Abstract
In T lymphocytes, the Src-family protein tyrosine kinase p56lck (Lck) is mostly associated with the cytoplasmic face of the plasma membrane. To determine how this distribution is achieved, we analyzed the location of Lck in lymphoid and in transfected nonlymphoid cells by immunofluorescence. We found that in T cells Lck was targeted correctly, independently of the cell surface proteins CD4 and CD8 with which it interacts. Similarly, in transfected NIH-3T3 fibroblasts, Lck was localized at the plasma membrane, indicating that T cell–specific proteins are not required for targeting. Some variation in subcellular distribution was observed when Lck was expressed in HeLa and MDCK cells. In these cells, Lck associated with both the plasma membrane and the Golgi apparatus, while subsequent expression of CD4 resulted in the loss of Golgi-associated staining. Together, these data indicate that Lck contains intrinsic signals for targeting to the plasma membrane. Furthermore, delivery to this site may be achieved via association with exocytic transport vesicles.
A mutant Lck molecule in which the palmitoylation site at cysteine 5 was changed to lysine (LC2) localized to the plasma membrane and the Golgi region in NIH3T3 cells. However, the localization of a mutant in which the palmitoylation site at cysteine 3 was changed to serine (LC1) was indistinguishable from wild-type Lck. Chimeras composed of only the unique domain of Lck linked to either c-Src or the green fluorescent protein similarly localized to the plasma membrane of NIH-3T3 cells. Thus, the targeting of Lck appears to be determined primarily by its unique domain and may be influenced by the use of different palmitoylation sites.
Alarge number of cytosolic proteins associate with membranes through long chain fatty acids covalently attached to their amino or carboxyl termini. Examples include members of the Src-family, the alpha subunits of heterotrimeric G proteins, small GTP-binding proteins, and retroviral matrix proteins. In a number of cases these proteins have been found to associate with a specific membrane compartment, e.g., c-Src associates with endosomal membranes (Kaplan et al., 1992), Gαi-3 with the Golgi complex (Ercolani et al., 1990), and the Gag protein of Moloney murine leukemia virus with the plasma membrane (Wills and Craven, 1991). However, little is known about the mechanisms that underlie the targeting of acylated proteins to their sites of function in the cell. To gain more insight into this, we have analyzed the cellular distribution of the myristoylated and palmitoylated Src-family member Lck.
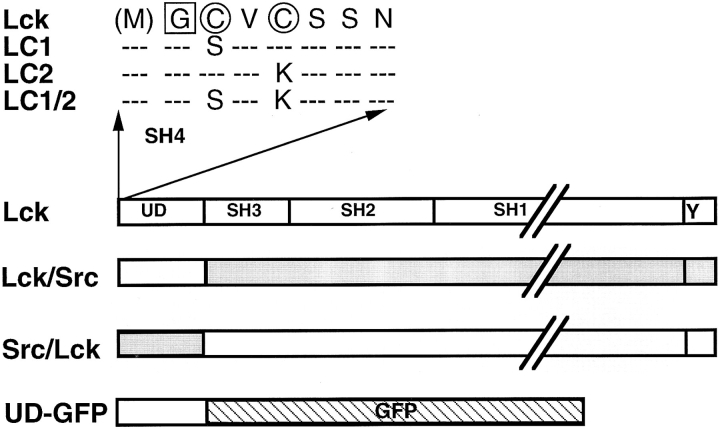
The Src-family of nonreceptor protein tyrosine kinases currently consists of nine proteins (Src, Fyn, Lyn, Yes, Fgr, Hck, Blk, Lck, and Yrk [for review see Rudd et al., 1993]) that play important roles in cell growth regulation and differentiation. These proteins have overlapping but distinct activities and tissue distributions. At the cellular level, Srcfamily members have discrete subcellular localizations that may in part determine the specific function of these proteins. V-Src has been found in focal adhesions (Rohrschneider, 1980), c-Src on endosomes (Kaplan et al., 1992), Fyn in the microtubule organizing center (Ley et al., 1994), Hck on secretory granules (Mohn et al., 1995), and Lck at the plasma membrane (Ley et al., 1994). Each Src-family protein contains a nonhomologous domain of ∼70 amino acids at the NH2 terminus (the unique domain), followed by a single Src homology 3 (SH3) domain, an SH2 domain, and the tyrosine kinase or SH1 domain (see Rudd et al., 1993) (Fig. 1). In addition, a short tyrosine-containing (Y505 in Lck) motif at the COOH terminus regulates the enzymatic activity of the protein (Cooper and Howell, 1993), and a conserved region (SH4 domain) at the extreme NH2 terminus contains the signal(s) for acylation (Fig. 1) (Resh, 1993). All family members are myristoylated and, with the exception of Src and Blk, contain one or two sites for palmitoylation (Koegl et al., 1994; Resh, 1994). Thus far palmitoylation has been demonstrated for Lck, Fyn, Hck, Yes, and Fgr (Paige et al., 1993; Alland et al., 1994; Koegl et al., 1994; Shenoy-Scaria et al., 1994).
Figure 1.
Domain organization of the Src-family proteins and of the Lck constructs used in this study. Lck is shown schematically as a representative of Src-family proteins. Indicated are the unique domain (UD), the Src Homology 3 (SH3), SH2, the tyrosine kinase (SH1) domains, and the short conserved COOHterminal region that contains the “regulatory” tyrosine (Y). The amino acid sequence of the conserved NH2-terminal region, also designated the SH4 domain, which contains the signals for acylation is shown for Lck. The attachment of myristic acid to glycine (boxed) requires the removal of the NH2-terminal methionine and the consensus sequence GXXXS/C. Palmitic acid is attached to cysteines (encircled) and requires a myristoylated NH2-terminal glycine and cysteine on position 3. Cysteine 5 in Lck is also a palmitate acceptor site.In this study we used Lck mutants with disruptions to the first (LC1), the second (LC2), or both (LC1/2) palmitoylation sites (Turner et al., 1990). Also depicted are the chimeras used in this study: Lck/Src contains the unique domain of Lck and the remainder of Src, Src/Lck contains the unique domain of Src and the remainder of Lck (Turner et al., 1990). The chimera UD-GFP is composed of the unique domain of Lck fused to the NH2 terminus of green fluorescent protein.
Lck is expressed in thymocytes and mature T lymphocytes and is crucial for the development (Molina et al., 1992; Levin et al., 1993) and function (Straus and Weiss, 1992) of these cells. The localization of Lck at the cytosolic side of the plasma membrane is consistent with its role in facilitating signaling through the T cell antigen receptor (TcR)1. It is well established that Lck interacts with CD4 and CD8 (Rudd et al., 1988; Veillette et al., 1988), the TcR coreceptors on helper and cytotoxic T cells, respectively, and that these interactions are important for T cell activation (Zamoyska et al., 1989; Glaichenhaus et al., 1991). Lck also plays a role in regulating the endocytic properties of CD4, thereby controlling the cellular distribution of this coreceptor (Pelchen-Matthews et al., 1992). The interaction of Lck with CD4 or CD8 is dependent on the presence of cysteine motifs in the cytoplasmic tails of the coreceptor molecules and in the unique domain of Lck (Shaw et al., 1990; Turner et al., 1990).
Here, we have sought to determine factors that are important for the plasma membrane distribution of Lck. The Lck domains confer a potential for numerous protein–protein and protein–lipid interactions, any of which might contribute to the subcellular localization of this protein. Palmitoylation and myristoylation at the SH4 domain play a key role in the association of Lck with membranes. The unique domain interacts with CD4 and CD8 (Rudd et al., 1988; Veillette et al., 1988), the SH2 domain with phospho-tyrosine–containing sequences (Pawson, 1995) and phosphatidylinositol (Rameh et al., 1995), the SH3 domain with proline-rich sequences (Pawson, 1995), and the kinase domain with its tyrosine-containing substrates. Furthermore, the regulatory COOH-terminal tyrosine is a substrate for c-Src kinase (Csk) (Nada et al., 1991), the CD45 phosphatases (Ostergaard et al., 1989) and, when phosphorylated, interacts with the SH2 domain (Sieh et al., 1993).
In addition to CD4 and CD8, Lck has been reported to interact with a variety of other cell surface proteins, both transmembrane and GPI-linked proteins, as well as with plasma membrane–associated caveolae (Shenoy-Scaria et al., 1994). Furthermore, an association of Lck with elements of the cytoskeleton has been suggested (Louie et al., 1988; Kinch et al., 1994). The role of these interactions in establishing and maintaining the subcellular distribution of Lck is also unclear.
We analyzed the distribution of Lck by immunofluorescence and found that neither CD4, CD8, nor other T cell– specific proteins are required for the plasma membrane localization of Lck in T cells and transfected NIH-3T3 fibroblasts. The use of chimeras demonstrated that the unique domain of Lck plays an important role in establishing the plasma membrane localization in NIH-3T3 cells. Palmitoylation of either Cys 3 or Cys 5 resulted in membrane association, confirming the result of others (Kwong et al., 1995; Yurchak et al., 1995). However, an Lck mutant that contains only the palmitoylation site at Cys 3 localized both to the Golgi region and the plasma membrane, suggesting that the position of the palmitic acid influences the subcellular distribution of Lck. We observed cell type–specific variation in the distribution of Lck: in HeLa cells, Lck was partially found at the Golgi complex. This may suggest that unidentified cellular components are involved in the correct sorting of Lck, and furthermore, that Lck may be transported to the plasma membrane via the exocytic pathway.
Materials and Methods
Reagents
Tissue culture reagents were from Gibco Ltd. (Paisley, UK), tissue culture plastic was from Falcon Plastics, and chemicals were from Sigma Chemical Co., (Poole, UK), unless indicated otherwise.
Cells
The human T cell lines, SupT1, BC7, A3.01, and A2.01, were cultured in RPMI 1640 supplemented with 10% FCS. BC7 cells (provided by J. Hoxie, University of Philadelphia, Philadelphia, PA) are CD4-negative derivatives of SupT1 cells (Endres et al., 1996). A3.01 cells were selected for HAT-sensitivity after mutagenesis of CEM T cells with 8-azaguanine (Folks et al., 1985). A2.01 cells are CD4-negative derivatives of A3.01 cells. A2.01 expressing CD4 without a cytoplasmic tail (A.201-CD4stop399) and A3.01 were kindly provided by D. Littman (Skirball Institute, New York).
NIH-3T3 fibroblasts and transfectants of these cells were grown in DME containing 10% FCS, 100 U/ml penicillin, 0.1 mg/ml streptomycin (Pen/Strep), and supplemented where appropriate with 1 mg/ml G418 and/or 0.2 mg/ml hygromycin (Boehringer Mannheim GmbH, Mannheim, Germany). NIH-3T3 cells transfected with human CD4 and either murine Lck, c-Src, Lck/Src, or Src/Lck have been described before (Pelchen-Matthews et al., 1992). HeLa cells and HeLa transfectants were cultured in DME containing 4% FCS, Pen/Strep (as above), and 1 mg/ml G418 where appropriate. The HeLa cells expressing human CD4 used in this study were made in our laboratory by Dr. C. Pitcher.
Transfections and DNA Constructs
Transfections of NIH-3T3 cells were carried out by electroporation in HEBS buffer (20 mM Hepes, pH 7.05, 137 mM NaCl, 5 mM KCl, 0.7 mM Na2HPO4, 6 mM d-glucose) using a Gene Pulser at 350 V, 250 μF, infinite Ω (Bio-Rad Laboratories, Richmond, CA). HeLa cells were transfected using 450 V. Murine Lck (Marth et al., 1985) and the constructs LC1, LC2, LC1/2, and Lck/Src (Turner et al., 1990) were all generously provided by D. Littman. These constructs were subcloned behind the SV-40 promotor of the pBabe/Hygro vector (Morgenstern and Land, 1990) and 10 μg DNA was used per transfection. Resistant colonies were selected on 0.2 mg/ml hygromycin. A cDNA encoding human CD4 was cloned into pSG5 (Stratagene Ltd., Cambridge, UK). A cDNA encoding CD4 with a stopcodon at position 399 (CD4Δcyt) was prepared by site-directed mutagenesis using the Kunkel method.
The GFP cDNA (Prasher et al., 1992), containing a Ser to Thr mutation at position 65 (Heim et al., 1995), was kindly provided by G. Gorrie (Medical Research Council Laboratory for Molecular Cell Biology, London). A murine Lck unique domain (UD)–green fluorescent protein (GFP) chimera was made by three-step PCR using Taq polymerase. The first PCR (PCR1) was performed using the Reverse Cycle primer (U.S. Biochemical Corp., Cleveland, OH) and the oligonucleotide (5′)TCCTTTACTCATCAGCGGGGATGC(3)′ (designated 413) with murine Lck cDNA cloned into Bluescript KS. One half of the latter primer overlaps with the last 12 nucleotides of the unique domain of Lck and the other half with the first 12 nucleotides of GFP. The second PCR (PCR2) was performed with GFP cDNA in pGW1, using oligonucleotide (5′)ATAAACAAGTTGGGCCAT(3′) (designated N2643) that anneals to the vector sequence and as 5′ primer (5′)GCATCCCCGCTGATGAGTAAAGGA(3′) (designated 412), which is complementary to 413. In the third PCR, the combined reaction products of PCR1 and PCR2 were used as templates for the Reverse Cycle primer (5′) and N2643 (3′). The resulting product was digested with EcoRI and cloned into pGW1 and sequenced using T7 sequenase (U.S. Biochemical Corp.).
Antibodies
The anti-CD4 mouse mAb Q4120 was provided by Q. Sattentau through the Medical Research Council AIDS Directed Reagents Programme (South Mimms, Potters Bar, UK). This antibody recognizes an epitope that is identical to, or overlaps with, the Leu3a epitope. Two rabbit polyclonal antisera against Lck were used, both raised against a synthetic peptide comprising residues 478–509. One (Lck I) was kindly provided by S. Ley (National Institute for Medical Research, Mill Hill, UK), (Ley et al., 1994) and used for immunofluorescence and electron microscopy. The other (Lck II) was raised in our laboratory (Pelchen-Matthews et al., 1992), affinity purified using the Lck-peptide immobilized on Reactigel (Pierce and Warriner, Chester, UK), and used for immunoblotting. Both antisera against Lck do not recognize other members of the Src-family. This was assessed by immunofluorescence and immunoblotting of T cells that do not express normal Lck (JCam.1 [Straus et al., 1992]) and untransfected NIH-3T3 cells. The mAb 327 against v-Src was purchased from Oncogene Science, Inc. (Manhassett, NY). The rat mAb 23C identifies β′-COP of the coatomer complex, under the conditions used (Willison et al., 1989; Harrison-Lavoie et al., 1993). Peroxidase-conjugated goat anti–rabbit and goat anti–mouse antibodies, as well as anti–mouse rhodamine, anti–rat rhodamine, and anti–rabbit FITC reagents, were from Pierce and Warriner.
Immunofluorescence
T cells were immobilized on 13-mm-diam glass coverslips coated with 1 mg/ml poly-l-lysine in PBS. Adherent NIH-3T3 and HeLa cells were plated on coverslips 2 d before analysis. Cells were fixed at room temperature with 3% paraformaldehyde for 15 min, quenched with 50 mM NH4Cl, and permeablilized in 0.1% Triton TX-100 for 10 min at room temperature. All solutions were made in PBS. After preincubation for 10 min in 0.2% gelatin, cells were incubated for 1 h with primary antibodies diluted in 0.2% gelatin: mAb Q4120 for CD4 (at 1.6 μg/ml), Lck I serum at 1:1,000 for Lck, and mAb 327 for Src at 0.1 μg/ml. Fluorescent second layer antibodies were used at 1:2,000 dilution in 0.2% gelatin, and incubations were again for 1 h. Coverslips were mounted in Moviol (Calbiochem-Novachem, [UK] Ltd, Beeston, UK) and observed either with a fluorescence microscope (Axioskop; Carl Zeiss, Inc., Thornwood, NY) or by confocal microscopy using an Optiphot-2 microscope (Nikon Inc., Melville, NY) equipped with an MRC 1024 laser scanning attachment (Bio-Rad Laboratories).
Microinjection
HeLa-Lck cells were seeded on 13-mm-diam coverslips 2 d before microinjection. Plasmid DNA containing cDNA for CD4 or CD4Δcyt was diluted in sterile PBS to a final concentration of 100 μg/ml and injected into the nuclei. During microinjection, the cells were maintained at 37°C, in 5% CO2. Typically, 50 cells were injected over a 15-min period and the cells were returned to the incubator for 3 h before fixation and staining.
Membrane Preparation
Cells (7.5 × 106) were washed once with ice-cold PBS, once with ice-cold TEA buffer (10 mM triethanolamine, 10 mM acetic acid, 250 mM sucrose, 1 mM EDTA, pH 7.45), and subsequently resuspended in 0.5 ml TEA buffer containing 1 mM PMSF. Cells were broken with a ball-bearing homogenizer (diameter: 0.1564 inch; ball: 0.1553 inch; H & Y Enterprise, Redwood City, CA) until 75% of nuclei were free (∼80 passes for T cells). Unbroken cells and nuclei were removed by centrifugation for 5 min at 2,000 rpm at 4°C. Postnuclear supernatants were subsequently centrifuged at 100,000 g for 40 min at 4°C in an ultracentrifuge (Optima™ TLX; Beckman Instruments, Fullerton, CA). Membrane pellets were dissolved in NP-40 lysis buffer. Proteins were precipitated by the addition of 4 vol −70°C acetone for 30 min, recovered by centrifugation at 13,000 g for 15 min at 4°C, and resuspended in SDS-PAGE sample buffer. Samples from membrane and supernatant preparations, representing equivalent numbers of cells, were separated on 10% nonreducing SDS–polyacrylamide gels.
Immunoblotting
After electrophoresis, proteins were transferred to nitrocellulose membranes (Schleicher and Schuell, Keene, NH). The membranes were incubated in blocking buffer (10% skimmed milk powder [Marvel, Premier Beverages, Adbaston, UK], 0.1% Tween-20 in PBS) overnight at 4°C. Incubation with primary antibody was for 1 h at room temperature. Lck was detected using affinity-purified Lck II at 0.15 μg/ml in blocking buffer, and CD4 using the mouse mAb Q4120 (1.6 μg/ml). HRP-conjugated goat anti–rabbit and goat anti–mouse antibodies, diluted 1:2,000 in blocking buffer, were used as secondary antibodies. Blots were developed using enhanced chemiluminescence (Amersham International plc, Little Chalfont, UK) and visualized using autoradiography film (Fuji Photo Film Co. Ltd., Tokyo, Japan).
Electron Microscopy
For immunolabeling of cryosections, HeLa cells (untransfected or transfected with Lck) were fixed in 4% paraformaldehyde in PBS containing 5% sucrose, for 1 h at room temperature. The cells were scraped from the culture dish, pelleted, and processed for cryo-EM. Small blocks of gelatine (10% wt/wt) embedded cells were infused with 2.3 M sucrose for 4 h at 4°C and then placed on specimen holders and frozen in liquid nitrogen. Ultrathin cryosections were cut using an ultracut E/FC4E low temperature sectioning system (Reichert Jung, Vienna, Austria) and the sections were collected with a mixture of methylcellulose and 2.3 M sucrose (Liou and Slot, 1994). Immunolabeling was performed as described (Slot et al., 1991) except that Lck I was used as primary antibody followed by goat anti–rabbit IgG conjugated to 10-nm gold. Sections were viewed using a transmission electron microscope (EM 400; Philips Electron Optic, Cambridge, UK).
Results
Distribution of Lck in T Cells
In T lymphocytes, the TcR coreceptors CD4 and CD8 are the principal binding determinants for Lck. This interaction involves two cysteines in the unique domain of Lck (at positions 20 and 23) and two cysteines in the cytoplasmic tail of CD4 (at positions 420 and 422 in human CD4) or the CD8α chain (at positions 194 and 196 in human CD8a) (Shaw et al., 1990; Turner et al., 1990). As both CD4 and CD8 are expressed at the cell surface, association with these proteins could promote plasma membrane localization of Lck. When expressed in the absence of CD4 or CD8 in transfected nonlymphoid cells, Lck has been found to associate with the plasma membrane to some extent (Kwong et al., 1995; Yurchak et al., 1995). Whether the distribution of Lck is influenced by coexpression of CD4 or CD8 has not been examined.
We compared the cellular distribution of Lck in a CD4positive human leukemia–derived T cell line, SupT1, and a CD4-negative derivative of this cell line, BC7. In addition, a second human CD4-positive (CD8-negative) CEMderived T cell line, A3.01, was compared with a derivative of this line, A2.01-CD4stop399, which expresses a CD4 molecule lacking 34 of the 38 amino acids from its cytoplasmic domain. This truncation, which removes C420 and C422, abolishes the ability of CD4 to interact with Lck (Turner et al., 1990). The relative amount of Lck that is associated with CD4 varies between T cell lines (Rudd et al., 1993). We determined this proportion to be at least 60 and 67% for SupT1 and A3.01 cells, respectively (not shown). This was established by immunoblotting cell lysates for Lck before and after depletion of CD4 by immunoprecipitation. The relative amount of CD4 that is associated with Lck in SupT1 cells was found to be at least 85% (not shown). Thus, the majority of Lck molecules are normally complexed with CD4 in these cells and vice versa.
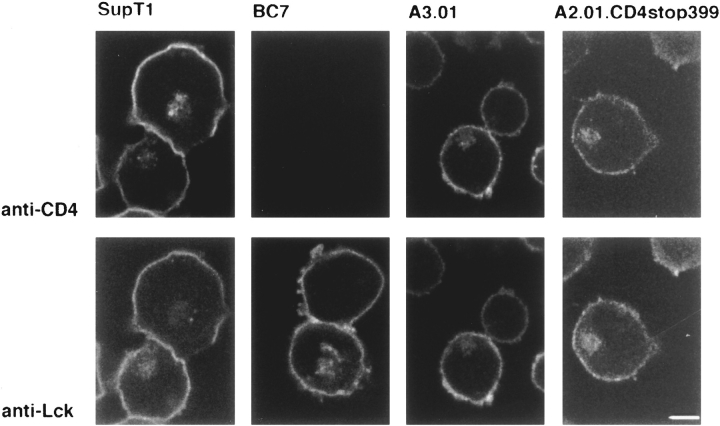
For immunofluorescence, T cells were immobilized on poly-l-lysine–coated coverslips, fixed, permeabilized, and stained with antibodies against the COOH-terminal domain of Lck and the NH2-terminal ecto-domain of CD4. In the CD4-positive cells SupT1 and A3.01, staining for Lck was only found in permeabilized cells and overlapped extensively with that of CD4 (Fig. 2), indicating that the majority of Lck was associated with the cytosolic side of the plasma membrane. The distribution of Lck in the absence of interacting CD4 was determined in BC7 and A2.01CD4stop399 cells. In both cell types Lck staining was again seen at the periphery of the cell. The clearest indication that this represents plasma membrane staining can be seen in A2.01-CD4stop399 cells, where Lck colocalizes with tailless CD4 (Fig. 2). The plasma membrane staining of Lck was evenly distributed in all cases and showed no apparent clustering. In addition to plasma membrane staining, some perinuclear staining was observed for Lck, both in the presence and absence of interactive CD4 (Fig. 2; the visibility of this staining depends on the plane of focus and is therefore not apparent in all the cell profiles in these fields). A similar perinuclear staining was previously described for Lck in Jurkat T cells and ascribed to late endosomes (Ley et al., 1994). By subcellular fractionation we found that this pool of Lck constitutes only a very minor fraction (<5%) of the total amount of Lck (not shown). CD4 staining was also found in the perinuclear area, partially overlapping the Lck staining (see Fig. 2, SupT1 cells). This localization may reflect the presence of CD4 in the exocytic pathway. We conclude that in T cells, Lck preferentially localizes to the plasma membrane independently of CD4 and, since CD8 is not expressed in A2.01CD4 stop399 cells, CD8.
Figure 2.
Distribution of Lck in T cells. Double indirect immunofluorescence staining of Lck and CD4 in the CD4-expressing T cell line SupT1 and its CD4-negative derivative, BC7, as well as for the CD4-positive T cell line A3.01 and its derivative A2.01-CD4stop399, that expresses CD4 without a cytoplasmic tail. Rabbit anti-Lck (Lck I) was detected with FITC-conjugated goat anti–rabbit antibodies, murine anti-CD4 (Q4120) with rhodamine-conjugated goat anti–mouse antibodies. Observation was by confocal microscopy of 3-μm-thick optical sections. Bar, 12.5 μm.
Distribution of Lck in NIH-3T3 Cells
In addition to CD4 and CD8, a number of other plasma membrane proteins, including CD2 (Bell et al., 1992; Carmo et al., 1993), CD5 (Raab et al., 1994), the IL-2 receptor (Hatakeyama et al., 1991), 4-1BB (Kim et al., 1993), and various glycosyl-phosphatidylinositol (GPI)–(Thy-1, CD48, CD55, and CD59) (Stefanova et al., 1991), have been shown to interact with Lck, albeit with low stoichiometry. To investigate whether any T cell–specific membrane protein is required for the cellular distribution of Lck, we stably transfected murine Lck cDNA into murine NIH-3T3 fibroblasts either with or without CD4. Neither CD4 nor Lck are normally expressed in these cells.
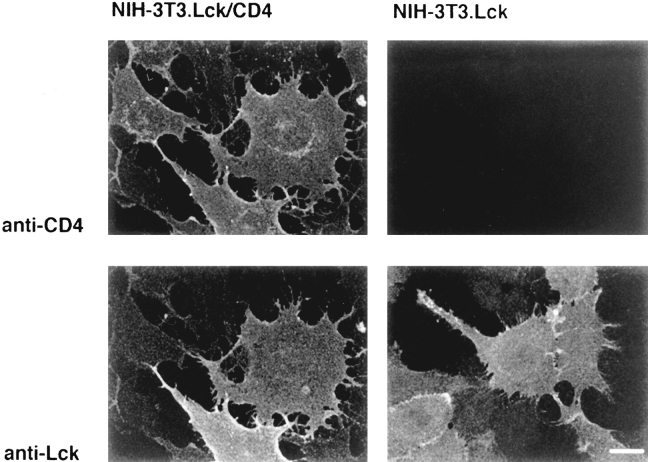
The amount of Lck associated with CD4 in the dual transfectants was found to be equivalent to that seen in T cells (not shown). Immunofluorescence staining of NIH3T3 cells that express CD4 and Lck showed colocalization of the two proteins at the plasma membrane (Fig. 3). As with the T cells, the plasma membrane staining of Lck in these flat fibroblastic cells was seen as a homogeneous, diffuse staining that was visible to the extremities of the cell. This staining was distinct from the cytosolic pattern seen with Lck mutants that are unable to bind to membranes (see Fig. 10). CD4 was also observed in a perinuclear pattern resembling that of Golgi-associated antigens and might represent CD4 in the exocytic pathway en route to the cell surface. When Lck was expressed in the absence of CD4, a homogeneous plasma membrane staining was again observed. This staining was indistinguishable from that seen in the presence of CD4 (Fig. 3). No clustering of Lck, indicative of association with plasma membrane microdomains, was apparent.
Figure 3.
Distribution of Lck in NIH-3T3 fibroblasts. Double indirect immunofluorescence staining of Lck and CD4 in fibroblasts either stably transfected with both CD4 and murine Lck (left) or with murine Lck alone (right). Primary and secondary antibodies were as described in the legend to Fig. 1. Optical sections (3 μm) were observed by confocal microscopy. Bar, 20 μm.
Figure 10.
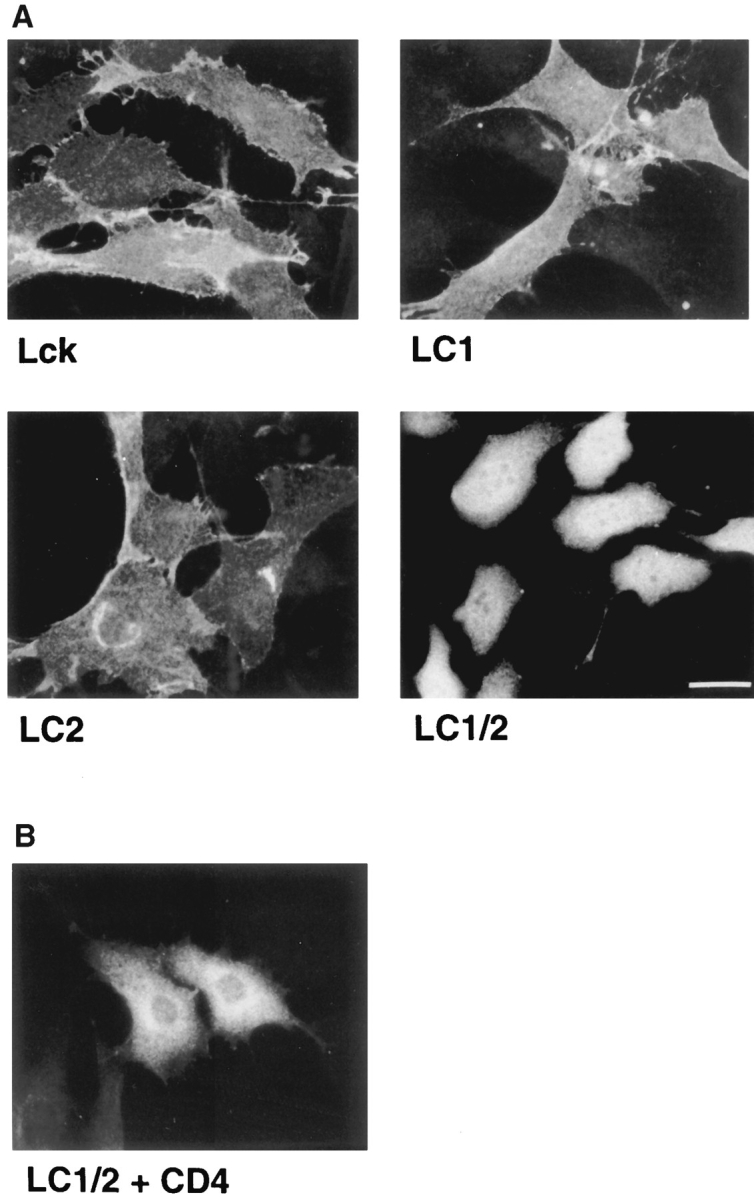
Cellular distribution of Lck palmitoylation mutants in NIH-3T3 cells. (A) NIH-3T3 cells were stably transfected with either wild-type Lck, LC1, LC2, or LC1/2 (see Fig. 1). Cells were stained by indirect immunofluorescence with an antibody against Lck (Lck I). (B) CD4-expressing NIH-3T3 cells were transfected with LC1/2 and stained for Lck with Lck I. Cells were observed by fluorescence microscopy. Bar, 25 μm.
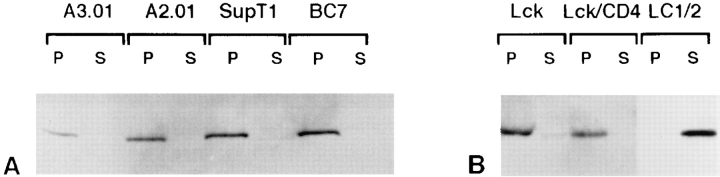
To quantitate the association of Lck with membranes in the presence and absence of CD4, CD4-positive and CD4negative T cell lines (Fig. 4 A) and NIH-3T3 cells (Fig. 4 B) were homogenized and fractionated into a 100,000-g membrane pellet and supernatant. Western blotting revealed that in all cell lines, Lck was exclusively present in the membrane fraction, independently of the presence of CD4. Association with CD4 therefore does not influence recruitment of Lck to membranes. Nonpalmitoylated Lck that does not associate with membranes (see Figs. 1 and 10) was found entirely in the supernatant fraction (Fig. 4 B).
Figure 4.
Membrane association of Lck in the presence and absence of CD4. Cells were broken by passage through a ball-bearing homogenizer. After removal of unbroken cells and nuclei, cellular membranes were recovered by centrifugation at 100,000 g (P, pellet) and separated from the soluble fraction (S, supernatant). For each cell line, samples representing equivalent amounts of cells were analyzed by SDS-PAGE and subsequent immunoblotting with the affinity-purified anti-Lck antibodies (Lck II). (A) T cell lines expressing CD4 (SupT1 and A3.01), tailless CD4 (A2.01) or no CD4 (BC7). (B) NIH-3T3 cells transfected with Lck alone, with both Lck and CD4, or with the palmitoylation mutant LC1/2.
We previously determined that the plasma membrane in BHK cells constitutes ∼18% of the total cellular membrane area (excluding inner mitochondria membranes [Griffiths et al., 1989]). As the surface areas of the membrane compartments in NIH-3T3 cells are likely to be similar to those in BHK cells, the exclusive presence of Lck at the plasma membrane suggests that Lck is specifically sorted to this compartment. This sorting appears to occur independently of the expression of T cell–specific proteins.
Distribution of Lck in HeLa Cells
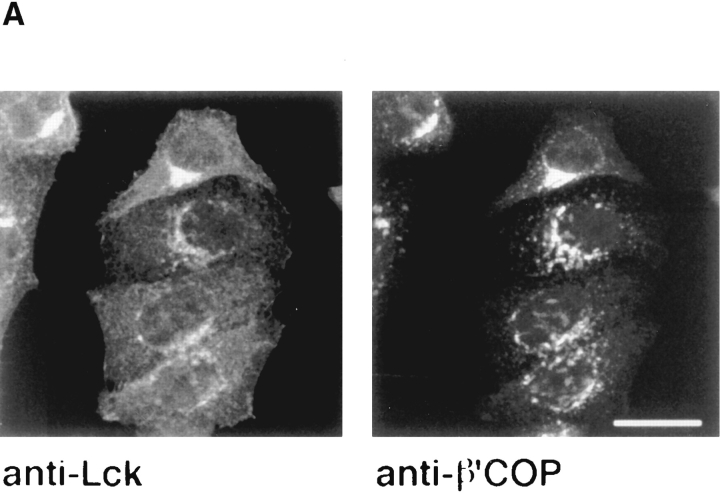
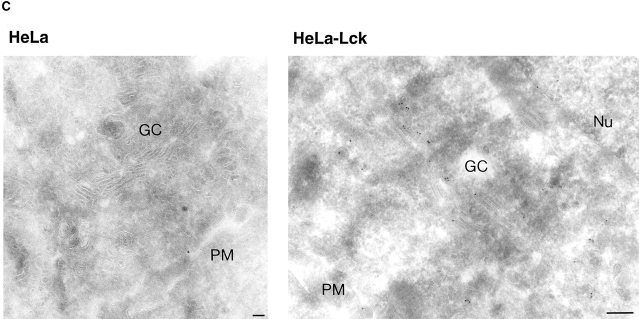
We stably expressed murine Lck in a second nonlymphoid cell line, HeLa. In these cells, Lck showed a distribution distinct from that seen in NIH-3T3 cells. The protein was not only located at the plasma membrane, but was also seen in the perinuclear region of the cells, in a pattern similar to that described for Golgi-associated proteins (Fig. 5 A). Costaining with an antibody against β′-COP indicated that this perinuclear staining of Lck overlapped with the Golgi complex (Fig. 5 A). This was confirmed by immunogold-electron microscopy. Frozen thin sections of HeLaLck cells, stained with anti-Lck antibodies and protein A gold showed label associated with elements of the Golgi apparatus (Fig. 5 C). The Golgi association was not a consequence of the heterologous expression of murine Lck in human cells, as it was also observed for human Lck in HeLa cells (Fig. 5 B). Nor was it due to overexpression of Lck, since high level expression driven by the cytomegalovirus immediate early promotor (Fig. 5) and moderate expression from the SV-40 early promotor showed similar distributions (not shown). The Golgi association was not unique to HeLa cells but was also observed when the protein was expressed in MDCK cells (not shown). It is possible that the cell type–specific variation in the cellular distribution of Lck reflects the involvement of cellular components that are present in T cells and NIH-3T3 cells but absent from HeLa and MDCK cells in establishing the distribution of Lck.
Figure 5.
Distribution of Lck in HeLa cells. (A) HeLa cells stably expressing murine Lck were double-stained with antibodies against Lck (Lck I) and β′-COP. Lck staining was detected with FITC-conjugated goat anti–rabbit antibodies, β′-COP staining with rhodamineconjugated goat anti–rat antibodies. (B) HeLa cells stably expressing human Lck were transfected with a cDNA encoding CD4. 2 d later, the cells were double-stained for Lck and CD4 (as in Fig. 1). Note the difference in distribution of Lck between cells that express CD4 (the four cells at the top) and cells that do not. Confocal images of a 3-μm optical section are shown. Bar, 20 μm. (C) Frozen thin sections of untransfected HeLa cells (left) or HeLa cells transfected with human Lck (right) were labeled with an antibody against Lck (Lck I) and as a second layer goat anti–rabbit IgG–conjugated gold (10 nm). Nu, nucleus; GC, Golgi complex; PM, plasma membrane. Bar, 0.1 μm.
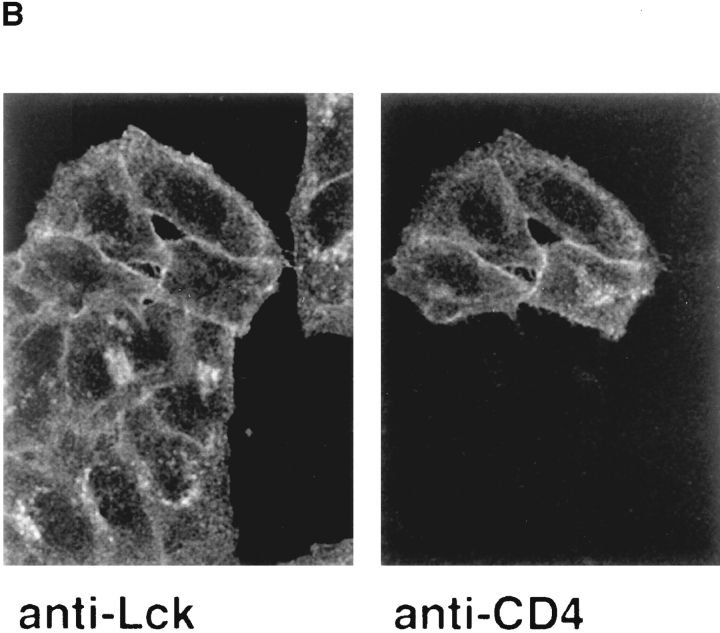
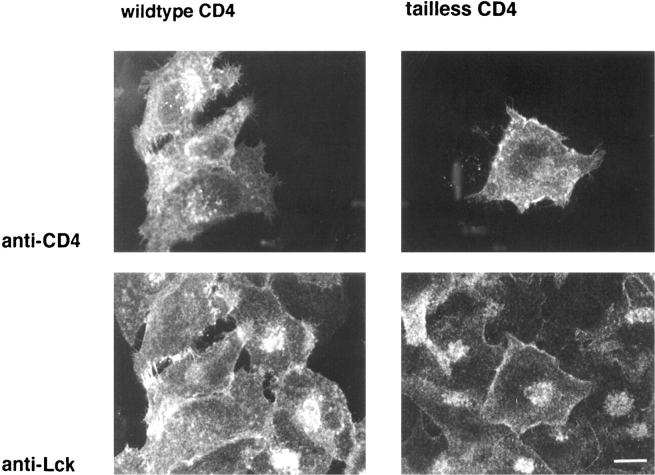
We investigated whether coexpression with CD4 would modulate the distribution of Lck. Hela-Lck cells were transfected with cDNA for human CD4 and analyzed 2 d later. We found that in the CD4-positive cells, Lck was exclusively located at the plasma membrane, while in adjacent CD4-negative cells, Lck was still observed in the Golgi region (Fig. 5 B). Similar results were observed for cells expressing either murine Lck or human Lck. Thus, association with CD4 either prevented deposition of Lck on Golgi membranes or induced redistribution of Lck that was already associated with the Golgi complex. To distinguish between these two possibilities, we expressed CD4 in HeLa-Lck cells by microinjection and analyzed the distribution of Lck soon after onset of CD4 synthesis. We observed that as early as 3 h after injection of cDNA for CD4, Golgi staining of Lck had disappeared and Lck was located only at the plasma membrane (Fig. 6, left). In contrast, injection of cDNA encoding a tailless form of CD4 did not change the distribution of Lck (Fig. 6, right). Previous experiments have estimated the half-life of Lck to be 20–30 h (Hurley and Sefton, 1989). The disappearance of the Golgi-associated Lck 3 h after microinjection of CD4 cDNA implies that newly synthesized CD4 can interact with previously synthesized Lck located on the Golgi membranes and facilitate the redistribution of this pool of Lck. Thus in HeLa cells, the interaction between Lck and CD4 can occur during transport of CD4 through the exocytic pathway.
Figure 6.
Microinjection of wild-type CD4 and tailless CD4 into Hela-Lck cells. HeLa cells stably expressing human Lck were microinjected with cDNA for wildtype CD4 (left) or tailless CD4 (right). Cells were fixed 3 h after injection and stained with antibodies against Lck and CD4 (as in Fig. 1). Note the difference in distribution of Lck between cells that were injected with wild-type CD4 cDNA (left, the three cells on the left) and cells that were not injected. Also note that injection with tailless CD4 cDNA has no effect on the distribution of Lck (right, the cell in the middle). Confocal images of 3-μm optical sections are shown. Bar, 15 μm.
The Role of the Unique Domain of Lck
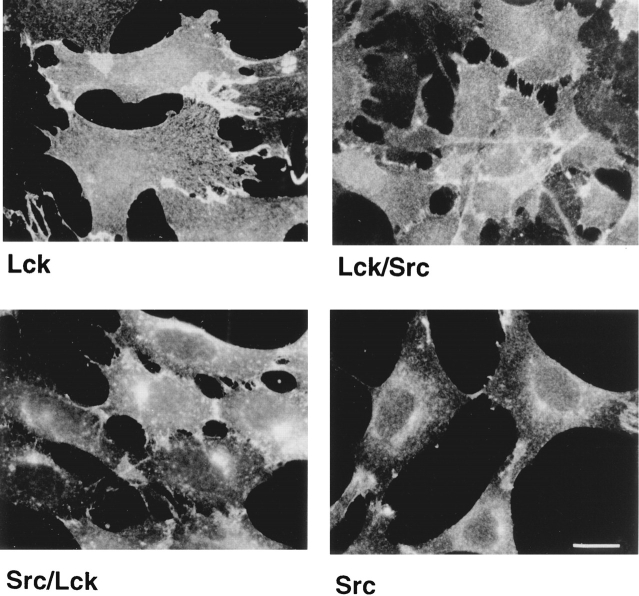
To determine the role of the unique domain of Lck in targeting to the plasma membrane, we studied the cellular distribution of chimeras between Lck and c-Src. These chimeras contain either the unique domain of murine Lck and the remainder of c-Src (Lck/Src) or the unique domain of c-Src and the remainder of Lck (Src/Lck) (Fig. 1) (Turner et al., 1990). The unique domains of Lck and c-Src are not homologous apart from the signal for myristoylation at the extreme NH2 terminus (Resh, 1993 and 1994). c-Src is modified by myristoylation only but contains an additional polybasic region that enhances membrane association (Silverman and Resh, 1992). In contrast, Lck is modified with both myristic and palmitic acid (Paige et al., 1993).
In cells expressing c-Src we observed the protein at the plasma membrane and in the perinuclear region (Fig. 7), a distribution clearly different from that of Lck (Fig. 7). A similar perinuclear distribution of c-Src was previously described (Kaplan et al., 1992) and attributed to association with late endosomes. The distribution of the Src/Lck chimera was different from that of Lck despite containing 88% of the Lck protein sequence (Fig. 7). The staining pattern for Src/Lck was more punctate than that of Lck and concentrated on one side of the nucleus. This distribution also differs from that of Src, but the specific location of this chimera remains to be identified. Nevertheless, it is clear that the presence of SH2, SH3, and kinase domains of Lck do not target this chimera exclusively to the plasma membrane. In contrast, the Lck/Src chimera was mainly found at the plasma membrane (Fig. 7) identical to Lck. Thus, the unique domain of Lck seems sufficient to target heterologous Src homology domains to the plasma membrane in NIH-3T3 cells.
Figure 7.
Cellular distribution of chimeras between Lck and Src. NIH-3T3 fibroblasts were stably transfected either with Lck, c-Src, Lck/Src, or Src/Lck as indicated. Src/Lck contains the unique domain of c-Src and the remainder of Lck, while Lck/Src contains the unique domain of Lck and the remainder of c-Src (see Fig. 1). Lck- and Src/Lck-expressing cells were stained with Lck I, and Src- and Lck/Src-expressing cells were stained with the anti-Src mAb 327. Cells were observed by fluorescence microscope. Bar, 20 μm.
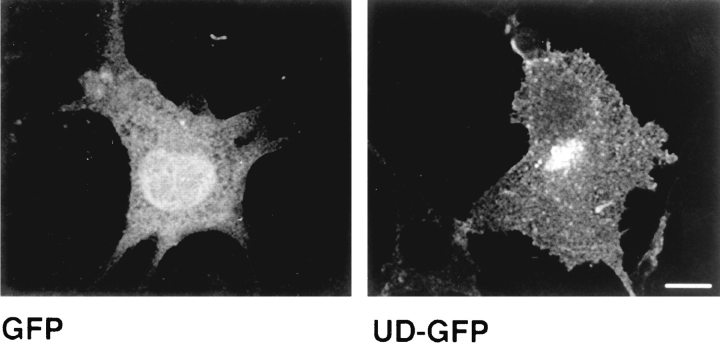
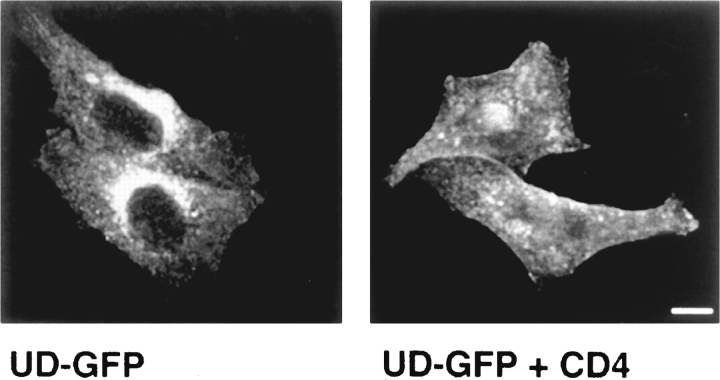
A similar result was obtained with a second chimera, composed of the unique domain of Lck linked to the NH2 terminus of the GFP of Aequoria victoria. cDNA's for this chimera (designated UD-GFP) and GFP were transfected separately into NIH-3T3 cells. The cells were fixed 2 d later and observed by fluorescence microscopy. We found that GFP localized to the cytoplasm and nucleus as described previously (Fig. 8) (Ogawa et al., 1995). However, UD-GFP was located at the plasma membrane, although some protein was observed in the perinuclear region as well (Fig. 8). In HeLa cells, UD-GFP again behaved similarly to Lck. In the absence of CD4 the protein was found at the plasma membrane and in the Golgi region (Fig. 9). When transfected into CD4-expressing cells, UD-GFP was located predominantly at the plasma membrane (Fig. 9). However, the extent of redistribution in the presence of CD4 was slightly less for UD-GFP than for Lck, as some internal staining of UD-GFP remained in CD4-expressing cells. Association of UD-GFP with CD4 could be demonstrated by coimmunoprecipitation with anti-CD4 antibodies (not shown).
Figure 8.
Cellular distribution of GFP and UDGFP in NIH-3T3 cells. NIH-3T3 cells were transfected either with GFP or UD-GFP, a chimera composed of the unique domain of Lck linked to GFP. Cells were fixed 2 d later and observed by fluorescent confocal microscopy. Note the plasma membrane distribution of UD-GFP as opposed to the cytosolic distribution of GFP. Confocal images of 3-μm optical sections are shown. Bar, 15 μm.
Figure 9.
Cellular distribution of UD-GFP in HeLa cells. UD-GFP was transfected into HeLa cells (left) or into HeLa cells that express CD4 (right). Cells were fixed 2 d later and 3-μm optical sections were observed by confocal microscopy. Bar, 10 μm.
We conclude that despite the potential of the other domains for extensive protein–protein interaction, the cellular distribution of Lck is determined primarily by its NH2terminal unique domain.
The Role of Palmitoylation
A short motif (SH4) at the NH2 terminus of Src-family kinases contains signals for myristoylation and palmitoylation (Fig. 1). Myristic acid is attached to an NH2-terminal glycine after removal of the methionine at position 1 (Fig. 10). In agreement with previous reports (Turner et al., 1990; Kwong and Lublin 1995), we found that the absence of both palmitoylation attachment sites in the Lck mutant LC1/2 (Fig. 1) localized the protein to the cytosol in stably transfected NIH-3T3 cells (Fig. 10 A), indicating that myristoylation alone is not sufficient for membrane association. Membrane association was also not detected by cell fractionation (Fig. 4 B). We further found that LC1/2 also localized to the cytosol when expressed in the presence of CD4 (Fig. 10 B). Thus, the interaction of Lck with CD4 requires initial membrane association of Lck.
Palmitoylation occurs on cysteines in the general motif Met-Gly-Cys, where Gly 2 is myristoylated (Resh, 1994; Milligan et al., 1995). In addition to Cys 3, Cys 5 in Lck and Cys 6 in Fyn (Alland et al., 1994) are also palmitate acceptor sites. For Lck, conflicting results have been reported on the relative contribution of the two cysteines to palmitoylation (Rodgers et al., 1994; Shenoy-Scaria et al., 1994). The constructs LC1 and LC2, with either one or the other palmitoylation site deleted (Fig. 1), both localized to the plasma membrane (Fig. 10 A) in stably transfected NIH-3T3 cells as was shown before by others (Yurchak et al., 1995). However, we observed a striking difference in distribution between LC1 and LC2: while the localization of LC1 was indistinguishable from that of wild-type Lck, LC2 was found at the plasma membrane and in the perinuclear region. The perinuclear staining of LC2 overlapped with that of β′-COP (not shown), suggesting that this mutant was associated with the Golgi complex in NIH-3T3 cells. A difference in localization between palmitoylation mutants was also observed by Yurchak et al. (1995). However, they found that in stably transfected rat 208F fibroblasts, a mutant without Cys 5 (=LC2) localized to a higher extent at the plasma membrane than wild-type Lck or a mutant that lacks Cys 3 (=LC1). Lck and the Cys 3 mutant localized partially to the perinuclear region in their experiments (Yurchak et al., 1995), but this staining does not resemble the Golgi staining described here for LC2. We do not know the cause for this discrepancy other than that cell type–specific differences might influence localization of Lck. Nevertheless, both sets of data show that the position of the palmitic acid can influence the association of Lck with specific membrane compartments.
Discussion
The tyrosine kinase Lck primarily associates with the cytosolic side of the plasma membrane in T cells. Here, we have studied the subcellular distribution of wild-type and mutant Lck in lymphoid and nonlymphoid cells to identify determinants that influence the localization of this protein. In particular, we have studied the contribution of CD4, the unique domain of Lck and palmitoylation (see Table I for summary).
Table I.
The Subcellular Distribution of Lck and Lck Constructs in T Cells and Transfected Cells
| T Cells | NIH-3T3 | HeLa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4 | − | + | − | + | − | + | ||||||
| Lck | Plasma membrane | Plasma membrane | Plasma membrane | Plasma membrane | Plasma membrane + Golgi | Plasma membrane | ||||||
| Lck/Src | — | — | Plasma membrane | Plasma membrane* | — | — | ||||||
| Src/Lck | — | — | Perinuclear | — | — | |||||||
| Src | — | — | Perinuclear | — | — | |||||||
| UD-GFP | — | — | Plasma membrane | — | Plasma membrane + Golgi | Plasma membrane | ||||||
| LC1 | — | — | Plasma membrane | — | — | — | ||||||
| LC2 | — | — | Plasma membrane + Golgi | — | — | — | ||||||
| LC1/2 | — | — | Cytosol | Cytosol | — | — | ||||||
Data not shown.
The association of Lck with CD4 and CD8, coreceptors of the TcR in helper and cytotoxic T cells, respectively, is well established. In the cells used in this study, at least 60– 70% of Lck is associated with CD4. Cysteine motifs in Lck and in CD4 (or CD8) are essential for the Lck-CD4 association; however, the precise nature of the interaction and where in the cell it is established is not understood. It has been shown that Lck can associate with CD4 at the ER, when CD4 is held in this compartment through association with an ER-retained HIV-gp120 (Crise and Rose, 1992). Furthermore, under conditions where Lck is overexpressed together with a construct containing the cytoplasmic tail of CD4, Lck was found to associate with an endo H–sensitive form of this construct (Shaw et al., 1989). These observations suggest that Lck and CD4 might interact early in the exocytic pathway and subsequently move together through the constitutive exocytic route to the plasma membrane. Consequently, CD4 could have an active role in establishing the cellular distribution of Lck. However, we found that in T cells, neither CD4 nor CD8 are required for targeting Lck to the plasma membrane.
In addition to CD4 and CD8, other T cell–specific transmembrane proteins such as CD2 (Bell et al., 1992; Carmo et al., 1993), CD5 (Raab et al., 1994), CD28 (August and Dupont, 1994), the IL-2 receptor (Hatakeyama et al., 1991), the IL-7 receptor (Page et al., 1995), and 4-1BB (Kim et al., 1993) associate with Lck, albeit with a lower stochiometry. The finding that Lck expressed in NIH-3T3 cells is also located primarily at the plasma membrane indicated that T cell–specific proteins are not required for its targeting.
Lck has also been shown to associate with various GPIlinked proteins, including CD48, CD55, CD59, and Thy-1 (Stefanova et al., 1991). A role for these ubiquitously expressed proteins in Lck targeting cannot be excluded at present. However, it should be noted that the association with GPI-linked proteins is not specific for Lck, but also occurs for other Src-family members that have different subcellular distributions to Lck (Shenoy et al., 1993; Stefanova et al., 1991; Ley et al., 1994). Furthermore, the interaction of Src-family kinases with GPI-linked proteins is not well understood and may reflect an ability of both GPI-linked and myristoylated/palmitoylated proteins to partition with specific membrane lipids under certain conditions rather than a direct interaction. For one of the GPI– linked proteins, CD59, we used anti-CD59 antibodies to induce cap formation on the CD4-negative T cells BC7. We failed to see cocapping of Lck, suggesting that the interaction between these two proteins in living cells is minimal (not shown). In contrast, cocapping of Lck with CD4 was observed (our unpublished data and Ley et al., 1994).
As far as the individual domains of Lck are concerned, we found that the unique domain of Lck is important for the subcellular distribution of the protein. A chimera of Lck and c-Src, in which the unique domain of c-Src was substituted for that of Lck, localized exclusively to the plasma membrane. In contrast, c-Src itself localizes to the plasma membrane and perinuclear region (Fig. 7). In addition, a chimera composed of the unique domain of Lck and GFP (UD-GFP) also localized to the plasma membrane, while GFP was found in the cytosol and nucleus (Fig. 8). Thus, the SH2 and SH3 domains do not appear to contain targeting information that is relevant under the conditions of these experiments. This is in contrast to v-Src, where the SH2 domain was found to be important for its localization to focal adhesion sites (Okamura and Resh, 1994), and the signaling molecules phospholipase Cγ and GRB2 where the SH3 domain is crucial for localizing to the actin cytoskeleton (Bar et al., 1993).
In HeLa and MDCK cells we found Lck located in the Golgi region and at the plasma membrane. Microinjection of a cDNA encoding Lck-interactive forms of CD4 led to the loss of Golgi-associated Lck within 3 h, indicating that newly synthesized CD4 can interact with this pool of Lck and presumably facilitate its transport to the cell surface. The notion that CD4 can provide a dominant signal for Lck localization may also explain why ER-retained CD4 can cause Lck to be located on the ER (Crise and Rose, 1992). Currently, we do not know why wild-type Lck associates with the Golgi in HeLa and MDCK cells but not in T cells or NIH 3T3 cells. One hypothesis is that T cells and NIH-3T3 cells express proteins that, in the absence of CD4, allow efficient transport of Lck to the plasma membrane and that these proteins are missing or limited in HeLa and MDCK cells. However, it is also possible that in HeLa and MDCK cells, Lck is retained in the Golgi complex due to aberrant interactions with Golgi components. Fusing NIH-3T3 cells with Lck-expressing HeLa cells might allow us to discriminate between these possibilities. The Golgi localization in HeLa cells might imply that newly synthesised Lck is initially directed to an intracellular membrane system and then transported to the plasma membrane on vesicular intermediates either with or without CD4/CD8.
In NIH-3T3 cells we also observed a Golgi distribution for an Lck construct that has a mutation of the palmitoylation site at Cys 5. A mutant with a disruption of the first palmitoylation site (Cys 3) showed a similar distribution to that of wild-type Lck (Fig. 10 A). We assume that these mutants were palmitoylated since they associated with membranes and either Cys 3 or Cys 5 can be used for palmitoylation (Kwong and Lublin, 1995; Rodgers et al., 1994; Yurchak and Sefton, 1995). One explanation for the difference in localization between the two Lck mutants could be that the use of different palmitoylation sites predisposes the protein to interact with the glycolipid domains that have been proposed to mediate sorting in the trans-Golgi network (Yoshimori et al., 1996). Some interaction of Lck with glycolipid domains has been reported (Shenoy et al., 1993; Rodgers et al., 1994), but the significance of this association remains to be determined. The position of the palmitic acids on Lck may also influence recognition by proteins that are involved in sorting Lck to the plasma membrane. Whether parts of the unique domain other than the SH4 region influence the distribution of Lck remains to be examined. The Golgi localization of LC2 again implies that Lck could normally associate transiently with elements of the exocytic pathway before being deposited at the plasma membrane, while inefficient transport results in retention of Lck on the Golgi complex.
Palmitoylation is crucial for stable association of Lck with membranes. So far, all palmitoyl transferase activities identified are membrane associated and, in some cases, have been localized to parts of the exocytic pathway. A palmitoyl transferase activity that acylates newly synthesized transmembrane proteins has been localized to the intermediate compartment (Bonatti et al., 1989). Furthermore, the cytosolic peripheral membrane protein glutamic acid decarboxylase (GAD65), requires targeting to the Golgi region for palmitoylation (Solimena et al., 1994). Recently, a palmitoyl transferase that is specific for myristoylated proteins and palmitoylates Fyn in vitro was partially purified from bovine brain membranes (Berthiaume and Resh, 1995). Together, these data imply that Lck is initially targeted to a membrane system, possibly belonging to the exocytic pathway, where palmitoylation occurs. After membrane binding, Lck moves to the plasma membrane. How Lck is sorted to the plasma membrane rather than other cellular membrane systems remains to be determined. However, the studies reported here provide a basis on which these mechanisms can start to be unravelled.
Acknowledgments
We thank colleagues in the Medical Research Council Laboratory for Molecular Cell Biology, for helpful discussions, Mark Shipman for assistance with confocal microscopy, Kate Nobes for instruction in microinjection, and Annegret Pelchen-Matthews for critically reading the manuscript. John Tite (GlaxoWellcome plc) provided constant support, and Dan Littman and Steve Ley provided crucial reagents.
This work was supported by Wellcome Foundation plc, the Leukaemia Research Campaign, the Medical Research Council, and by a long term European Molecular Biology Organization fellowship to M.-J.J.E. Bijlmakers.
Abbreviations used in this paper
- GFP
green fluorescent protein
- Lck
Src-family protein tyrosine kinase p56lck
- TcR
T cell antigen receptor
- UD
unique domain
Footnotes
Address all correspondence to Mark Marsh, MRC Laboratory for Molecular Cell Biology and Department of Biochemistry, University College London, Gower Street, London WC1E 6BT, United Kingdom. Tel.: (0044) 0171 380 7807. Fax: (0044) 0171 380 7805. E-mail: m.marsh@ucl.ac.uk
References
- Alland L, Peseckis SM, Atherton RE, Berthiaume L, Resh MD. Dual myristylation and palmitylation of Src family member p59fyn affects subcellular localization. J Biol Chem. 1994;24:16701–16705. [PubMed] [Google Scholar]
- August A, Dupont B. Activation of Src family kinase Lck following CD28 crosslinking in the Jurkat leukemic cell line. Biochem Biophys Res Commun. 1994;199:1466–1473. doi: 10.1006/bbrc.1994.1396. [DOI] [PubMed] [Google Scholar]
- Bar SD, Rotin D, Batzer A, Mandiyan V, Schlessinger J. SH3 domains direct cellular localization of signaling molecules. Cell. 1993;74:83–91. doi: 10.1016/0092-8674(93)90296-3. [DOI] [PubMed] [Google Scholar]
- Bell GM, Bolen JB, Imboden JB. Association of Src-like protein tyrosine kinases with the CD2 cell surface molecule in rat T lymphocytes and natural killer cells. Mol Cell Biol. 1992;12:5548–5554. doi: 10.1128/mcb.12.12.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthiaume L, Resh MD. Biochemical characterization of a palmitoyl acyltransferase activity that palmitoylates myristoylated proteins. J Biol Chem. 1995;270:22399–22405. doi: 10.1074/jbc.270.38.22399. [DOI] [PubMed] [Google Scholar]
- Bonatti S, Migliaccio G, Simons K. Palmitylation of viral membrane glycoproteins takes place after exit from the endoplasmic reticulum. J Biol Chem. 1989;264:12590–12595. [PubMed] [Google Scholar]
- Carmo AM, Mason DW, Beyers AD. Physical association of the cytoplasmic domain of CD2 with the tyrosine kinases p56lck and p59fyn. Eur J Immunol. 1993;23:2196–2201. doi: 10.1002/eji.1830230922. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Howell B. The when and how of Src regulation. Cell. 1993;73:1051–1054. doi: 10.1016/0092-8674(93)90634-3. [DOI] [PubMed] [Google Scholar]
- Crise B, Rose JK. Human immunodeficiency virus type 1 glycoprotein precursor retains a CD4-p56lckcomplex in the endoplasmic reticulum. J Virol. 1992;66:2296–2301. doi: 10.1128/jvi.66.4.2296-2301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres MJA, Clapham PR, Marsh M, Ahuja M, Davis-Turner J, McKnight A, Thomas J, Stoebenau-Haggarty B, Choe S, Vance PJ, et al. CD4-independent infection by HIV–2 is mediated by fusin/CXCR4. Cell. 1996;877:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- Ercolani L, Stow JL, Boyle JF, Holtzman EJ, Lin H, Grove JR, Ausiello DA. Membrane localization of the pertussis toxin-sensitive G-protein subunit αi-2 and αi-3 and expression of a metallothionein-αi-2 fusion gene in LLC-PK1 cells. Proc Natl Acad Sci USA. 1990;87:4635–4639. doi: 10.1073/pnas.87.12.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folks T, Benn S, Rabson A, Theodore T, Hoggan MD, Martin M, Lightfoote M, Sell K. Characterization of a continuous T-cell line susceptible to the cytopathic effects of the acquired immunodeficiency syndrome (AIDS)-associated retrovirus. Proc Natl Acad Sci USA. 1985;82:4539–4543. doi: 10.1073/pnas.82.13.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaichenhaus N, Shastri N, Littman DR, Turner JM. Requirement for association of p56lck with CD4 in antigen-specific signal transduction in T cells. Cell. 1991;64:511–520. doi: 10.1016/0092-8674(91)90235-q. [DOI] [PubMed] [Google Scholar]
- Griffiths G, Back R, Marsh M. A quantitative analysis of the endocytic pathway in baby hamster kidney cells. J Cell Biol. 1989;109:2703–2720. doi: 10.1083/jcb.109.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison-Lavoie KJ, Lewis VA, Hynes GM, Collison KS, Nutland E, Willison KR. A 102 kDa subunit of a Golgi-associated particle has homology to β subunits of trimeric G proteins. EMBO (Eur Mol Biol Organ) J. 1993;12:2847–2853. doi: 10.1002/j.1460-2075.1993.tb05946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama M, Kono T, Kobayashi N, Kawahara A, Levin SD, Perlmutter RM, Taniguchi T. Interaction of the IL-2 receptor with the srcFamily kinase p56lck: identification of novel intermolecular association. Science (Wash DC) 1991;252:1523–1528. doi: 10.1126/science.2047859. [DOI] [PubMed] [Google Scholar]
- Heim, R., A.B. Cubitt, and R.Y. Tsien. 1995. Improved green fluorescence. Nature (Lond.). 373:663–664. (Letter.) [DOI] [PubMed]
- Hurley TR, Sefton BM. Analysis of the activity and phosphorylation of the Lck protein in lymphoid cells. Oncogene. 1989;4:265–272. [PubMed] [Google Scholar]
- Kaplan K, Swedlow JR, Varmus HE, Morgan DO. Association of p60c-src with endosomal membranes in mammalian fibroblasts. J Cell Biol. 1992;118:321–333. doi: 10.1083/jcb.118.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-J, Pollok KE, Zhou Z, Shaw A, Bohlen JB, Fraser M, Kwon BS. Novel T cell antigen 4-1BB associates with the protein tyrosine kinase p56lck. J Immunol. 1993;151:1255–1262. [PubMed] [Google Scholar]
- Kinch MS, Sanfridson A, Doyle C. The protein tyrosine kinase p56(lck) regulates cell adhesion mediated by CD4 and major histocompatibility complex class II proteins. J Exp Med. 1994;180:1729–1739. doi: 10.1084/jem.180.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegl M, Zlatkine P, Ley SC, Courtneidge SA, Magee AI. Palmitoylation of multiple Src-family kinases at a homologous N-terminal motif. Biochem J. 1994;303:749–753. doi: 10.1042/bj3030749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong J, Lublin DM. Amino-terminal palmitate or polybasic domain can provide required second signal for membrane binding of p56lck . Biochem Biophys Res Commun. 1995;207:868–876. doi: 10.1006/bbrc.1995.1266. [DOI] [PubMed] [Google Scholar]
- Levin SD, Anderson SJ, Forbush KA, Perlmutter RM. A dominant-negative transgene defines a role for p56lck in thymopoiesis. EMBO (Eur Mol Biol Organ) J. 1993;12:1671–1680. doi: 10.1002/j.1460-2075.1993.tb05812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley SC, Marsh M, Bebbington CR, Proudfoot K, Jordan P. Distinct intracellular localization of Lck and Fyn protein tyrosine kinases in human T lymphocytes. J Cell Biol. 1994;125:639–649. doi: 10.1083/jcb.125.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou W, Slot JW. Improved fine structure in immunolabeled cryosections after modifying the sectioning and pick-up conditions. Proc Int Conf Elect Microsc. 1994;13:253–254. [Google Scholar]
- Louie RR, King CS, Macauley A, Marth JD, Perlmutter RM, Eckhart W, Cooper JA. p56lck protein-tyrosine kinase is cytoskeletal and does not bind to polyomavirus middle T antigen. J Virol. 1988;62:4673–4679. doi: 10.1128/jvi.62.12.4673-4679.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth JD, Peet R, Krebs EG, Perlmutter RM. A lymphocytespecific protein tyrosine kinase gene is rearranged and overexpressed in the murine T cell lymphoma LSTRA. Cell. 1985;43:393–404. doi: 10.1016/0092-8674(85)90169-2. [DOI] [PubMed] [Google Scholar]
- Milligan G, Parenti M, Magee AI. The dynamic role of palmitoylation in signal transduction. Trends Biochem Sci. 1995;20:181–186. doi: 10.1016/s0968-0004(00)89004-0. [DOI] [PubMed] [Google Scholar]
- Mohn H, Le-Cabec V, Fischer S, Maridonneau-Parini ITI. The Src-family protein-tyrosine kinase p59hck is located on the secretory granules in human neutrophils and translocates towards the phagosome during cell activation. Biochem J. 1995;15:657–665. doi: 10.1042/bj3090657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina TJ, Kishihara K, Siderovski DP, van Ewijk W, Narendran A, Timms E, Wakeham A, Paige CJ, Hartmann K-U, Veillette A, et al. Profound block in thymocyte development in mice lacking p56lck. Nature (Lond) 1992;357:161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- Morgenstern JP, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nada S, Okada M, MacAuley A, Cooper JA, Nakagawa H. Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature (Lond) 1991;351:69–72. doi: 10.1038/351069a0. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Inouye S, Tsuji FI, Yasuda K, Umesono K. Localization, trafficking, and temperature-dependence of the Aequoreagreen fluorescent protein in cultured vertebrate cells. Proc Natl Acad Sci USA. 1995;92:11899–11903. doi: 10.1073/pnas.92.25.11899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura H, Resh MD. Differential binding of pp60c-src and pp60v-src to cytoskeleton is mediated by SH2 and catalytic domains. Oncogene. 1994;9:2293–2303. [PubMed] [Google Scholar]
- Ostergaard HL, Shackelford DA, Hurley TR, Johnson P, Hyman R, Sefton BM, Trowbridge IS. Expression of CD45 alters phosphorylation of the Lck-encoded tyrosine protein kinase in murine lymphoma T-cell lines. Proc Natl Acad Sci USA. 1989;86:8959–8963. doi: 10.1073/pnas.86.22.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page TH, Lali FV, Foxwell BM. Interleukin-7 activates p56lck and p59fyn, two tyrosine kinases associated with the p90 interleukin-7 receptor in primary human T cells. Eur J Immunol. 1995;25:2956–2960. doi: 10.1002/eji.1830251036. [DOI] [PubMed] [Google Scholar]
- Paige LA, Nadler MJS, Harrison ML, Cassady JM, Geahlen RL. Reversible palmitoylation of the protein-tyrosine kinase p56lck. J Biol Chem. 1993;268:8669–8674. [PubMed] [Google Scholar]
- Pawson T. Protein modules and signalling networks. Nature (Lond) 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- Pelchen-Matthews A, Boulet I, Littman DR, Fagard R, Marsh M. The protein tyrosine kinase p56lck inhibits CD4 endocytosis by preventing entry of CD4 into coated pits. J Cell Biol. 1992;117:279–290. doi: 10.1083/jcb.117.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasher DC, Eckenrode VK, Ward WW, Prendergast FG, Cormier MJ. Primary structure of the Aequorea victoriagreen-fluorescent protein. Gene. 1992;111:229–233. doi: 10.1016/0378-1119(92)90691-h. [DOI] [PubMed] [Google Scholar]
- Raab M, Yamamoto M, Rudd CE. The T-cell antigen CD5 acts as a receptor and substrate for the protein-tyrosine kinase p56lck. Mol Cell Biol. 1994;14:2862–2870. doi: 10.1128/mcb.14.5.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameh LE, Chen CS, Cantley LC. Phosphatidylinositol (3,4,5)P3 interacts with SH2 domains and modulates PI-3 kinase association with tyrosine phosphorylated proteins. Cell. 1995;83:821–830. doi: 10.1016/0092-8674(95)90195-7. [DOI] [PubMed] [Google Scholar]
- Resh MD. Interaction of tyrosine kinase oncoproteins with cellular membranes. Biochim Biophys Acta. 1993;1155:307–322. doi: 10.1016/0304-419x(93)90012-2. [DOI] [PubMed] [Google Scholar]
- Resh MD. Myristylation and palmitylation of Src family members: the fats of the matter. Cell. 1994;76:411–413. doi: 10.1016/0092-8674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Rodgers W, Crise B, Rose JK. Signals determining protein tyrosine kinase and glycosyl-phosphatidylinositol-anchored protein targeting to a glycolipid-enriched membrane fraction. Mol Cell Biol. 1994;14:5384–5391. doi: 10.1128/mcb.14.8.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider LR. Adhesion plaques of Rous sarcoma virus–transformed cells contain the src gene product. Proc Natl Acad Sci USA. 1980;77:3514–3518. doi: 10.1073/pnas.77.6.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd CE, Janssen O, Prasad KVS, Raab M, da Silva A, Telfer JC, Yamamoto M. Src-related protein tyrosine kinases and their surface receptors. Biochim Biophys Acta. 1993;1155:239–266. doi: 10.1016/0304-419x(93)90007-y. [DOI] [PubMed] [Google Scholar]
- Rudd CE, Trevillyan JM, Dasgupta JD, Wong LL, Schlossman SF. The CD4 receptor is complexed in detergent lysates to a proteintyrosine kinase (pp58) from human T lymphocytes. Proc Natl Acad Sci USA. 1988;85:5190–5194. doi: 10.1073/pnas.85.14.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AS, Amrein KE, Hammond C, Stern DF, Sefton BM, Rose JK. The Lck tyrosine protein kinase interacts with the cytoplasmic tail of the CD4 glycoprotein through its unique amino-terminal domain. Cell. 1989;59:627–636. doi: 10.1016/0092-8674(89)90008-1. [DOI] [PubMed] [Google Scholar]
- Shaw AS, Chalupny J, Whitney JA, Hammond C, Amrein KE, Kavathas P, Sefton BM, Rose JK. Short related sequences in the cytoplasmic domains of CD4 and CD8 mediate binding to the amino-terminal domain of the p56lck tyrosine protein kinase. Mol Cell Biol. 1990;10:1853–1862. doi: 10.1128/mcb.10.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy-Scaria AM, Gauen LK, Kwong J, Shaw AS, Lublin DM. Palmitylation of an amino-terminal cysteine motif of protein tyrosine kinases p56lck and p59fyn mediates interaction with glycosyl-phosphatidylinositol-anchored proteins. Mol Cell Biol. 1993;13:6385–6392. doi: 10.1128/mcb.13.10.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy-Scaria AM, Dietzen DJ, Kwong J, Link DC, Lublin DM. Cysteine3 of Src family protein tyrosine kinases determines palmitoylation and localization in caveolae. J Cell Biol. 1994;126:353–363. doi: 10.1083/jcb.126.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieh M, Bolen JB, Weiss A. CD45 specifically modulates binding of Lck to a phosphopeptide encompassing the negative regulatory tyrosine of Lck. EMBO (Eur Mol Biol Organ) J. 1993;12:315–321. doi: 10.1002/j.1460-2075.1993.tb05659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman L, Resh MD. Lysine residues form an integral component of a novel NH2-terminal membrane targeting motif for myristoylated pp60v-src. J Cell Biol. 1992;119:415–425. doi: 10.1083/jcb.119.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot JW, Geuze HJ, Gigengack S, Lienhard GE, James DE. Immunolocalization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol. 1991;113:123–135. doi: 10.1083/jcb.113.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solimena M, Dirkx R, Jr, Radzynski M, Mundigl O, De Camilli P. A signal located within amino acids 1-27 of GAD65 is required for its targetting to the Golgi complex region. J Cell Biol. 1994;126:331–341. doi: 10.1083/jcb.126.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova I, Horejsi V, Ansotegui IJ, Knapp W, Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science (Wash DC) 1991;254:1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- Straus DB, Weiss A. Genetic evidence for the involvement of the Lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- Turner JM, Brodsky MH, Irving BA, Levin SD, Perlmutter RM, Littman DR. Interaction of the unique N-terminal region of tyrosine kinase p56lck with cytoplasmic domains of CD4 and CD8 is mediated by cysteine motifs. Cell. 1990;60:755–765. doi: 10.1016/0092-8674(90)90090-2. [DOI] [PubMed] [Google Scholar]
- Veillette A, Bookman MA, Horak EM, Bolen JB. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988;55:301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Willison K, Lewis V, Zuckerman KS, Cordell J, Dean C, Miller K, Lyon MF, Marsh M. The t-complex polypeptide-1 (TCP-1) is associated with the cytoplasmic aspect of Golgi membranes. Cell. 1989;57:621–632. doi: 10.1016/0092-8674(89)90131-1. [DOI] [PubMed] [Google Scholar]
- Wills JW, Craven RC. Form, function, and use of retroviral Gag proteins. AIDS. 1991;5:639–654. doi: 10.1097/00002030-199106000-00002. [DOI] [PubMed] [Google Scholar]
- Yoshimori T, Keller P, Roth M, Simons K. Different biosynthetic transport routes to the plasma membrane in BHK and CHO cells. J Cell Biol. 1996;133:247–256. doi: 10.1083/jcb.133.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchak LK, Sefton BM. Palmitoylation of either Cys-3 or Cys-5 is required for the biological activity of the Lck tyrosine protein kinase. Mol Cell Biol. 1995;15:6914–6922. doi: 10.1128/mcb.15.12.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamoyska R, Derham P, Gorman SD, von Hoegen P, Bolen JB, Veilette A, Parnes JR. Inability of CD8α′ polypeptides to associate with p56lck correlates with impaired function in vitro and lack of expression in vivo. . Nature (Lond) 1989;342:278–281. doi: 10.1038/342278a0. [DOI] [PubMed] [Google Scholar]