Abstract
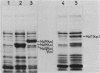
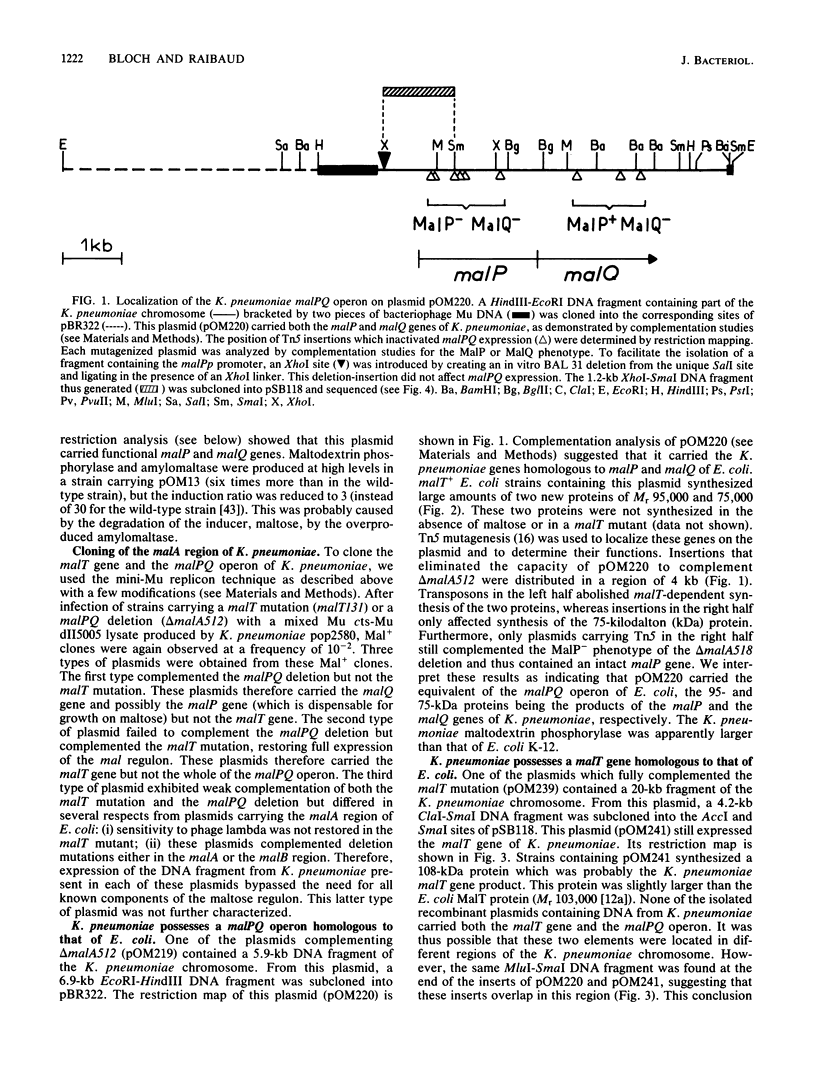
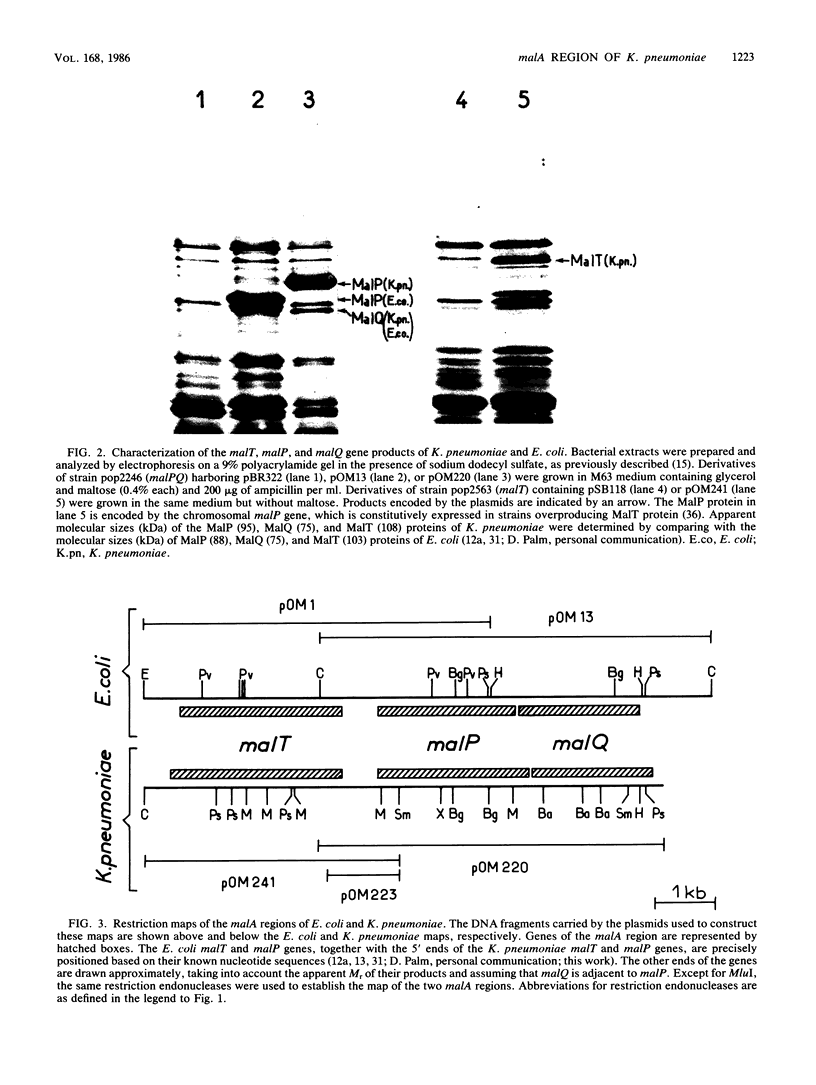
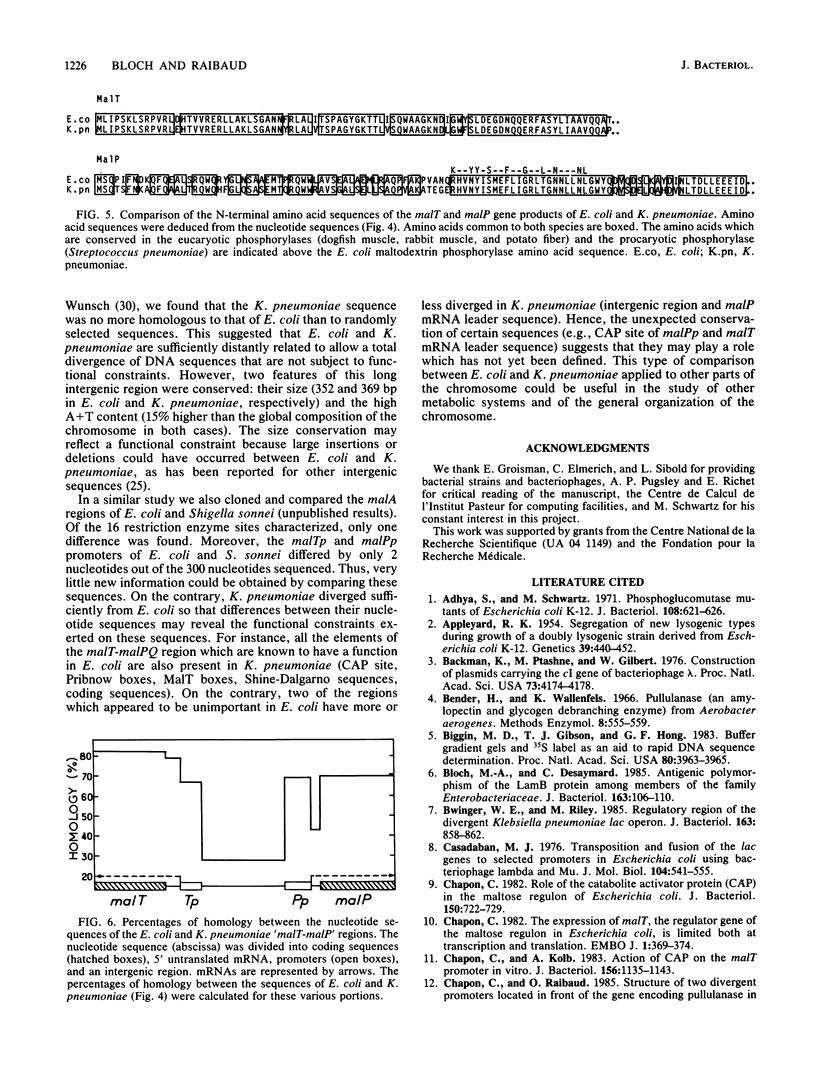
Using the mini-Mu-duction technique, we cloned the malA regions from Escherichia coli K-12 and Klebsiella pneumoniae. A comparison of the structures of the cloned DNAs indicated that the malT, malP, and malQ genes, encoding the transcriptional activator of the maltose regulon, maltodextrin phosphorylase, and amylomaltase, respectively, are similarly organized in both species; malP and malQ constitute an operon divergent from the malT gene. We sequenced 1,200 nucleotides encompassing the beginnings of the malT and malP genes, their promoters, and the intergenic region. The DNA sequences from the two species were very different; the levels of homology ranged from 28 to 80%, depending on the region. The sequences of the coding regions and of elements known to be important for the functions of these two promoters in E. coli were well conserved between the two bacteria, whereas the sequence of the malT-malP intergenic region had totally diverged.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Schwartz M. Phosphoglucomutase mutants of Escherichia coli K-12. J Bacteriol. 1971 Nov;108(2):621–626. doi: 10.1128/jb.108.2.621-626.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman K., Ptashne M., Gilbert W. Construction of plasmids carrying the cI gene of bacteriophage lambda. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4174–4178. doi: 10.1073/pnas.73.11.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch M. A., Desaymard C. Antigenic polymorphism of the LamB protein among members of the family Enterobacteriaceae. J Bacteriol. 1985 Jul;163(1):106–110. doi: 10.1128/jb.163.1.106-110.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buvinger W. E., Riley M. Regulatory region of the divergent Klebsiella pneumoniae lac operon. J Bacteriol. 1985 Sep;163(3):858–862. doi: 10.1128/jb.163.3.858-862.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Chapon C. Expression of malT, the regulator gene of the maltose region in Escherichia coli, is limited both at transcription and translation. EMBO J. 1982;1(3):369–374. doi: 10.1002/j.1460-2075.1982.tb01176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapon C., Kolb A. Action of CAP on the malT promoter in vitro. J Bacteriol. 1983 Dec;156(3):1135–1143. doi: 10.1128/jb.156.3.1135-1143.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapon C., Raibaud O. Structure of two divergent promoters located in front of the gene encoding pullulanase in Klebsiella pneumoniae and positively regulated by the malT product. J Bacteriol. 1985 Nov;164(2):639–645. doi: 10.1128/jb.164.2.639-645.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapon C. Role of the catabolite activator protein in the maltose regulon of Escherichia coli. J Bacteriol. 1982 May;150(2):722–729. doi: 10.1128/jb.150.2.722-729.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S. T., Raibaud O. The nucleotide sequence of the malT gene encoding the positive regulator of the Escherichia coli maltose regulon. Gene. 1986;42(2):201–208. doi: 10.1016/0378-1119(86)90297-0. [DOI] [PubMed] [Google Scholar]
- Debarbouille M., Cossart P., Raibaud O. A DNA sequence containing the control sites for gene malT and for the malPQ operon. Mol Gen Genet. 1982;185(1):88–92. doi: 10.1007/BF00333795. [DOI] [PubMed] [Google Scholar]
- Débarbouillé M., Raibaud O. Expression of the Escherichia coli malPQ operon remains unaffected after drastic alteration of its promoter. J Bacteriol. 1983 Mar;153(3):1221–1227. doi: 10.1128/jb.153.3.1221-1227.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Débarbouillé M., Shuman H. A., Silhavy T. J., Schwartz M. Dominant constitutive mutations in malT, the positive regulator gene of the maltose regulon in Escherichia coli. J Mol Biol. 1978 Sep 15;124(2):359–371. doi: 10.1016/0022-2836(78)90304-2. [DOI] [PubMed] [Google Scholar]
- Freundlieb S., Boos W. Alpha-amylase of Escherichia coli, mapping and cloning of the structural gene, malS, and identification of its product as a periplasmic protein. J Biol Chem. 1986 Feb 25;261(6):2946–2953. [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- Groisman E. A., Castilho B. A., Casadaban M. J. In vivo DNA cloning and adjacent gene fusing with a mini-Mu-lac bacteriophage containing a plasmid replicon. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1480–1483. doi: 10.1073/pnas.81.5.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez C., Chapon C., Schwartz M. Indirect effects of the 3'-5' cyclic adenosine monophosphate binding protein (CAP) on the transcription of the malPQ operon in Escherichia coli. Biochimie. 1985 Jan;67(1):145–148. doi: 10.1016/s0300-9084(85)80241-8. [DOI] [PubMed] [Google Scholar]
- Hatfield D., Hofnung M., Schwartz M. Genetic analysis of the maltose A region in Escherichia coli. J Bacteriol. 1969 May;98(2):559–567. doi: 10.1128/jb.98.2.559-567.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield D., Hofnung M., Schwartz M. Nonsense mutations in the maltose A region of the genetic map of Escherichia coli. J Bacteriol. 1969 Dec;100(3):1311–1315. doi: 10.1128/jb.100.3.1311-1315.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofnung M., Schwartz M., Hatfield D. Complementation studies in the maltose-A region of the Escherichia coli K12 genetic map. J Mol Biol. 1971 Nov 14;61(3):681–694. doi: 10.1016/0022-2836(71)90072-6. [DOI] [PubMed] [Google Scholar]
- Linder D., Kurz G., Bender H., Wallenfels K. 1, 4-alpha-Glucan phosphorylase from Klebsiella pneumoniae purification, subunit structure and amino acid composition. Eur J Biochem. 1976 Nov 1;70(1):291–303. doi: 10.1111/j.1432-1033.1976.tb10981.x. [DOI] [PubMed] [Google Scholar]
- MARMUR J., FALKOW S., MANDEL M. NEW APPROACHES TO BACTERIAL TAXONOMY. Annu Rev Microbiol. 1963;17:329–372. doi: 10.1146/annurev.mi.17.100163.001553. [DOI] [PubMed] [Google Scholar]
- MacFarlane S. A., Merrick M. The nucleotide sequence of the nitrogen regulation gene ntrB and the glnA-ntrBC intergenic region of Klebsiella pneumoniae. Nucleic Acids Res. 1985 Nov 11;13(21):7591–7606. doi: 10.1093/nar/13.21.7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Michaelis S., Chapon C., D'Enfert C., Pugsley A. P., Schwartz M. Characterization and expression of the structural gene for pullulanase, a maltose-inducible secreted protein of Klebsiella pneumoniae. J Bacteriol. 1985 Nov;164(2):633–638. doi: 10.1128/jb.164.2.633-638.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Palm D., Goerl R., Burger K. J. Evolution of catalytic and regulatory sites in phosphorylases. Nature. 1985 Feb 7;313(6002):500–502. doi: 10.1038/313500a0. [DOI] [PubMed] [Google Scholar]
- Palmer T. N., Ryman B. E., Whelan W. J. The action pattern of amylomaltase from Escherichia coli. Eur J Biochem. 1976 Oct 1;69(1):105–115. doi: 10.1111/j.1432-1033.1976.tb10863.x. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Débarbouillé M., Schwartz M. Use of deletions created in vitro to map transcriptional regulatory signals in the malA region of Escherichia coli. J Mol Biol. 1983 Jan 25;163(3):395–408. doi: 10.1016/0022-2836(83)90065-7. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Gutierrez C., Schwartz M. Essential and nonessential sequences in malPp, a positively controlled promoter in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1201–1208. doi: 10.1128/jb.161.3.1201-1208.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raibaud O., Roa M., Braun-Breton C., Schwartz M. Structure of the malB region in Escherichia coli K12. I. Genetic map of the malK-lamB operon. Mol Gen Genet. 1979 Jul 24;174(3):241–248. doi: 10.1007/BF00267796. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Schwartz M. Restriction map of the Escherichia coli malA region and identification of the malT product. J Bacteriol. 1980 Aug;143(2):761–771. doi: 10.1128/jb.143.2.761-771.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibold L. The polar effect on nifM of mutations in the nifU,-S,-V genes of Klebsiella pneumoniae depends on their plasmid or chromosomal location. Mol Gen Genet. 1982;186(4):569–571. doi: 10.1007/BF00337966. [DOI] [PubMed] [Google Scholar]
- Silhavy T. J., Brickman E., Bassford P. J., Jr, Casadaban M. J., Shuman H. A., Schwartz V., Guarente L., Schwartz M., Beckwith J. R. Structure of the malB region in Escherichia coli K12. II. Genetic map of the malE,F,G operon. Mol Gen Genet. 1979 Jul 24;174(3):249–259. doi: 10.1007/BF00267797. [DOI] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Toussaint A., Lefebvre N., Scott J. R., Cowan J. A., de Bruijn F., Bukhari A. I. Relationships between temperate phages Mu and P1. Virology. 1978 Aug;89(1):146–161. doi: 10.1016/0042-6822(78)90048-x. [DOI] [PubMed] [Google Scholar]
- Vidal-Ingigliardi D., Raibaud O. A convenient technique to compare the efficiency of promoters in Escherichia coli. Nucleic Acids Res. 1985 Aug 26;13(16):5919–5926. doi: 10.1093/nar/13.16.5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Wöhner G., Wöber G. Subcellular distribution of enzymes involved in alpha-glucan utilization in Klebsiella pneumoniae. Proteins of cytopasm, periplasm, cytoplasmic and outer membrane. Arch Microbiol. 1978 Mar;116(3):311–316. doi: 10.1007/BF00417857. [DOI] [PubMed] [Google Scholar]
- de Bruijn F. J., Lupski J. R. The use of transposon Tn5 mutagenesis in the rapid generation of correlated physical and genetic maps of DNA segments cloned into multicopy plasmids--a review. Gene. 1984 Feb;27(2):131–149. doi: 10.1016/0378-1119(84)90135-5. [DOI] [PubMed] [Google Scholar]