Abstract
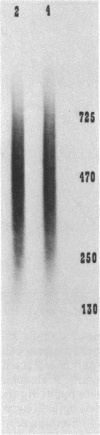
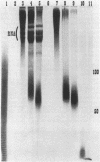
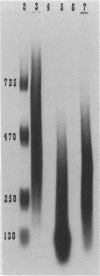
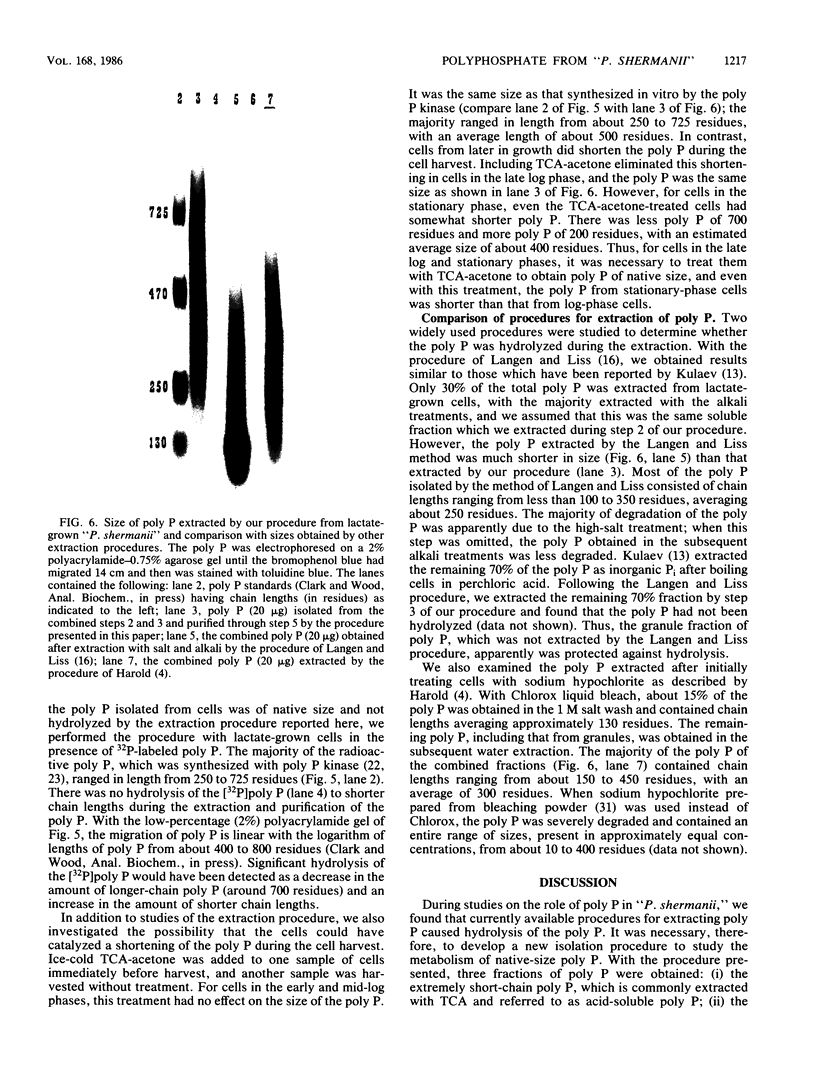
A procedure is presented for the isolation of intact polyphosphate (poly P) from "Propionibacterium shermanii." It is demonstrated, by including [32P]poly P during the extraction, that this procedure does not hydrolyze the poly P, and it is shown that two other widely used procedures do cause breakdown of the poly P. The procedure presented allows isolation of three fractions, short-chain poly P which is soluble in trichloroacetic acid, long-chain poly P which is soluble at neutral pH, and long-chain poly P which is present in volutin granules. Cells which had been grown on lactate did not contain short-chain poly P but did contain a high amount of long-chain poly P, which accumulated to 3% of the cell dry weight. At least 70% of this poly P was present in volutin granules. The poly P ranged in length from 250 to 725 phosphate residues and was the same average size as that synthesized in vitro by the poly P kinase from "P. shermanii". This indicates that the poly P kinase is responsible for catalyzing the synthesis of the poly P. In contrast to cells grown on lactate, those which had been grown on glucose did not contain volutin granules, did contain short-chain poly P and had 100-fold less long-chain poly P than lactate-grown cells. We propose that during the fermentation of glucose, the amount of poly P is lower than during growth on lactate because it is continuously utilized as a substrate in the phosphorylation of glucose.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cramer C. L., Vaughn L. E., Davis R. H. Basic amino acids and inorganic polyphosphates in Neurospora crassa: independent regulation of vacuolar pools. J Bacteriol. 1980 Jun;142(3):945–952. doi: 10.1128/jb.142.3.945-952.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. B., Davidian N. M., Penniall R. Studies of phosphorus metabolism by isolated nuclei. VII. Identification of polyphosphate as a product. J Biol Chem. 1965 Nov;240(11):4427–4434. [PubMed] [Google Scholar]
- HAROLD F. M., HAROLD R. L. DEGRADATION OF INORGANIC POLYPHOSPHATE IN MUTANTS OF AEROBACTER AEROGENES. J Bacteriol. 1965 May;89:1262–1270. doi: 10.1128/jb.89.5.1262-1270.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAROLD F. M. INORGANIC POLYPHOSPHATE OF HIGH MOLECULAR WEIGHT FROM AEROBACTER AEROGENES. J Bacteriol. 1963 Oct;86:885–887. doi: 10.1128/jb.86.4.885-887.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L., Halmann M., Yariv J. The molecular composition of the volutin granule of yeast. Biochem J. 1982 Mar 1;201(3):473–479. doi: 10.1042/bj2010473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. E., Chambers L. A. Localized intracellular polyphosphate formation by Desulfovibrio gigas. J Gen Microbiol. 1975 Jul;89(1):67–72. doi: 10.1099/00221287-89-1-67. [DOI] [PubMed] [Google Scholar]
- Josse J. Constitutive inorganic pyrophosphatase of Escherichia coli. 1. Purification and catalytic properties. J Biol Chem. 1966 May 10;241(9):1938–1947. [PubMed] [Google Scholar]
- Karl D. M. Cellular nucleotide measurements and applications in microbial ecology. Microbiol Rev. 1980 Dec;44(4):739–796. doi: 10.1128/mr.44.4.739-796.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulaev I. S., Vagabov V. M. Polyphosphate metabolism in micro-organisms. Adv Microb Physiol. 1983;24:83–171. doi: 10.1016/s0065-2911(08)60385-9. [DOI] [PubMed] [Google Scholar]
- Kulaev I. S., Vorob'eva L. I., Konovalova L. V., Bobyk M. A., Konoshenko G. I. Fermenty polifosfatnogo obmena v protsesse razvitiia Propionibacterium shermanii v norme i v prisutstvii polimiksina M. Biokhimiia. 1973 Jul-Aug;38(4):712–717. [PubMed] [Google Scholar]
- LANGEN P., LISS E. Uber Bildung und Umsatz der Polyphosphate der Hefe. Biochem Z. 1958;330(6):455–466. [PubMed] [Google Scholar]
- Pepin C. A., Wood H. G. Polyphosphate glucokinase from Propionibacterium shermanii. Kinetics and demonstration that the mechanism involves both processive and nonprocessive type reactions. J Biol Chem. 1986 Apr 5;261(10):4476–4480. [PubMed] [Google Scholar]
- Pepin C. A., Wood H. G., Robinson N. A. Determination of the size of polyphosphates with polyphosphate glucokinase. Biochem Int. 1986 Jan;12(1):111–123. [PubMed] [Google Scholar]
- Rao N. N., Roberts M. F., Torriani A. Amount and chain length of polyphosphates in Escherichia coli depend on cell growth conditions. J Bacteriol. 1985 Apr;162(1):242–247. doi: 10.1128/jb.162.1.242-247.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisner A. H., Nemes P., Bucholtz C. The use of Coomassie Brilliant Blue G250 perchloric acid solution for staining in electrophoresis and isoelectric focusing on polyacrylamide gels. Anal Biochem. 1975 Apr;64(2):509–516. doi: 10.1016/0003-2697(75)90461-3. [DOI] [PubMed] [Google Scholar]
- Robinson N. A., Goss N. H., Wood H. G. Polyphosphate kinase from Propionibacterium shermanii: formation of an enzymatically active insoluble complex with basic proteins and characterization of synthesized polyphosphate. Biochem Int. 1984 Jun;8(6):757–769. [PubMed] [Google Scholar]
- Robinson N. A., Wood H. G. Polyphosphate kinase from Propionibacterium shermanii. Demonstration that the synthesis and utilization of polyphosphate is by a processive mechanism. J Biol Chem. 1986 Apr 5;261(10):4481–4485. [PubMed] [Google Scholar]
- Steck G., Leuthard P., Bürk R. R. Detection of basic proteins and low molecular weight peptides in polyacrylamide gels by formaldehyde fixation. Anal Biochem. 1980 Sep 1;107(1):21–24. doi: 10.1016/0003-2697(80)90486-8. [DOI] [PubMed] [Google Scholar]
- TEWARI K. K., KRISHNAN P. S. Further studies on the metachromatic reaction of metaphosphate. Arch Biochem Biophys. 1959 May;82(1):99–106. doi: 10.1016/0003-9861(59)90094-3. [DOI] [PubMed] [Google Scholar]
- Tijssen J. P., Beekes H. W., Van Steveninck J. Localization of polyphosphates at the outside of the yeast cell plasma membrane. Biochim Biophys Acta. 1981 Dec 21;649(3):529–532. doi: 10.1016/0005-2736(81)90156-5. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D. H., WILKINSON J. F. The isolation and estimation of the poly-beta-hydroxybutyrate inclusions of Bacillus species. J Gen Microbiol. 1958 Aug;19(1):198–209. doi: 10.1099/00221287-19-1-198. [DOI] [PubMed] [Google Scholar]
- Wood H. G., Goss N. H. Phosphorylation enzymes of the propionic acid bacteria and the roles of ATP inorganic pyrophosphate, and polyphosphates. Proc Natl Acad Sci U S A. 1985 Jan;82(2):312–315. doi: 10.1073/pnas.82.2.312. [DOI] [PMC free article] [PubMed] [Google Scholar]