes in p65tpr-met-expressing C2 clones. Total RNA from proliferating (lanes 1) and differentiating (lanes 2, 3, and 4; 24-, 48-, and 72-h postserum deprivation, respectively) cultures was hybridized with the indicated probes. To estimate if equal amounts of RNA were loaded, the same filter was probed with a constitutively expressed gene (GAPDH).
In the p65tpr-met moderate expressing clones, the transcripts for MyoD and myogenin are detectable, but the relative hybridization signals have an intensity lower than those of C2 parental cells, suggesting that the degree of myogenic inhibition correlates with the levels of p65tpr-met expressed (Fig. 8). Control C2 cells stably transfected with the empty pMT2 construct did not show any growth and differentiating alterations (data not shown).
Effect of Heterologous h-met and h-HGF/SF Double Expression in C2 Cells
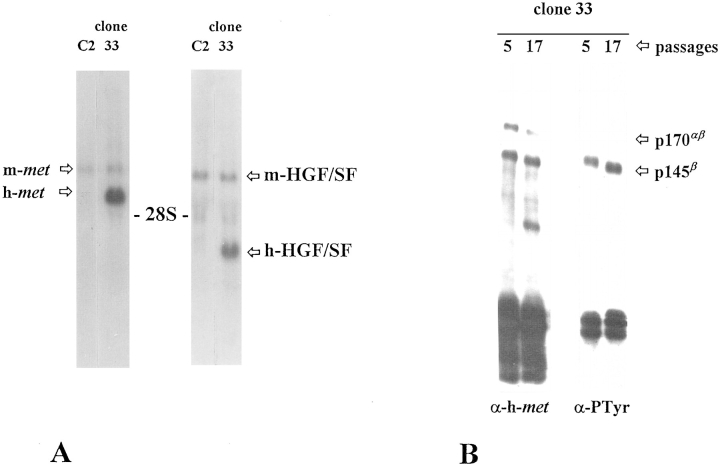
To determine whether the expression of an autocrine loop for HGF/SF released from differentiation control could result in a phenotype similar to that of p65tpr-met-expressing C2 cells, we analyzed the properties of C2 cells double transfected with the heterologous h-met and h-HGF/SF coding sequences. The choice to use the human isoforms allowed us to distinguish them from the endogenous ones both at mRNA and protein levels. Stable transfection of C2 cells was performed using pRK5-met, pPEB-HGF/SF, and pRSV-neo constructs at a 5:5:1 ratio. From 40 clones selected for G418 resistance, only two of them (clones 9 and 33) resulted positive for the synthesis of both h-met and h-HGF/SF mRNAs. The remaining 38 G418-resistant clones expressed h-met (eight clones), h-HGF/SF (nine clones), or neither of them (data not shown). Subsequent studies have revealed that, after three to five passages, clone 9 did not express the h-met mRNA any more; the loss of h-met transcript is probably the result of an unstable genomic integration of human DNA sequence. For this reason, only clone 33 should be considered as a real double transfectant (Fig. 9 A). As determined by Western blot analysis, clone 33 expresses a highly tyrosine-phosphorylated form of h-met (Fig. 9 B). This is consistent with the productive binding and activation by h-HGF/SF.
Figure 9.
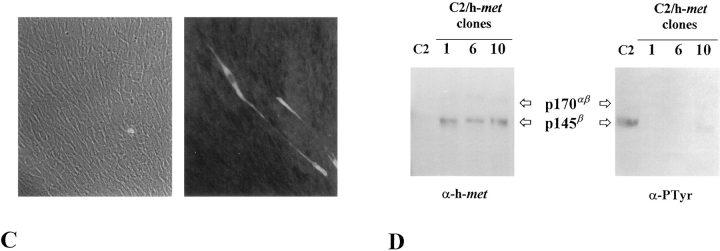
Double ectopic expression of h-met and h-HGF/ SF is required for the constitutive activation of h-met kinase. (A) Northern blot analysis of RNA expression levels of h-met and h-HGF/SF in clone 33 selected from C2 h-met/h-HGF/SF double transfection. Exogenous transcripts are larger than endogenous ones. (B) The h-met receptor expressed by clone 33 is phosphorylated on tyrosine residues. Cells were lysed at two different culture passages and immunoprecipitated with anti–human met antibodies. Proteins were resolved by 8% reducing SDSPAGE and immunoblotted with anti–human met (α-hmet) or anti-phosphotyrosine (α-PTyr) antibodies. (C) Phase-contrast and anti-MHC immunofluorescence micrographs of clone 33 cells exposed for 48 h in differentiation medium containing 10% HS. (D) Cell lysates from C2 parental cells and three single h-met–expressing C2 (C2/ h-met) clones were immunoprecipitated with anti–murine met antiserum (C2 cells) or with anti-human met antiserum (C2/h-met clones), respectively. The immunocomplexes were resolved by 8% reducing SDS-PAGE and immunoblotted with anti–human (α-h-met) or anti-phosphotyrosine mAbs (α-PTyr). The almost undetectable tyrosine phosphorylation exhibited by h-met in comparison with the endogenous receptor suggests that it is not efficiently activated by murine HGF/SF.
Cells from clone 33 show several alterations in the growth properties, particularly in the ability to grow in soft agar, whereas C2 clones expressing only h-met or h-HGF/ SF, as well as C2 parental cells, are not able to grow independently from anchorage (data not shown).
The clone 33 was also tested for its differentiating potential. Confluent cells were transferred in DME containing 10% HS. After a 48-h incubation in this medium, cells were analyzed for the expression of MHC by indirect immunofluorescence staining. As shown in Fig. 9 C, clone 33 presents a significant inhibition of the differentiating process, since few cells express myosin and most of them are mononucleated and fusiform, similar to those observed in p65tpr-met-expressing clones. In differentiation studies performed by shifting to very low percentages of serum (0.5% or 1% FCS), we have observed a partial restoration of the differentiating capacity in clone 33 (data not shown). The reacquiring of the differentiated phenotype can be explained by the prevention of HGF/SF processing in these culture conditions, clearly indicating a lack of HGF/SF biological activity (Naka et al., 1992; Naldini et al., 1992, 1995).
In view of these data, although they refer to only one h-met/h-HGF/SF–expressing clone, we can infer that a constitutive autocrine loop for h-HGF/SF causes growth alterations and myogenic inhibition.
The single stable expression of h-met or h-HGF/SF in C2 cells was not found to induce appreciable phenotypic changes in proliferative conditions, indicating that a high species-specificity of ligand–receptor interaction is required to activate met signaling. Indeed, C2 clones selected for expression of the human isoform of the receptor showed consistent protein levels of h-met, which, in a different way from the endogenous m-met, is weakly or not at all phosphorylated on tyrosine residues (Fig. 9 D). This is in accordance with previous data obtained using mouse fibroblast cells (Rong et al., 1992). Likewise, no substantial alterations of muscle differentiation were observed in C2/h-met and C2/h-HGF/SF clones in comparison with C2 parental cells (data not shown).
Although we have had no particular problems in selecting C2 clones expressing p65tpr-met kinase, we presume that the simultaneous expression of h-met and h-HGF/SF results in severe cell growth disturbances and is therefore more restrictive for C2 myoblast survival. Two lines of evidence support this hypothesis, with the first being the low ratio of positive double transfectants as compared with that of C2/h-met and C2/h-HGF/SF single transfectants. Here it should be noted that the h-met/h-HGF/SF transfection was repeated a second time yet without success (from 30 clones selected, none were double positive). Secondly, cells of clone 33 are subject to cell death when cultured in growth-restrictive conditions. Preliminary experiments indicate that the observed cell death is to be classified as apoptosis (data not shown).
Discussion
It is well known that muscle development is strongly influenced by serum components, first of all by peptide growth factors (for review see Florini and Magri, 1989; Florini et al., 1991; Olson, 1992; Maione and Amati, 1996). The functional relationships between growth factors and muscle differentiation have been extensively investigated by using in vitro cultured muscle cells and taking the advantage of the myogenic process study in monitored conditions. Myogenesis in tissue culture is accompanied by a terminal and irreversible withdrawal from cell cycle so that the postmitotic cells become committed to fusion and form multinucleated myotubes (Nadal-Ginard, 1978). The morphological events in muscle differentiation are associated with the expression of an array of muscle-specific gene products, especially structural proteins of the contractile apparatus.
In this context, the local concentration of growth factors (of own production or added exogenously to the culture medium) results in being critical for maintaining cells as proliferating myoblasts or inducing them to acquire the differentiated phenotype. Until now, much of the work done was centered on distinct growth factors such as FGF, TGF-β, and the somatomedins (insulin, insulin-like growth factor-1 [IGF-1], and insulin-like growth factor-2 [IGF-2]) (Florini and Magri, 1989; Florini et al., 1991; Olson, 1992; Maione and Amati, 1996). All of them have been recognized to exert a key function in the control of in vitro, and presumably also in vivo, muscle differentiation. Nevertheless, it cannot be excluded that additional factors exist that contribute to the regulatory pathways governing the myogenic process.
In the present work we have addressed the question of whether HGF/SF, a pleiotropic protein able to elicit multiple biological responses (for review see Goldberg and Rosen, 1993), could play a role in the differentiation of the myogenic mouse C2 cell line.
We have shown that C2 myoblasts express both HGF/ SF and its receptor, met tyrosine kinase. Despite the fact that HGF/SF has been considered essentially as a paracrine factor secreted by mesenchymal cells and effective on epithelial cells, some examples of natural autocrine cells for HGF/SF have been described (Adams et al., 1991; Rong et al., 1992, 1993; Tsao et al., 1993; Ferracini et al., 1995; Woolf et al., 1995; Maier et al., 1996). We show that an autocrine loop for HGF/SF is present and active in C2 cells since the high levels of tyrosine phosphorylation exhibited by met receptor are dependent from the endogenously produced ligand. Further experiments have produced evidence of the coexpression of HGF/SF and its related receptor also in another myogenic cell line and in mouse primary satellite cells.
Different studies indicate that HGF/SF can exert an important function in muscle development. It has been shown that met and HGF/SF transcripts are present in muscle formation sites during mouse embryogenesis (Sonnenberg et al., 1993). Bladt et al. (1995) reported that mice homozygous for a null mutation of the met locus fail to form muscles in the limb anlage, in the diaphragm, and at the tip of the tongue, because of the inability of myogenic precursor cells to migrate from the somites to these sites. A detailed work by Yang et al. (1996) confirms that, in Pax 3–deficient mice, the loss of met gene expression in somitic myogenic precursors correlates with the lack of limb bud colonization. Similar results were obtained by Maina et al. (1996) by using mice carrying met receptor variants that are defective in the transduction of HGF/SF signal. In addition, they reveal a novel role of met kinase also in promoting the proliferation of fetal myoblasts just before the formation of the secondary fibers during the late stages of muscle development. However, no clear hint about a putative autocrine condition for HGF/SF in myogenic cell lineage was given in these works. Rather, a general paracrine control by this growth factor was postulated. Our results appear in disagreement with this interpretation since we have observed an autocrine loop for HGF/SF both in C2 myoblasts and in mouse primary satellite cells. Since C2 myoblasts were also derived from satellite cells (Yaffe and Saxel, 1977), it can be hypothesized that the autocrine loop for HGF/SF could represent an intrinsic characteristic of satellite cells. According to this assumption, it is expected that the HGF/SF autocrine loop would become established only when satellite cells are induced to replicate and migrate to where damaged muscle fibers must be replaced. In this regard, a study by Jennische et al. (1993) points out an induction of HGF/SF gene expression in rat skeletal-regenerating muscle after ischemic injury. A second element of concordance is that HGF/SF stimulates the growth of satellite cells otherwise quiescent (Allen et al., 1995). It should not be completely excluded that the coexpression of met and HGF/SF genes could occur in the somitic precursors and in other cells belonging to the myogenic lineage without preventing them from responding also to an external HGF/SF gradient. The finding that an inappropriate expression of HGF/SF in transgenic mice causes ectopic muscle formation in the central nervous system (Takayama et al., 1996) strengthens the view that a spatially and temporally regulated HGF/SF signaling would be required for a proper myogenic process, during both embryogenesis and muscle regeneration. Work in this field will be extremely informative for better understanding the complexity of HGF/SF action in muscle development.
We have shown that the expression of HGF/SF and met genes is subordinated to the proliferative state of myoblast cells since a transcriptional decline for both of them was consistently observed after induction of differentiation. The modulation of genes encoding for growth factors and growth factor receptors seems to be quite a common strategy adopted by muscle cells when they enter the differentiating pathway (Florini and Magri, 1989; Florini et al., 1991; Olson, 1992). For example, the endogenous expression of FGF and TGF-β, as well as that of their cognate receptors, is downregulated during myogenesis (Ewton et al., 1988; Olwin and Hauschka, 1988; Hu and Olson, 1990: Lafyatis et al., 1991; Moore et al., 1991).
The disappearance of growth factor receptors with the onset of differentiation is important in ensuring an irreversible withdrawal from cell cycle and, consequently, a stable expression of muscle-specific phenotype. In fact, fusion-defective muscle cells differentiate with neither terminal commitment nor receptor decline in such a way that they can reverse muscle phenotype after growth factor exposure (Hu and Olson, 1990). However, existing data suggest that the loss of responsiveness to growth factors occurs also at a postreceptor level (Maione and Amati, 1996). An opposite kind of regulation has been evidenced for the myogenic stimulators IGF-1, IGF-2, and their related receptors, whose expression increases coordinately in differentiating cells (Tollefsen et al., 1989a ,b).
The observed HGF/SF and met gene downregulation suggests a functional shut-off in the autocrine stimulation by HGF/SF during C2 myogenic differentiation. Our data are in agreement with the previous observation that in rodents HGF/SF and met receptor are expressed in skeletal muscle tissue during embryonic development and in the first days after birth, while the levels of the relative transcripts are weak or not detectable in adult skeletal muscle (Jennische et al., 1993; Sonnenberg et al., 1993). Hence, HGF/SF could generate a signal interfering with the differentiating program, just like FGF and TGF-β. Research is in progress to determine the possible role of myogenic factors in the downregulation of both met and HGF/SF during C2 differentiation.
We have found a similar differentiation-dependent downregulation of HGF/SF-met system in other met-HGF/SF coexpressing cells, C3H-10T1/2 mouse fibroblasts converted to myoblasts by stably expressed MyoD. This finding further supports the incompatibility between met kinase signaling and the differentiating program.
The transcriptional downregulation of HGF/SF and met genes during muscle differentiation allows us to think that a premature met kinase signaling interruption could result in a major propension of cells to differentiate. However, our attempts to force C2 myoblasts to enter the differentiating pathway by cultivating them in growth medium supplemented with anti-HGF/SF neutralizing antibodies were not successful; in fact, no sign of precocious myogenesis was detected (data not shown). It is possible that the high levels of HGF/SF produced by C2 cells would make the interruption of the autocrine stimulation by the neutralizing antibodies very difficult, at least in the conditions we used. Furthermore, met kinase inactivation could be necessary, but not by itself sufficient, to permit muscle differentiation since additional inhibitory constraints would continue to function in these conditions.
Nevertheless, strong evidence for a negative effect of met kinase signaling on myogenic differentiation comes from the characterization of C2 clones expressing the constitutively activated p65tpr-met fusion protein. As determined by immunofluorescence staining, these clones are defective for the synthesis of muscle-specific structural proteins (MHC) and myotube formation. The extent of this myogenesis inhibition corresponds with the expression levels of p65tpr-met.
A reduced expression of different myogenic helix-loophelix transcription factors, such as MyoD and myogenin, was also detected in these clones. Once again, the p65tpr-met highly expressing clones exhibit the most extreme phenotype with a complete failure in myogenin gene activation and with the silencing of MyoD gene expression. A negative regulation of both gene expression and functional activity of MyoD has been already reported for FGF and TGF-β (Vaidya et al., 1989). The mechanisms by which the activation of the growth factor pathways interfere with the myogenic program are under extensive investigation (for reviews see Olson, 1992; Maione and Amati, 1996).
In addition, we have shown that the activity of p65tpr-met kinase induces remarkable changes in cell morphology. Particularly in p65tpr-met highly expressing clones, cells appear spindle-shaped and refractile, denoting a poor adherence to substrate, witnessed also by a major sensitivity to trypsinization. The presence of a network of threadlike cytoplasmic extensions is reminiscent of the scattered appearance of epithelial cells upon HGF/SF stimulation, indicating that p65tpr-met signaling triggers an increased motogenic response.
Attempting to obtain further insight into the role of HGF/SF in C2 differentiation and to overcome the differentiation-dependent decrease of HGF/SF and met endogenous genes, we have also studied the effects of an ectopic constitutive expression of the human isoforms of HGF/SF and of its cognate receptor in C2 myoblasts.
Clones selected for the single expression of h-met or h-HGF/SF do not exhibit significant phenotypic alterations compared with those of the parental cell line, although they produce detectable quantities of each product. From the results it may be inferred that a species-specific correspondence between the ligand and its related receptor is required to create an effective autocrine loop for h-HGF/ SF in C2 myoblasts. This is noteworthy if we consider the high degree of homology between murine and human HGF/SF proteins, which is >90% (Liu et al., 1993). However, a low binding affinity of HGF/SF to heterospecific receptor molecules has been originally reported by Rong et al. (1992) to explain the tumorigenicity induced by met proto-oncogene in a murine fibroblast cell line. The finding that in C2/h-met clones the human isoform of the receptor is weakly or not at all tyrosine-phosphorylated is in line with this high species-specificity, since murine HGF/SF is not efficient in activating h-met.
On the other hand, the establishment of an autocrine loop for h-HGF/SF through its coexpression with h-met in C2 cells results in soft agar growth ability and in a simultaneous myogenic inhibition, just as for p65tpr-met-expressing clones. In this case, h-met exhibits a quantitative tyrosine phosphorylation, indicating a full activation by h-HGF/SF.
In light of these observations, we believe that a met receptor stably activated through an autocrine HGF/SF stimulation could be equivalent to the expression of tprmet kinase, despite the fact that p65tpr-met has a cytoplasmic localization and a constitutive kinase activity independent from HGF/SF. Yet some differences must be present, since we were able to select only one C2 clone expressing simultaneously h-met and h-HGF/SF from two distinct transfection assays. This difficulty was further confirmed by the loss of one human product in a second clone and by the cell death observed when cells of clone 33 were transferred to differentiation medium. It appears therefore that the establishment of a species-specific autocrine loop for h-HGF/SF, released from differentiation-dependent control, has a major negative action on C2 myoblasts.
In sum, our results support the notion that HGF/SF plays a role in the motogenic and growth properties of myogenic cells through an autocrine loop that needs to be downregulated during differentiation, since the constitutive activation of met kinase is incompatible with myogenesis.
Acknowledgments
We are grateful to Dr. Carola Ponzetto for making available the tpr-met oncogene and for valuable suggestions, to Dr. Ermanno Gherardi for the generous gift of anti–mouse HGF/SF serum, and to Dr. Giulio Cossu for providing the mouse primary satellite cells. The help of Angelo Peschiaroli in some of the experiments is also acknowledged.
This work has been supported by grants of the Associazione Italiana Ricerche sul Cancro, Progetto Finalizzato Applicazioni Cliniche della Ricerca Oncologica-Consiglio Nazionale delle Ricerche and Ministero dell'Università e della Ricerca Scientifica e Tecnologica Roma, to P. Comoglio and P. Amati.
Footnotes
1. Abbreviations used in this paper: HGF, hepatocyte growth factor; h-HGF/SF, human HGF/SF; h-met, human isoform of met receptor; HS, horse serum; IGF, insulin-like growth factor; LTR, long terminal repeat; MHC, myosin heavy chain; MHCe, embryonic MHC; SF, scatter factor.
Please address all correspondence to Paolo Amati, Dipartimento di Biotecnologie Cellulari ed Ematologia, Sezione di Genetica Molecolare, Università di Roma La Sapienza, Viale Regina Elena 324, 00161 Roma, Italy. Tel.: (39) 6-490393. Fax: (39) 6-4462891. e-mail: amati@dbu.uniroma1.it
References
- Adams JC, Furlong RA, Watt FM. Production of scatter factor by ndk, a strain of epithelial cells, and inhibition of scatter factor activity by suramin. J Cell Sci. 1991;98:385–394. doi: 10.1242/jcs.98.3.385. [DOI] [PubMed] [Google Scholar]
- Allen RE, Sheehan SM, Taylor RG, Kendall TL, Rice GM. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J Cell Physiol. 1995;165:307–312. doi: 10.1002/jcp.1041650211. [DOI] [PubMed] [Google Scholar]
- Bader D, Masaki T, Fischman DA. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982;95:763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellusci S, Moens G, Gaudino G, Comoglio PM, Nakamura T, Thiery J-P, Jouanneau J. Creation of an hepatocyte growth factor/scatter factor autocrine loop in carcinoma cells induces invasive properties associated with increased tumorigenicity. Oncogene. 1994;9:1091–1099. [PubMed] [Google Scholar]
- Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-metreceptor in the migration of myogenic precursor cells into the limb bud. Nature (Lond) 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- Boon, T., M.E. Buckingham, D.L. Dexter, H. Jacob, and F. Jacob. 1974. Mouse teratocarcinoma: isolation and characterization of two muscle cell lines. Ann. Microbiol. 125B(1):13–28. [PubMed]
- Bottaro DP, Rubin JS, Faletto DL, Chan AML, Kmiecik TE, Vande GF, Woude, Aaronson SA. Identification of the hepatocyte growth factor receptor as the metproto-oncogene product. Science (Wash DC) 1991;251:802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- Braun T, Buschhausen G, Denker, Bober E, Tannich E, Arnold HH. A novel human muscle factor related to but distinct from MyoD1 induces myogenic conversion in 10T1/2 fibroblasts. EMBO (Eur Mol Biol Organ) J. 1989;8:701–709. doi: 10.1002/j.1460-2075.1989.tb03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119:629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AM-L, King HWS, Deakin EA, Tempest PR, Hilkens J, Kroezen V, Edwards DR, Wills AJ, Brookes P, Cooper CS. Characterization of the mouse met proto-oncogene. Oncogene. 1988;2:593–599. [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Comoglio PM, Boccaccio C. The HGF receptor family: unconventional signal transducers for invasive cell growth. Genes Cells. 1996;1:347–354. doi: 10.1046/j.1365-2443.1996.37037.x. [DOI] [PubMed] [Google Scholar]
- Cooper CS, Park M, Blair D, Tainsky MA, Huebner K, Croce CM, Vande GF, Woude Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature (Lond) 1984;311:29–33. doi: 10.1038/311029a0. [DOI] [PubMed] [Google Scholar]
- Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- DeFrances MC, Wolf H, Michalopoulos GK, Zarnegar R. The presence of hepatocyte growth factor in the developing rat. Development (Camb) 1992;116:387–395. doi: 10.1242/dev.116.2.387. [DOI] [PubMed] [Google Scholar]
- Di Renzo MF, Narsimhan RP, Olivero M, Bretti S, Giordano S, Medico E, Gaglia P, Zara P, Comoglio PM. Expression of the Met/HGF receptor in normal and neoplastic human tissues. Oncogene. 1991;6:1997–2003. [PubMed] [Google Scholar]
- Ewton DZ, Spizz G, Olson EN, Florini JR. Decrease in transforming growth factor-β binding and action during differentiation in muscle cells. J Biol Chem. 1988;263:4029–4032. [PubMed] [Google Scholar]
- Ferracini R, Di Renzo MF, Scotlandi K, Baldini N, Olivero M, Lollini PL, Cremona O, Campanacci M, Comoglio PM. The met/HGF receptor is over-expressed in human osteosarcomas and is activated by either a paracrine or an autocrine circuit. Oncogene. 1995;10:739–749. [PubMed] [Google Scholar]
- Florini JR, Magri KA. Effects of growth factors on myogenic differentiation. Am J Physiol. 1989;256:C701–C711. doi: 10.1152/ajpcell.1989.256.4.C701. [DOI] [PubMed] [Google Scholar]
- Florini JR, Ewton DZ, Magri KA. Hormones, growth factors, and myogenic differentiation. Annu Rev Physiol. 1991;53:201–216. doi: 10.1146/annurev.ph.53.030191.001221. [DOI] [PubMed] [Google Scholar]
- Gherardi E, Gray J, Stoker M, Perryman M, Furlong R. Purification of scatter factor, a fibroblast-derived basic protein that modulates epithelial interactions and movement. Proc Natl Acad Sci USA. 1989;86:5844–5848. doi: 10.1073/pnas.86.15.5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano S, Ponzetto C, Di Renzo MF, Cooper CS, Comoglio PM. Tyrosine kinase receptor indistinguishable from the metprotein. Nature (Lond) 1989a;339:155–156. doi: 10.1038/339155a0. [DOI] [PubMed] [Google Scholar]
- Giordano S, Di Renzo MF, Narsimhan RP, Cooper CS, Rosa C, Comoglio PM. Biosynthesis of the protein encoded by the metprotooncogene. Oncogene. 1989b;4:1383–1388. [PubMed] [Google Scholar]
- Goldberg, I.D., and E.M. Rosen, editors. 1993. Hepatocyte Growth FactorScatter Factor (HGF-SF) and the c-Met Receptor. Birkäuser-Verlag, Basel, Switzerland. 397 pp.
- Gonzatti-Haces M, Seth A, Park M, Copeland T, Oroszlan S, Vande GF, Woude Characterization of TPR-MET oncogene p65 and the MET protooncogene p140 protein-tyrosine kinases. Proc Natl Acad Sci USA. 1988;85:21–25. doi: 10.1073/pnas.85.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant DS, Kleinman HK, Goldberg ID, Bhargava MM, Nickoloff BJ, Kinsella JL, Polverini P, Rosen EM. Scatter factor induces blood vessel formation in vivo. Proc Natl Acad Sci USA. 1993;90:1937–1941. doi: 10.1073/pnas.90.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann G, Naldini L, Weidner KM, Sachs M, Vigna E, Comoglio PM, Birchmeier W. A functional domain in the heavy chain of scatter factor/hepatocyte growth factor binds the c-met receptor and induces cell dissociation but not mitogenesis. Proc Natl Acad Sci USA. 1992;89:11574–11578. doi: 10.1073/pnas.89.23.11574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JS, Olson EN. Functional receptors for transforming growth factor-β are retained by biochemically differentiated C2 myocytes in growth factor-deficient medium containing EGTA but down-regulated during terminal differentiation. J Biol Chem. 1990;265:7914–7919. [PubMed] [Google Scholar]
- Igawa T, Kanda S, Kanetake H, Saito Y, Ichichara A, Tomita Y, Nakamura T. Hepatocyte growth factor is a potent mitogen for cultured rabbit renal tubular epithelial cells. Biochem Biophys Res Commun. 1991;174:831–838. doi: 10.1016/0006-291x(91)91493-v. [DOI] [PubMed] [Google Scholar]
- Ishiki Y, Ohnishi H, Muto Y, Matsumoto K, Nakamura T. Direct evidence that hepatocyte growth factor is a hepatotrophic factor for liver regeneration and has potent anti-hepatitis effect in vivo. Hepatology. 1992;16:1227–1235. [PubMed] [Google Scholar]
- Iyer A, Kmiecik TE, Park M, Daar I, Blair D, Dunn KJ, Sutrave P, Ihle JN, Bodescot M, Vande GF, Woude Structure, tissue-specific expression, and transforming activity of the mouse met proto-oncogene. Cell Growth Differ. 1990;1:87–95. [PubMed] [Google Scholar]
- Jennische E, Ekberg S, Matejka GL. Expression of hepatocyte growth factor in growing and regenerating rat skeletal muscle. Am J Physiol. 1993;265:C122–C128. doi: 10.1152/ajpcell.1993.265.1.C122. [DOI] [PubMed] [Google Scholar]
- Kan M, Zhang G, Zarnegar R, Michalopoulos G, Myoken Y, McKeehan WL, Stevens JI. Hepatocyte growth factor/hepatopoietin A stimulates the growth of rat kidney proximal tubule epithelial cells (RPTE), rat nonparenchymal liver cells, human melanoma cells, mouse keratinocytes, and stimulates anchorage-independent growth of SV-40 transformed RPTE. Biochem Biophys Res Commun. 1991;174:331–337. doi: 10.1016/0006-291x(91)90524-b. [DOI] [PubMed] [Google Scholar]
- Kawaida K, Matsumoto K, Shimazu H, Nakamura T. Hepatocyte growth factor prevents acute renal failure and accelerates renal regeneration in mice. Proc Natl Acad Sci USA. 1994;91:4357–4361. doi: 10.1073/pnas.91.10.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafyatis R, Lechleider R, Roberts AB, Sporn MB. Secretion and transcriptional regulation of transforming growth factor-β3 during myogenesis. Mol Cell Biol. 1991;11:3795–3803. doi: 10.1128/mcb.11.7.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Michalopoulos GK, Zarnegar R. Molecular cloning and characterization of cDNA encoding mouse hepatocyte growth factor. Biochim Biophys Acta. 1993;1216:299–303. doi: 10.1016/0167-4781(93)90159-b. [DOI] [PubMed] [Google Scholar]
- Lokker NA, Mark MR, Luis EA, Bennett GL, Robbins KA, Baker JB, Godowski PJ. Structure-function analysis of hepatocyte growth factor: identification of variants that lack mitogenic activity yet retain high affinity receptor binding. EMBO (Eur Mol Biol Organ) J. 1992;11:2503–2510. doi: 10.1002/j.1460-2075.1992.tb05315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier JAM, Mariotti M, Comoglio PM, Soria MR. Interleukin 1 induces an autocrine loop Hepatocyte Growth Factor/c-Met in murine Kaposi-like spindle cells. Oncogene. 1996;13:1009–1015. [PubMed] [Google Scholar]
- Maina F, Casagranda F, Audero E, Simeone A, Comoglio PM, Klein R, Ponzetto C. Uncoupling of Grb2 from the Met receptor in vivo reveals complex roles in muscle development. Cell. 1996;87:531–542. doi: 10.1016/s0092-8674(00)81372-0. [DOI] [PubMed] [Google Scholar]
- Maione R, Amati P. Interdependence between muscle differentiation and cell-cycle control. Published online: . Biochim Biophys Acta Reviews on Cancer. 1996;1332(1):M19–M30. doi: 10.1016/s0304-419x(96)00036-4. [DOI] [PubMed] [Google Scholar]
- Maione R, Fimia GM, Amati P. Inhibition of in vitro myogenic differentiation by a polyomavirus early function. Oncogene. 1992;7:85–93. [PubMed] [Google Scholar]
- Matsumoto K, Tajima H, Nakamura T. Hepatocyte growth factor is a potent stimulator of human melanocyte DNA synthesis and growth. Biochem Biophys Res Commun. 1991;176:45–51. doi: 10.1016/0006-291x(91)90887-d. [DOI] [PubMed] [Google Scholar]
- Medico E, Mongiovi AM, Huff J, Jelinek M-A, Follenzi A, Gaudino G, Parsons JT, Comoglio PM. The tyrosine kinase receptors Ron and Sea control “scattering” and morphogenesis of liver progenitor cells in vitro. Mol Biol Cell. 1996;7:495–504. doi: 10.1091/mbc.7.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos GK, Zarnegar R. Hepatocyte growth factor. Hepatology. 1992;15:149–155. doi: 10.1002/hep.1840150125. [DOI] [PubMed] [Google Scholar]
- Miyazawa K, Tsubouchi H, Naka D, Takahashi K, Okigaki M, Arakaki N, Nakayama H, Hirono S, Sakiyama O, Gohda E, et al. Molecular cloning and sequence analysis of cDNA for human hepatocyte growth factor. Biochem Biophys Res Commun. 1989;163:967–973. doi: 10.1016/0006-291x(89)92316-4. [DOI] [PubMed] [Google Scholar]
- Montesano R, Matsumoto K, Nakamura T, Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991;67:901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- Moore JW, Dionne C, Jaye M, Swain JL. The mRNAs encoding acidic FGF, basic FGF and FGF receptor are coordinately downregulated during myogenic differentiation. Development (Camb) 1991;111:741–748. doi: 10.1242/dev.111.3.741. [DOI] [PubMed] [Google Scholar]
- Morgenstern JP, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan RC, Berg P. Expression of a bacterial gene in mammalian cells. Science (Wash DC) 1980;209:1422–1427. doi: 10.1126/science.6251549. [DOI] [PubMed] [Google Scholar]
- Nadal-Ginard B. Committment, fusion and biochemical differentiation of a myogenic cell line. Cell. 1978;15:855–863. doi: 10.1016/0092-8674(78)90270-2. [DOI] [PubMed] [Google Scholar]
- Nagaike M, Hirao S, Tajima H, Noji S, Taniguchi S, Matsumoto K, Nakamura T. Renotropic functions of hepatocyte growth factor in renal regeneration after unilateral nephrectomy. J Biol Chem. 1991;266:22781–22784. [PubMed] [Google Scholar]
- Naka D, Ishii T, Yoshiyama Y, Miyazawa K, Hara H, Hishida T, Kitamura N. Activation of hepatocyte growth factor by proteolitic conversion of a single chain form to a heterodimer. J Biol Chem. 1992;267:20114–20119. [PubMed] [Google Scholar]
- Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, Tashiro K, Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature (Lond) 1989;342:440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- Naldini L, Vigna E, Narsimhan RP, Gaudino G, Zarnegar R, Michalopoulos GK, Comoglio PM. Hepatocyte growth factor (HGF) stimulates the tyrosine kinase activity of the receptor encoded by the protooncogene c-MET. Oncogene. 1991a;6:501–504. [PubMed] [Google Scholar]
- Naldini L, Weidner KM, Vigna E, Gaudino G, Bardelli A, Ponzetto C, Narsimhan RP, Hartmann G, Zarnegar R, Michalopoulos GK, et al. Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO (Eur Mol Biol Organ) J. 1991b;10:2867–2878. doi: 10.1002/j.1460-2075.1991.tb07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L, Tamagnone L, Vigna E, Sachs M, Hartmann G, Birchmeier W, Daikuhara Y, Tsubouchi H, Blasi F, Comoglio PM. Extracellular proteolytic cleavage by urokinase is required for activation of hepatocyte growth factor/scatter factor. EMBO (Eur Mol Biol Organ) J. 1992;11:4825–4833. doi: 10.1002/j.1460-2075.1992.tb05588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L, Vigna E, Bardelli A, Follenzi A, Galimi F, Comoglio PM. Biological activation of pro-HGF (hepatocyte growth factor) by urokinase is controlled by a stoichiometric reaction. J Biol Chem. 1995;270:603–611. doi: 10.1074/jbc.270.2.603. [DOI] [PubMed] [Google Scholar]
- Olson EN. Interplay between proliferation and differentiation within the myogenic lineage. Dev Biol. 1992;154:261–272. doi: 10.1016/0012-1606(92)90066-p. [DOI] [PubMed] [Google Scholar]
- Olwin BB, Hauschka SD. Cell surface fibroblast growth factor and epidermal growth factor receptors are permanently lost during skeletal muscle terminal differentiation in culture. J Cell Biol. 1988;107:761–769. doi: 10.1083/jcb.107.2.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Dean M, Cooper CS, Schmidt M, O'Brien SJ, Blair DG, Vande GF, Woude Mechanism of metoncogene activation. Cell. 1986;45:895–904. doi: 10.1016/0092-8674(86)90564-7. [DOI] [PubMed] [Google Scholar]
- Piechaczyk M, Blanchard JM, Marty L, Dani C, Panabieres F, El S, Sabouty, Fort P, Jeanteur P. Post-transcriptional regulation of glyceraldehyde-3-phosphate-dehydrogenase gene expression in rat tissues. Nucleic Acids Res. 1984;12:6951–6963. doi: 10.1093/nar/12.18.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzetto C, Giordano S, Peverali F, Della G, Valle, Abate ML, Vaula G, Comoglio PM. C-met is amplified but not mutated in a cell line with an activated met tyrosine kinase. Oncogene. 1991;6:553–559. [PubMed] [Google Scholar]
- Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla Zonca P, Giordano S, Graziani A, Panayotou G, Comoglio PM. A multifunctional docking site mediates signalling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77:261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- Prat M, Narsimhan RP, Crepaldi T, Nicotra MR, Natali PG, Comoglio PM. The receptor encoded by the human c-Met oncogene is expressed in hepatocytes, epithelial cells and solid tumors. Int J Cancer. 1991;49:323–328. doi: 10.1002/ijc.2910490302. [DOI] [PubMed] [Google Scholar]
- Rodriguez GA, Park M. Dimerization mediated through a leucine zipper activates the oncogenic potential of the metreceptor tyrosine kinase. Mol Cell Biol. 1993;13:6711–6722. doi: 10.1128/mcb.13.11.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong S, Bodescot M, Blair D, Dunn J, Nakamura T, Mizuno K, Park M, Chan A, Aaronson S, Vande GF, Woude Tumorigenicity of the met proto-oncogene and the gene for hepatocyte growth factor. Mol Cell Biol. 1992;12:5152–5158. doi: 10.1128/mcb.12.11.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong S, Jeffers M, Resau JH, Tsarfaty I, Oskarsson M, Vande GF, Woude Met expression and sarcoma tumorigenicity. Cancer Res. 1993;53:5355–5360. [PubMed] [Google Scholar]
- Rong S, Segal S, Anver M, Resau JH, Vande GF, Woude Invasiveness and metastasis of NIH3T3 cells induced by met-hepatocyte growth factor/scatter factor autocrine stimulation. Proc Natl Acad Sci USA. 1994;91:4731–4735. doi: 10.1073/pnas.91.11.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin JS, Chan AM-L, Bottaro DP, Burgess WH, Taylor WG, Cech AC, Hirschfield DW, Wong J, Miki T, Finch PW, et al. A broadspectrum human lung fibroblast-derived mitogen is a variant of hepatocyte growth factor. Proc Natl Acad Sci USA. 1991;88:415–419. doi: 10.1073/pnas.88.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatori G, Ferrari G, Mezzogiorno A, Servidei S, Coletta M, Tonali P, Giavazzi R, Cossu G, Mavilio F. Retroviral vector-mediated gene transfer into human primary myogenic cells leads to expression in muscle fibers in vivo. . Hum Gene Therapy. 1993;4:713–723. doi: 10.1089/hum.1993.4.6-713. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., E.F. Fritsch, and T. Maniatis. 1989. Molecular Cloning: A Laboratory Manual. Second edition. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. 545 pp.
- Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature (Lond) 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- Shiota G, Rhoads DB, Wang TC, Nakamura T, Schmidt EV. Hepatocyte growth factor inhibits growth of hepatocellular carcinoma cells. Proc Natl Acad Sci USA. 1992;89:373–377. doi: 10.1073/pnas.89.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg E, Meyer D, Weidner KM, Birchmeier C. Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol. 1993;123:223–235. doi: 10.1083/jcb.123.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker M, Gherardi E, Perryman M, Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature (Lond) 1987;327:239–242. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- Sturani E, Zippel R, Toschi L, Morello L, Comoglio PM, Alberghina L. Kinetics and regulation of the tyrosine phosphorylation of epidermal growth factor receptor in intact A431cells. Mol Cell Biol. 1988;8:1345–1351. doi: 10.1128/mcb.8.3.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama H, LaRochelle WJ, Anver M, Bockman DE, Merlino G. Scatter factor/hepatocyte growth factor as a regulator of skeletal muscle and neural crest development. Proc Natl Acad Sci USA. 1996;93:5866–5871. doi: 10.1073/pnas.93.12.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro K, Hagiya M, Nishizawa T, Seki T, Shimonishi M, Shimizu S, Nakamura T. Deduced primary structure of rat hepatocyte growth factor and expression of the mRNA in rat tissues. Proc Natl Acad Sci USA. 1990;87:3200–3204. doi: 10.1073/pnas.87.8.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefsen SE, Lajara R, McCusker RH, Clemmons DR, Rotwein P. Insulin-like growth factors (IGF) in muscle development. Expression of IGF-1, the IGF-1 receptor, and an IGF binding protein during myoblast differentiation. J Biol Chem. 1989a;264:13810–13817. [PubMed] [Google Scholar]
- Tollefsen SE, Sadow JL, Rotwein P. Coordinate expression of insulin-like growth factor II and its receptor during muscle differentiation. Proc Natl Acad Sci USA. 1989b;86:1543–1547. doi: 10.1073/pnas.86.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao MS, Zhu H, Giaid A, Viallet J, Nakamura T, Park M. Hepatocyte growth factor/scatter factor is an autocrine factor for human normal bronchial, epithelial, and lung carcinoma cells. Cell Growth Differ. 1993;4:571–579. [PubMed] [Google Scholar]
- Tsarfaty I, Resau JH, Rulong S, Keydar I, Faletto DL, Vande GF, Woude The metproto-oncogene receptor and lumen formation. Science (Wash DC) 1992;257:1258–1261. doi: 10.1126/science.1387731. [DOI] [PubMed] [Google Scholar]
- Vaidya TB, Rhodes SJ, Taparowsky EJ, Konieczny SF. Fibroblast growth factor and transforming growth factor β repress transcription of the myogenic regulatory gene MyoD1. Mol Cell Biol. 1989;9:3576–3579. doi: 10.1128/mcb.9.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner KM, Behrens J, Vandekerckhove J, Birchmeier W. Scatter factor: molecular characteristics and effect on the invasiveness of epithelial cells. J Cell Biol. 1990;111:2097–2108. doi: 10.1083/jcb.111.5.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner KM, Arakaki N, Hartmann G, Vandekerckhove J, Weingart S, Rieder H, Fonatsch C, Tsubouchi H, Hishida T, Daikuhara Y, Birchmeier W. Evidence for the identity of human scatter factor and human hepatocyte growth factor. Proc Natl Acad Sci USA. 1991;88:7001–7005. doi: 10.1073/pnas.88.16.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner KM, Sachs M, Birchmeier W. The met receptor tyrosine kinase transduces motility, proliferation, and morphogenic signals of scatter factor/hepatocyte growth factor in epithelial cells. J Cell Biol. 1993;121:145–154. doi: 10.1083/jcb.121.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H. The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell. 1993;75:1241–1244. doi: 10.1016/0092-8674(93)90610-3. [DOI] [PubMed] [Google Scholar]
- Weydert A, Daubas P, Lazaridis I, Barton P, Garner I, Leader DP, Bonhomme F, Catalan J, Simon D, Guenet JL, et al. Genes for skeletal muscle myosin heavy chains are clustered and are not located on the same mouse chromosome as a cardiac myosin heavy chain gene. Proc Natl Acad Sci USA. 1985;82:7183–7187. doi: 10.1073/pnas.82.21.7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M, Silverstein S, Lee L, Pellicer A, Cheg YC, Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977;11:223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Wolf HK, Zarnegar R, Michalopoulos GK. Localization of hepatocyte growth factor in human and rat tissues: an immunohistochemical study. Hepatology. 1991;14:488–494. [PubMed] [Google Scholar]
- Woolf AS, Kolatsi-Joannou M, Hardman P, Andermarcher E, Moorby C, Fine LG, Jat PS, Noble MD, Gherardi E. Roles of hepatocyte growth factor and the met receptor in the early development of the metanephros. J Cell Biol. 1995;128:171–184. doi: 10.1083/jcb.128.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature (Lond) 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Yanagita K, Matsumoto K, Sekiguchi K, Ishibashi H, Niho Y, Nakamura T. Hepatocyte growth factor may act as a pulmotrophic factor on lung regeneration after acute lung injury. J Biol Chem. 1993;268:21212–21217. [PubMed] [Google Scholar]
- Yang X-M, Vogan K, Gros P, Park M. Expression of the metreceptor tyrosine kinase in muscle progenitor cells in somites and limbs is absent in Splotch mice. Development (Camb) 1996;122:2163–2171. doi: 10.1242/dev.122.7.2163. [DOI] [PubMed] [Google Scholar]
- Zarnegar R, Muga S, Rahija R, Michalopoulos G. Tissue distribution of hepatopoietin-A: a heparin-binding polypeptide growth factor for hepatocytes. Proc Natl Acad Sci USA. 1990;87:1252–1256. doi: 10.1073/pnas.87.3.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]