Abstract
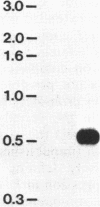
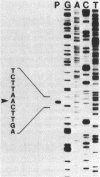
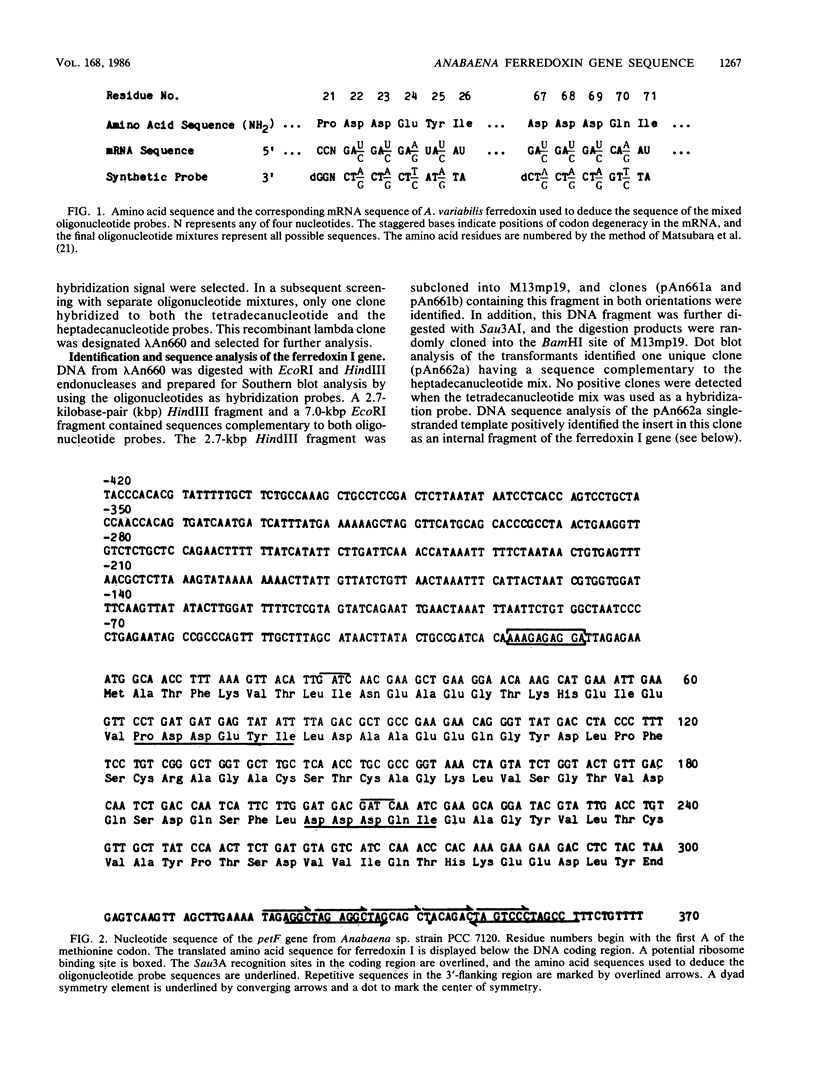
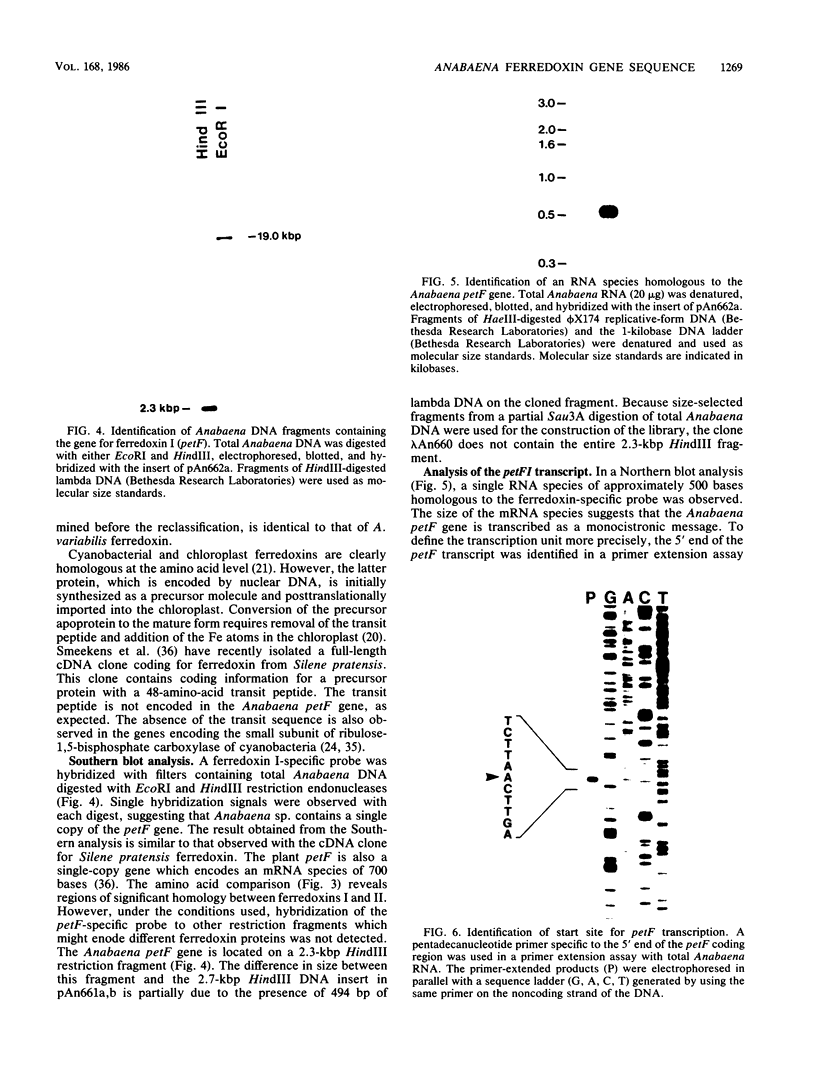
The structural gene for ferredoxin I, petF, from the cyanobacterium Anabaena sp. strain PCC 7120 has been isolated from a recombinant lambda library. Mixtures of tetradecanucleotides and heptadecanucleotides, each containing all possible DNA sequences corresponding to two separate regions of the ferredoxin amino acid sequence, were synthesized and used as hybridization probes to identify a genomic clone containing the coding sequence for the petF gene. The sequence of the entire petF coding region and portions of the 3'- and 5'-flanking regions was determined. The DNA sequence of petF suggests that, in contrast to the nucleus-encoded plant protein, cyanobacterial apoferredoxin is not synthesized as a higher-molecular-weight precursor. The Anabaena petF gene is a single-copy gene. During growth on complete medium it was transcribed into a monocistronic mRNA species of approximately 500 bases that initiated 100 base pairs upstream from the petF coding region.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- André E., Kessler M., Briquel M. E., Alexandre P., Hurault de Ligny B., Huriet C. Intérêt pratique du dosage des produits de dégradation de la fibrine urinaire dans la surveillance précoce des transplantés rénaux. Pathol Biol (Paris) 1983 Jan;31(1):23–27. [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B. The ferredoxin/thioredoxin system: a key element in the regulatory function of light in photosynthesis. Bioscience. 1984 Jun;34(6):378–383. [PubMed] [Google Scholar]
- Cammack R., Rao K. K., Bargeron C. P., Hutson K. G., Andrew P. W., Rogers L. J. Midpoint redox potentials of plant and algal ferredoxins. Biochem J. 1977 Nov 15;168(2):205–209. doi: 10.1042/bj1680205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis S. E., Haselkorn R. Isolation and sequence of the gene for the large subunit of ribulose-1,5-bisphosphate carboxylase from the cyanobacterium Anabaena 7120. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1835–1839. doi: 10.1073/pnas.80.7.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBS M. B., BECKER E. L. Quantitation of haemagglutination by enumeration of free cells by an electronic counter. Nature. 1963 Apr 6;198:90–90. doi: 10.1038/198090a0. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Methods Enzymol. 1983;100:333–342. doi: 10.1016/0076-6879(83)00066-x. [DOI] [PubMed] [Google Scholar]
- Hase T., Inoue K., Matsubara H., Williams M. M., Rogers L. J. Amino acid sequence of Synechocystis 6714 ferredoxin: a unique structural feature of unicellular blue-green algal ferredoxin. J Biochem. 1982 Nov;92(5):1357–1362. doi: 10.1093/oxfordjournals.jbchem.a134059. [DOI] [PubMed] [Google Scholar]
- Hase T., Matsubara H., Hutber G. N., Rogers L. J. Amino acid sequences of Nostoc strain MAC ferredoxins I and II. J Biochem. 1982 Nov;92(5):1347–1355. doi: 10.1093/oxfordjournals.jbchem.a134058. [DOI] [PubMed] [Google Scholar]
- Hutson K. G., Rogers L. J. Two plant-type ferredoxins in light-grown and dark-grown cells of the blue-green bacterium Nostoc strain MAC. Biochem Soc Trans. 1975;3(3):377–379. doi: 10.1042/bst0030377. [DOI] [PubMed] [Google Scholar]
- Lea P. J., Miflin B. J. Alternative route for nitrogen assimilation in higher plants. Nature. 1974 Oct 18;251(5476):614–616. doi: 10.1038/251614a0. [DOI] [PubMed] [Google Scholar]
- Loenen W. A., Brammar W. J. A bacteriophage lambda vector for cloning large DNA fragments made with several restriction enzymes. Gene. 1980 Aug;10(3):249–259. doi: 10.1016/0378-1119(80)90054-2. [DOI] [PubMed] [Google Scholar]
- Mazur B. J., Rice D., Haselkorn R. Identification of blue-green algal nitrogen fixation genes by using heterologous DNA hybridization probes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):186–190. doi: 10.1073/pnas.77.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierzwicki-Bauer S. A., Curtis S. E., Haselkorn R. Cotranscription of genes encoding the small and large subunits of ribulose-1,5-bisphosphate carboxylase in the cyanobacterium Anabaena 7120. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5961–5965. doi: 10.1073/pnas.81.19.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D., Mazur B. J., Haselkorn R. Isolation and physical mapping of nitrogen fixation genes from the cyanobacterium Anabaena 7120. J Biol Chem. 1982 Nov 10;257(21):13157–13163. [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- SAN PIETRO A., LANG H. M. Photosynthetic pyridine nucleotide reductase. I. Partial purification and properties of the enzyme from spinach. J Biol Chem. 1958 Mar;231(1):211–229. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Trebst A. The mechanism of photosynthetic sulfate reduction by isolated chloroplasts. Biochim Biophys Acta. 1969 Aug 5;180(3):529–535. doi: 10.1016/0005-2728(69)90031-0. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Sugiura M. The gene for the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase is located close to the gene for the large subunit in the cyanobacterium Anacystis nidulans 6301. Nucleic Acids Res. 1983 Oct 25;11(20):6957–6964. doi: 10.1093/nar/11.20.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S., van Binsbergen J., Weisbeek P. The plant ferredoxin precursor: nucleotide sequence of a full length cDNA clone. Nucleic Acids Res. 1985 May 10;13(9):3179–3194. doi: 10.1093/nar/13.9.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie R. M. Isolation of two proteins with chloroplast ferredoxin activity from a blue-green alga. Biochem Biophys Res Commun. 1965 Sep 8;20(5):621–629. doi: 10.1016/0006-291x(65)90445-6. [DOI] [PubMed] [Google Scholar]
- Smith R. V., Noy R. J., Evans M. C. Physiological electron donor systems to the nitrogenase of the blue-green alga Anabaena cylindrica. Biochim Biophys Acta. 1971 Nov 2;253(1):104–109. doi: 10.1016/0005-2728(71)90238-6. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Tomioka N., Sugiura M. The complete nucleotide sequence of a 16S ribosomal RNA gene from a blue-green alga, Anacystis nidulans. Mol Gen Genet. 1983;191(1):46–50. doi: 10.1007/BF00330888. [DOI] [PubMed] [Google Scholar]
- Wood P. M. Interchangeable copper and iron proteins in algal photosynthesis. Studies on plastocyanin and cytochrome c-552 in Chlamydomonas. Eur J Biochem. 1978 Jun 1;87(1):9–19. doi: 10.1111/j.1432-1033.1978.tb12346.x. [DOI] [PubMed] [Google Scholar]
- van der Mark F., van den Briel W., Huisman H. G. Phytoferritin is synthesized in vitro as a high-molecular-weight precursor. Studies on the synthesis and the uptake in vitro of the precursors of ferritin and ferredoxin by intact chloroplasts. Biochem J. 1983 Sep 15;214(3):943–950. doi: 10.1042/bj2140943. [DOI] [PMC free article] [PubMed] [Google Scholar]