Abstract
The gene encoding thioredoxin in Anabaena sp. strain PCC 7119 was cloned in Escherichia coli based on the strategy that similarity between the two thioredoxins would be reflected both in the gene sequence and in functional cross-reactivity. DNA restriction fragments containing the Anabaena thioredoxin gene were identified by heterologous hybridization to the E. coli thioredoxin gene following Southern transfer, ligated with pUC13, and used to transform an E. coli strain lacking functional thioredoxin. Transformants that complemented the trxA mutation in E. coli were identified by increased colony size and confirmed by enzyme assay. Expression of the cloned Anabaena thioredoxin gene in E. coli was substantiated by subsequent purification and characterization of the algal protein from E. coli. The amino acid sequence derived from the DNA sequence of the Anabaena gene was identical to the known amino acid sequence of Anabaena thioredoxin. The E. coli strains which expressed Anabaena thioredoxin complemented the TrxA- phenotype in every respect except that they did not support bacteriophage T7 growth and had somewhat decreased ability to support bacteriophages M13 and f1.
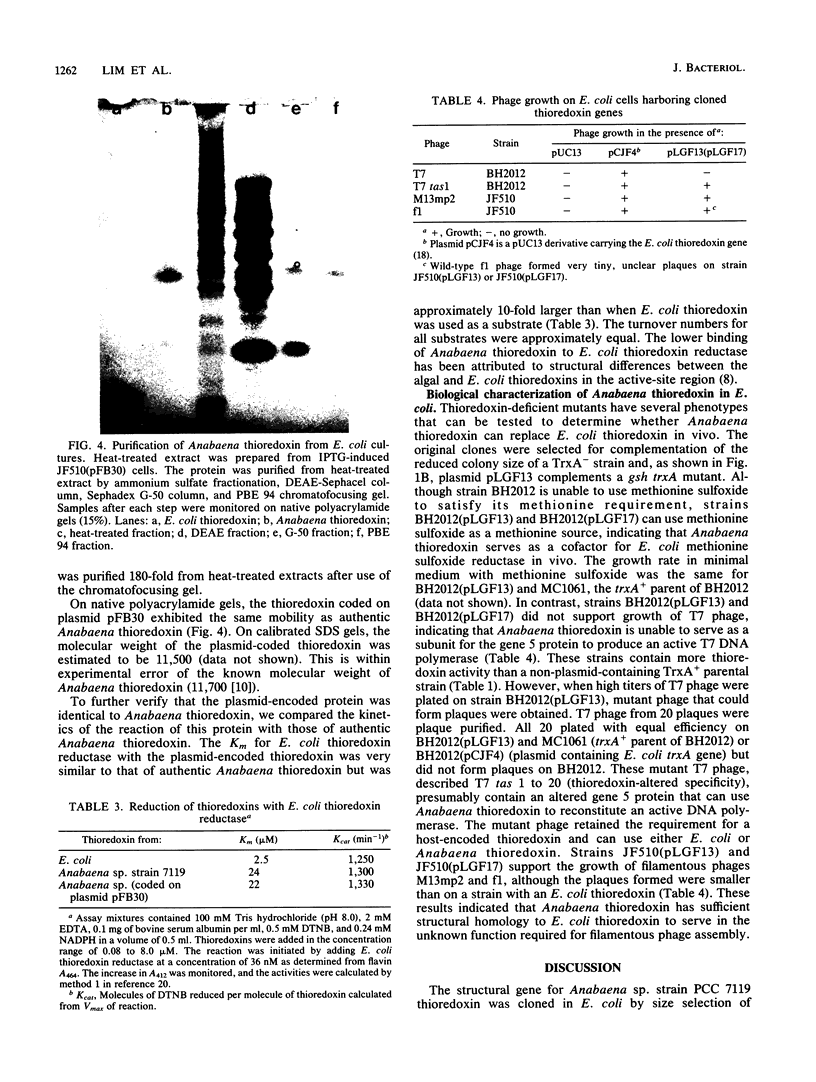
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton A. R., Brennan T., Anderson L. E. Thioredoxin-like Activity of Thylakoid Membranes: THIOREDOXIN CATALYZING THE REDUCTIVE INACTIVATION OF GLUCOSE-6-PHOSPHATE DEHYDROGENASE OCCURS IN BOTH SOLUBLE AND MEMBRANE-BOUND FORM. Plant Physiol. 1980 Oct;66(4):605–608. doi: 10.1104/pp.66.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Curtis S. E., Haselkorn R. Isolation and sequence of the gene for the large subunit of ribulose-1,5-bisphosphate carboxylase from the cyanobacterium Anabaena 7120. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1835–1839. doi: 10.1073/pnas.80.7.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R., Tuli R., Haselkorn R. A cloned cyanobacterial gene for glutamine synthetase functions in Escherichia coli, but the enzyme is not adenylylated. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3393–3397. doi: 10.1073/pnas.78.6.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason F. K., Frick T. D. Adenosylcobalamin-dependent ribonucleotide reductase from the blue-green alga, Anabaena sp. Purification and partial characterization. J Biol Chem. 1980 Aug 25;255(16):7728–7733. [PubMed] [Google Scholar]
- Gleason F. K., Holmgren A. Isolation and characterization of thioredoxin from the cyanobacterium, Anabaena sp. J Biol Chem. 1981 Aug 25;256(16):8306–8309. [PubMed] [Google Scholar]
- Gleason F. K., Whittaker M. M., Holmgren A., Jörnvall H. The primary structure of thioredoxin from the filamentous cyanobacterium Anabaena sp. 7119. J Biol Chem. 1985 Aug 15;260(17):9567–9573. [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- Heidecker G., Messing J., Gronenborn B. A versatile primer for DNA sequencing in the M13mp2 cloning system. Gene. 1980 Jun;10(1):69–73. doi: 10.1016/0378-1119(80)90145-6. [DOI] [PubMed] [Google Scholar]
- Holmgren A., Kallis G. B., Nordström B. A mutant thioredoxin from Escherichia coli tsnC 7007 that is nonfunctional as subunit of phage T7 DNA polymerase. J Biol Chem. 1981 Mar 25;256(6):3118–3124. [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J Biol Chem. 1979 Oct 10;254(19):9627–9632. [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Kodaki T., Katagiri F., Asano M., Izui K., Katsuki H. Cloning of phosphoenolpyruvate carboxylase gene from a cyanobacterium, Anacystis nidulans, in Escherichia coli. J Biochem. 1985 Feb;97(2):533–539. doi: 10.1093/oxfordjournals.jbchem.a135088. [DOI] [PubMed] [Google Scholar]
- Lim C. J., Geraghty D., Fuchs J. A. Cloning and nucleotide sequence of the trxA gene of Escherichia coli K-12. J Bacteriol. 1985 Jul;163(1):311–316. doi: 10.1128/jb.163.1.311-316.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C. J., Haller B., Fuchs J. A. Thioredoxin is the bacterial protein encoded by fip that is required for filamentous bacteriophage f1 assembly. J Bacteriol. 1985 Feb;161(2):799–802. doi: 10.1128/jb.161.2.799-802.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthman M., Holmgren A. Rat liver thioredoxin and thioredoxin reductase: purification and characterization. Biochemistry. 1982 Dec 21;21(26):6628–6633. doi: 10.1021/bi00269a003. [DOI] [PubMed] [Google Scholar]
- Maeda K., Tsugita A., Dalzoppo D., Vilbois F., Schürmann P. Further characterization and amino acid sequence of m-type thioredoxins from spinach chloroplasts. Eur J Biochem. 1986 Jan 2;154(1):197–203. doi: 10.1111/j.1432-1033.1986.tb09379.x. [DOI] [PubMed] [Google Scholar]
- Mazur B. J., Chui C. F. Sequence of the gene coding for the beta-subunit of dinitrogenase from the blue-green alga Anabaena. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6782–6786. doi: 10.1073/pnas.79.22.6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevarech M., Rice D., Haselkorn R. Nucleotide sequence of a cyanobacterial nifH gene coding for nitrogenase reductase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6476–6480. doi: 10.1073/pnas.77.11.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrich P., Richardson C. C. Bacteriophage T7 deoxyribonucleic acid replication invitro. Bacteriophage T7 DNA polymerase: an an emzyme composed of phage- and host-specific subunits. J Biol Chem. 1975 Jul 25;250(14):5515–5522. [PubMed] [Google Scholar]
- Nordström B., Randahl H., Slaby I., Holmgren A. Characterization of bacteriophage T7 DNA polymerase purified to homogeneity by antithioredoxin immunoadsorbent chromatography. J Biol Chem. 1981 Mar 25;256(6):3112–3117. [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Russel M., Model P. Characterization of the cloned fip gene and its product. J Bacteriol. 1984 Feb;157(2):526–532. doi: 10.1128/jb.157.2.526-532.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]