Abstract
The highly efficient nature of dendritic cells (DC) as antigen-presenting cells raises the possibility of uncovering in tumor-bearing hosts very low levels of T cell reactivity to poorly immunogenic tumors that are virtually undetectable by other means. Here, we demonstrate the in vitro and in vivo capacities of murine bone marrow-derived, cytokine-driven DC to elicit potent and specific anti-tumor responses when pulsed with whole tumor lysates. Stimulation of naive spleen-derived T cells by tumor lysate-pulsed DC generated tumor-specific proliferative cytokine release and cytolytic reactivities in vitro. In addition, in two separate strains of mice with histologically distinct tumors, s.c. injections of DC pulsed with whole tumor lysates effectively primed these animals to reject subsequent lethal challenges with viable parental tumor cells and, important to note, also mediated significant reductions in the number of metastases established in the lungs. Tumor rejection depended on host-derived CD8+ T cells and, to a lesser extent, CD4+ T cells. Spleens from mice that had rejected their tumors contained specific precursor cytotoxic T lymphocytes. The use of whole tumor lysates as a source of tumor-associated antigen(s) for pulsing of DC circumvents several limitations encountered with other methods as well as provides certain distinct advantages, which are discussed. These data serve as rationale for our recent initiation of a phase I clinical trial of immunization with autologous tumor lysate-pulsed DC in adult and pediatric cancer patients.
Recent attempts to improve immunotherapy for cancer have included the genetic modification of tumor-infiltrating lymphocytes (TIL) to express exogenous genes encoding for either antitumor cytokines (1) or new “chimeric” receptors to redirect tumor antigen specificity (2). Moreover, cytotoxic T lymphocyte (CTL)-defined tumor peptides (3) and gene-modified tumor cells (4) have been used as immunogens to generate more potent TIL or tumor-draining lymph node cells or to impact directly on established metastatic disease by serving as “therapeutic vaccines.” We have been investigating approaches to enhance the activity of tumor vaccines to increase the frequency of tumor-reactive T cells and to overcome tumor-induced immune suppression (5, 6). Our recent studies have involved the use of dendritic cells (DC) as antigen-presenting cells (APC) in an attempt to stimulate both primary and secondary immune responses to poorly immunogenic tumors.
DC are of bone marrow origin, develop from myeloid (7) or lymphoid (8) precursors, and possess strong APC function. In this regard, DC have been shown to stimulate both primary and secondary T and B cell responses (9) and to internalize, process, and present antigens efficiently (7). DC pulsed with defined tumor-associated peptides or proteins as well as with model “tumor” antigens have been shown to elicit potent antitumor T cell responses both in vitro and in vivo (10). We had reported earlier that murine epidermal Langerhans cells and splenic DC could present efficiently antigens associated with tumor cell lysates to primed CD4+ T cells in vitro (11, 12).
It is generally accepted that tumors growing in vivo naturally provide antigen(s) to APC either by shedding from the surface of viable cells or by fragmentation of dead tumor cells. Such processes elicit the induction of cellular immune responses by mechanisms that include “cross-priming” (13). The use of tumor cell lysates as a possible source of tumor antigen(s) for DC pulsing has several potential advantages, which include mimicking the physiologic processes by which a growing tumor induces an immune response (albeit low) in vivo. In practical terms, tumor lysates circumvent the need for viable fresh tumor cells and for the establishment of tumor cell lines in vitro, which for some human tumors (e.g., breast carcinoma) has been difficult, as well as avoid the necessity for molecular characterization of the tumor antigen(s) for effective immunization (11, 12). Because human cancers have been shown to elicit multiple specific immune responses in the autologous patient (14), the approach of using tumor lysates pulsed onto DC would offer the potential advantage of augmenting a broader T cell immune response to tumor-associated antigens that would not be obtained by pulsing DC with a single or perhaps several defined tumor peptides. This strategy potentially lessens the possibility of tumor escape by the broader elicited immune response yet increases the potential to trigger T cell reactivity to those particular antigens, which results in actual tumor regression in vivo (namely, “tumor rejection” antigens) (3, 15). In addition, greater potential exists for simultaneous presentation of “CTL-defined” and “T helper-defined” epitopes by virtue of a whole tumor lysate serving as the source for pulsing DC, which highly express both major histocompatibility complex (MHC) class I and II molecules. In this regard, although several distinct peptides have been identified in human tumors, few, to date, are MHC class II, CD4+ T cell-defined (3, 16). Yet, in several murine tumor models, the adoptive transfer of immune CD4+ T cells, when successfully elicited, mediates potent antitumor therapeutic effects in vivo (17). Moreover, certain melanoma vaccines generated from mechanical lysates stimulate both CD4+ and CD8+ T cell activity in immunized cancer patients (18).
Here, we investigated the capacity of bone marrow-derived, cytokine-driven DC pulsed with tumor lysates to elicit antitumor responses both in vitro and in vivo in two syngeneic murine tumor models. We show that tumor lysate-pulsed DC elicit specific proliferative and cytolytic T cell reactivities in vitro. In addition, immunization of mice with tumor lysate-pulsed DC can mediate effective immune priming in vivo and successfully treats established visceral lung metastases from either a syngeneic sarcoma or a breast carcinoma.
MATERIALS AND METHODS
Animals.
C57BL/6 (denoted B6) and BALB/c mice (6- to 8-week-old female) were purchased from the Jackson Laboratory and housed at the Animal Maintenance Facility of the University of Michigan Medical Center. The animals were used for experiments between 10 and 14 weeks of age.
Medium and Cytokines.
Complete medium (CM) consisted of RPMI medium 1640 with 10% heat-inactivated fetal calf serum and supplements, as described (11, 12). Recombinant cytokines were used at the following concentrations diluted in CM: recombinant mouse granulocyte/macrophage (GM) colony-stimulating factor (CSF) 10 ng/ml (specific activity: ≥5 × 106 units/mg; Immunex); recombinant mouse interleukin (IL)-4, 10 ng/ml (specific activity: 2.8 × 108 units/mg; Schering-Plough); recombinant human IL-7, 5 ng/ml (specific activity: 5 × 107 units/mg; Sanofi, Paris); rhIL-2, 20 units/ml (specific activity: 18 × 106 units/mg; Chiron).
Tumor Cell Lines.
The 3-methylcholanthrene (MCA)-102 and -207 tumors are weakly immunogenic, MCA-induced fibrosarcomas of B6 origin (19). Tumor cell suspensions were prepared from solid tumors by enzymatic digestion, as described (19). MT-7 is a cultured murine tumor cell line derived from a dimethylbenzanthracene-induced mammary carcinoma in the BALB/c strain (20). A subline, denoted MT-901, was derived from an early in vivo passage of cultured MT-7 tumor injected s.c. This tumor is weakly immunogenic, and it expresses surface MHC class I (but not MHC class II) molecules. All tumor cells subsequently were cultured in CM before use, as described (11, 12).
Generation of Bone Marrow-Derived DC.
Erythrocyte-depleted mouse bone marrow cells from flushed marrow cavities were cultured in CM with 10 ng/ml GM-CSF and 10 ng/ml IL-4 at 1 × 106 cells/ml (21). On day 7, DC were harvested by gentle pipetting and were enriched by 14.5% (by weight) metrizamide (Sigma) CM gradients. The low density interface containing the DC was collected by gentle pipette aspiration. The DC were washed twice with CM, enumerated [purity >90% by positive coexpression of MHC class II, CD40, CD80, CD86, and CD11c by fluorescence-activated cell sorter (FACS), not shown], and cultured in CM with added cytokines for further studies.
Antibodies.
Cell surface staining used direct immunofluorescence and was analyzed by flow cytometry (FACScan, Becton Dickinson). Staining was performed with the following fluorescein isothiocyanate-labeled mouse antibodies: I-Ab, I-Ad, H-2Kb, H-2Kd, CD3, CD4, CD8, CD11c, CD80, and CD86 (PharMingen). Primary antibodies were directed toward a panel of cell surface markers and were compared with the appropriate isotype-matched controls (PharMingen). For studies of blocking of CTL reactivity to MCA-207 tumor cell targets in vitro, the following unconjugated mAbs were used: anti-H-2Kb/H-2Db and, as a negative control, anti-H-2Dd (both from PharMingen).
Antigen Pulsing of DC.
Day 7 DC were incubated with freeze-thawed tumor lysates at a ratio of three tumor cell equivalents to one DC (i.e., 3:1) in CM, as described (11, 12). After 18 hr of incubation, DC were harvested, irradiated with 2,000 rad (Gamma Cell 1000; Nordion, Kanata, Canada), washed twice in Hank’s balanced salt solution (GIBCO), and resuspended in Hank’s balanced salt solution for further studies.
Responder T Cells.
Splenocytes obtained from naive B6 mice were treated with ammonium chloride-potassium lysing buffer for 1 min to deplete erythrocytes and were washed twice with Hank’s balanced salt solution. These splenocytes then were incubated in CM for 90 min, and nonadherent cells (mostly T and B cells) were removed gently and incubated on nylon wool columns for 1 hr at 37°C. After elution, the resulting cells were ≈90% CD3+ by FACS analysis (not shown).
Induction of Proliferative T Cell Responses in Vitro.
B6 mouse bone marrow-derived DC (1 × 105/ml) pulsed with MCA-207 tumor lysate were added to naive responder T cells (1 × 106/ml) in 24-well tissue culture plates (Costar) at a responder-to-stimulator ratio of 20:1. After 5 days, cultures were resuspended and harvested, adjusted to 1 × 106 cells/ml, and placed in CM containing IL-2 at 20 units/ml and IL-7 at 5 ng/ml (22). Cell cultures were counted routinely and replenished with fresh CM and cytokines every 2–3 days. These cultures were restimulated twice with irradiated MCA-207 tumor lysate-pulsed DC (at the 20:1 responder-to-stimulator ratio) every 7 days. After 19 days, the T cells (1 × 106/ml) were harvested and cocultured with either MCA-207 tumor lysate-pulsed DC, unpulsed DC alone, tumor lysate alone, or DC pulsed with an irrelevant (control) MCA-102 tumor lysate for 3 days in 96-well U-bottom microtiter plates (Costar). At 24 hr before assay completion, tritiated deoxythymidine (1 μCi/well; NEN) was added to each microtiter well. At completion, plates were harvested with a semiautomated Tomtec cell harvester (Orange, CT) and measured by a Wallac 1205 Betaplate liquid scintillation counter (Gaithersburg, MD). Responses were reported as mean cpm ± SEM from triplicate samples.
Induction of Tumor-Specific CTL in Vitro.
After 19 days of in vitro culture (described above), T cells also were tested for cytolytic activity in a standard 4-hr 51Cr-release assay, as described (22, 23). Each assay was performed in triplicate, and spontaneous release was <20% of the maximal release by detergent in all assays. In blocking/inhibition experiments using anti-MHC mAb, MCA-207 target cells first were incubated at 37°C for 30 min with anti-H-2Kb/H-2Db or anti-H-2Dd mAb (as negative control) before addition in the cytotoxicity assay. The final mAb concentration was 50 μg/ml.
Cytokine Analysis.
After 19 days of in vitro culture (described above), 1 × 106 T cells were restimulated with either 1 × 105 MCA-207 tumor lysate-pulsed DC, unpulsed DC alone, tumor lysate alone, or DC pulsed with an irrelevant (control) MCA-102 tumor lysate in 2 ml of CM in 24-well tissue culture plates. After 24 hr, culture supernatants were collected for measurements of GM-CSF release in triplicate by using standard ELISA (23). Cytokine release was reported as mean picograms of GM-CSF ± SEM from triplicate samples.
Induction of Tumor-Specific CTL in Tumor Lysate-Pulsed, DC-Treated Mice.
Nylon wool-separated, spleen-derived T cells were isolated from mice effectively immunized by tumor lysate-pulsed DC and remaining tumor-free 30 days after challenge with a lethal dose of MCA-207 tumor. These splenic T cells (1 × 106 cells/ml) were cultured for 5 days in vitro with irradiated (10,000 rad) MCA-207 tumor cells (1 × 105 cells/ml) in 24-well culture plates (Costar) at a responder-to-stimulator ratio of 10:1. After 5 days of restimulation, these splenocytes were harvested and tested for cytolytic activity in the presence or absence of anti-MHC blocking mAb by using a standard 4-hr 51Cr-release assay (22, 23).
In Vivo Immunization and Tumor Challenge.
B6 or BALB/c mice were immunized s.c. in the right flank with 1 × 106 MCA-207 or 1 × 106 MT-901 tumor lysate-pulsed DC, respectively, twice at 7-day intervals. In some experiments, B6 or BALB/c mice were depleted of CD4+ or CD8+ T cells by i.v. injection of 200 μl anti-CD4 (GK1.5, rat IgG2b) or anti-CD8 (2.43, rat IgG2b) mAbs (American Type Culture Collection), respectively, 4 and 3 days before receiving the first immunization. Control mice received isotype-matched rat IgG (Sigma). Antibody treatment continued 3, 7, and 10 days after the first immunization to ensure chronic depletion of the desired cell type. The efficacy of the antibody depletion was analyzed by FACS and was determined to be completely effective (data not shown). Mice then were rechallenged 7 days after the last immunization with a lethal dose of 1 × 105 MCA-207 (for B6 mice) or 3 × 105 MT-901 (for BALB/c mice) viable tumor cells by s.c. injections into the left flank. The size of the tumors was assessed in a blinded, coded fashion twice weekly and recorded as tumor area (in square mm) by measuring the largest perpendicular diameters with calipers. Data are reported as the average tumor area ± SEM (five or more mice per group).
In Vivo Immunization for Treatment of Pulmonary Metastases.
B6 or BALB/c mice received 1.5 × 105 MCA-207 or 2 × 105 MT-901 viable tumor cells, respectively, i.v. in the lateral tail vein to establish pulmonary metastases, as described (19). The mice then were immunized s.c. with, respectively, 1 × 106 MCA-207 tumor lysate-pulsed DC three times on days 3, 7, and 11 or 1 × 106 MT-901 tumor lysate-pulsed DC twice on days 3 and 7 after tumor injection and were killed on days 14 and 17, respectively. On days 4 and 3 before the first immunization, groups of mice received 200 μl of ascites antibody i.v. (GK1.5, 2.43, or rat IgG) to deplete CD4+ or CD8+ T cells (described above). Antibody treatment continued 0, 4, and 7 days after the first immunization to ensure chronic depletion of the desired cell type. Pulmonary metastases were enumerated on day 15 (MCA-207) or 14 (MT-901), as described (19). Data are reported as the mean number of metastases ± SEM (five or more mice per group).
RESULTS
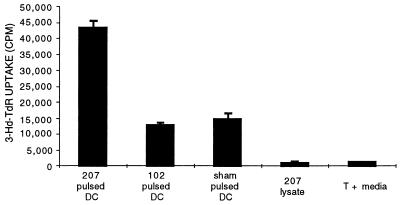
We first investigated the capacity of MCA-207 tumor lysate-pulsed, bone marrow-derived DC to prime naive syngeneic T cells in vitro, as measured by a tumor-specific proliferative response. T cells from B6 mouse spleen were stimulated in vitro by MCA-207 tumor lysate-pulsed DC, as described in Materials and Methods. As shown by the representative experiment of Fig. 1, repetitive stimulation of naive, syngeneic B6 T cells with MCA-207 tumor lysate-pulsed DC resulted in a significant proliferative response to MCA-207 tumor lysate-pulsed DC, compared with all control groups (P < 0.01). Additional control experiments demonstrated a lack of specific proliferative response to MCA-207 tumor lysate-pulsed DC when naive T cells were stimulated repetitively in vitro with either unpulsed DC alone, tumor lysate alone, or irradiated intact MCA-207 tumor cells alone (data not shown).
Figure 1.
Bone marrow-derived DC pulsed with tumor lysate can elicit a tumor-specific T cell proliferative response in vitro. DC were generated from B6 mouse bone marrow cells, were pulsed with tumor lysate, and were added to naive spleen T cells. Stimulated T cells were generated and tested as described in Materials and Methods. Responses are reported as mean cpm ± SEM of triplicate wells.
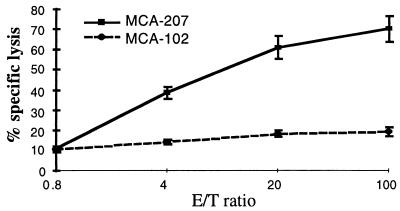
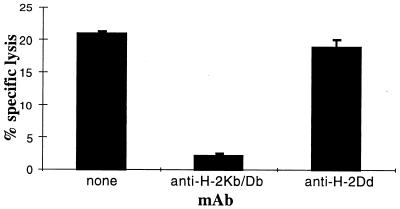
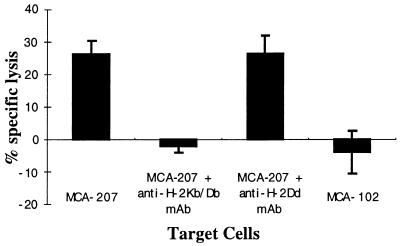
We also analyzed the capacity of MCA-207 tumor lysate-pulsed DC to generate MCA-207 tumor-specific CTL in vitro. T cells obtained from normal B6 mice were primed in vitro by three consecutive stimulations with MCA-207 tumor lysate-pulsed DC in the presence of IL-2 and IL-7, and the resulting T cells were evaluated for their cytolytic reactivity against MCA-207 and MCA-102 target cells. As shown in the representative experiment of Fig. 2, naive T cells repetitively stimulated with MCA-207 tumor lysate-pulsed DC were able to lyse efficiently and specifically MCA-207, but not MCA-102 tumor target cells. In contrast, repetitive stimulation of naive T cells with either control unpulsed DC alone, MCA-207 tumor lysate alone, or irradiated intact MCA-207 tumor cells alone failed to result in the generation of CTL (data not shown). Of importance, the anti-MCA-207 tumor cytolytic activity of these effector T cells was blocked by the addition of anti-H-2Kb/H-2Db, but not anti-H-2Db, mAb, indicating that these CTL were indeed MHC class I-restricted (Fig. 3).
Figure 2.
Bone marrow-derived DC pulsed with tumor lysate can generate tumor-specific cytotoxic T cells in vitro. Splenic T cells obtained from naive B6 mice were primed in vitro by MCA-207 tumor lysate-pulsed DC, as described in Materials and Methods, and the resulting effector cells were evaluated for cytolytic activity against 51Cr-labeled MCA-207 and MCA-102 target cells at the effector-to-target (E/T) ratios indicated. Values are the mean ± SEM of triplicate wells.
Figure 3.
Cytolytic T cell activity against MCA-207 tumor target cells is MHC class I-restricted. Tumor cells were preincubated with either haplotype-specific (H-2Kb/H-2Db) or nonspecific (H-2Dd) mAb for 30 min before the addition of T cells. Cytolytic T cells were generated by repetitive stimulation of naive B6 mouse splenic T cells with MCA-207 tumor lysate-pulsed DC in vitro. The assay was performed at an effector-to-target ratio of 4:1. Values are the mean ± SEM of triplicate wells.
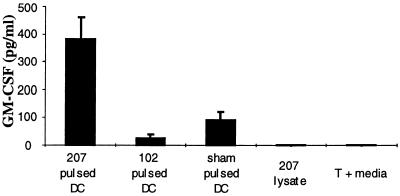
We and others reported that TILs could secrete cytokines upon specific tumor stimulation in vitro, which appeared to correlate with their antitumor therapeutic efficacy in vivo (23). T cells primed by MCA-207 tumor lysate-pulsed DC in vitro were restimulated for 24 hr with either MCA-207 tumor lysate-pulsed DC, unpulsed DC alone, tumor lysate alone, or irrelevant (control) MCA-102 tumor lysate-pulsed DC. As shown in Fig. 4, MCA-207 tumor lysate-pulsed DC induced specific GM-CSF production by primed T cells (>350 pg/106 cells) in response to stimulation by MCA-207 tumor lysate-pulsed DC compared with controls.
Figure 4.
Tumor-specific GM-CSF production by T cells primed in vitro by MCA-207 tumor lysate-pulsed DC. Responder T cells were cultured with either MCA-207 tumor lysate-pulsed DC, unpulsed DC alone, tumor lysate alone, or DC pulsed with a control, irrelevant MCA-102 tumor lysate. After 24 hr, culture supernatants were collected for measurement of GM-CSF release by ELISA. Cytokine release is reported in picograms (mean ± SEM of triplicate samples).
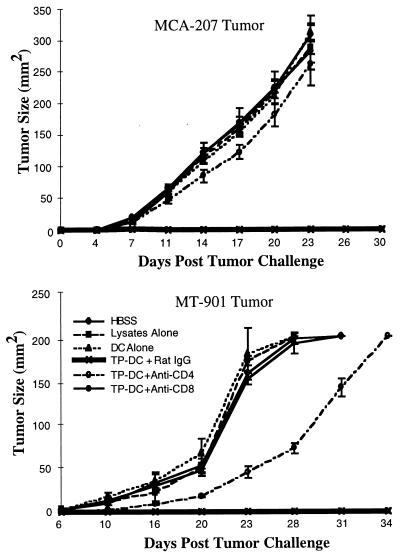
The capacity of tumor lysate-pulsed DC to immunize effectively naive mice was assessed in vivo by using the MCA-207 sarcoma and the MT-901 mammary carcinoma, syngeneic to B6 and BALB/c mice, respectively (Fig. 5). In representative experiments, s.c. immunization of mice with MCA-207 (Fig. 5 Upper) or MT-901 (Fig. 5 Lower) tumor lysate-pulsed DC resulted in strong protection against subsequent s.c. challenge with a lethal dose of the respective viable parental tumor cells (mice remained tumor-free for at least 100 days posttumor challenge; not shown). In contrast, control groups of mice either left untreated or immunized with unpulsed DC alone or tumor lysate alone all harbored large tumors >100 mm2 by day 24 after tumor rechallenge (P < 0.01). As shown in Fig. 5, selective depletion of CD8+ T cells or, to a lesser extent, CD4+ T cells by specific mAb administration prevented the effective immune priming by tumor lysate-pulsed DC. All immunized mice depleted of either CD4+ or CD8+ T cells succumbed to progressive MCA-207 or MT-901 tumor growth on challenge. By FACS analysis, spleens of mice treated with specific anti-CD4 or anti-CD8 mAb were chronically devoid of the respective T cell subset for at least the duration of tumor measurements shown in Fig. 6 (data not shown). In a separate experiment, immunization of mice with MT-901 tumor lysate-pulsed DC had no detectable effect on the growth of an unrelated syngeneic tumor (data not shown).
Figure 5.
Immunization with tumor lysate-pulsed DC protects B6 (Upper) and BALB/c (Lower) mice from a subsequent lethal challenge with syngeneic MCA-207 sarcoma and MT-901 breast carcinoma cells, respectively, and is T cell mediated. B6 and BALB/c mice were immunized s.c. with or without chronic depletion of CD4+ or CD8+ T cells, as described in Materials and Methods. Data are reported as the average tumor area ± SEM of five or more mice per group.
Figure 6.
Generation of tumor-specific, MHC class I-restricted cytotoxic T cells in tumor-free mice after immunization with tumor lysate-pulsed DC. Details of the generation and testing of these CTL are provided in Materials and Methods. The data are presented at an effector-to-target ratio of 4:1. Values are the mean ± SEM of triplicate wells.
Because CD8+ T cells were found by mAb depletion studies to play a major role in tumor rejection in vivo after immunization with tumor lysate-pulsed DC, we evaluated the capacity of spleen cells obtained from tumor-free mice to mediate specific CTL activity in vitro. Spleen cells from mice immunized s.c with tumor lysate-pulsed DC and rendered tumor-free for at least 30 days after MCA-207 tumor challenge were restimulated in vitro with irradiated tumor cells and tested for cytolytic activity. As shown in Fig. 6, these effector cells efficiently lysed MCA-207, but not MCA-102, tumor target cells. Importantly, cytolytic T cell activity was blocked completely by the addition of the appropriate haplotype-specific anti-MHC class I mAb. Control spleen cells from naive mice stimulated in vitro with irradiated MCA-207 tumor cells or spleen cells from mice rendered immune and tumor-free by tumor lysate-pulsed DC vaccination but not restimulated in vitro with irradiated MCA-207 tumor cells failed to demonstrate CTL activity in vitro (data not shown).
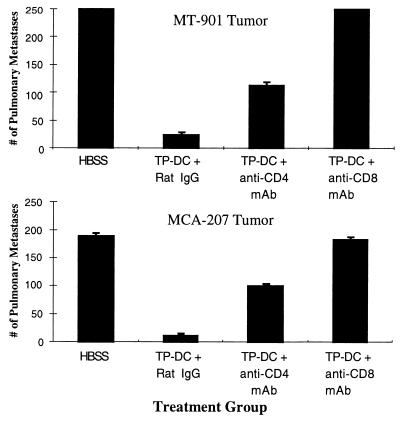
We next investigated whether tumor lysate-pulsed DC administration could mediate a therapeutic benefit on distant, established visceral metastases. In this treatment model, pulmonary metastases were established first by the i.v. injection of either MCA-207 sarcoma or MT-901 breast carcinoma cells in B6 and BALB/c mice, respectively. At day 3, when numerous micrometastases were evident in the lungs (19), the mice received the first s.c. injection of the respective MCA-207 or MT-901 tumor lysate-pulsed DC. Control mice received a similar course of s.c. injections of tumor lysate alone or unpulsed DC alone, or were left untreated. As shown by the representative experiment in Fig. 7, mice receiving MCA-207 (Fig. 7 Lower) or MT-901 (Fig. 7 Upper) tumor lysate-pulsed DC demonstrated ≈90% reduction in the number of established pulmonary metastases compared with the control groups (P < 0.01). Mice immunized with tumor lysates alone or (unpulsed) DC alone showed no reduction in the number of MCA-207 or MT-901 lung metastases (data not shown). In preliminary experiments, vaccination with tumor lysate-pulsed DC also could mediate significant reductions (by 25–70%) in the number of grossly visible, 7-day pulmonary metastases from MCA-207 and MT-901 tumors (data not shown).
Figure 7.
Immunization with tumor lysate-pulsed DC mediates regression of established pulmonary metastases via host-derived T cells. Induction of tumor nodules and their treatment are detailed in Materials and Methods. Values represent mean number of metastases ± SEM of five or more mice per group.
Fig. 7 also shows the effect of selective CD4+ and CD8+ T cell depletion by specific mAb administration in vivo. In both MCA-207 and MT-901 tumor models, complete elimination of the therapeutic efficacy of tumor lysate-pulsed DC on 3-day pulmonary metastases was observed after depletion of host-derived CD8+ T cells. In contrast, depletion of CD4+ T cells resulted in a partial, but significant, inhibition of the antitumor effect.
DISCUSSION
DC both can generate antigen-specific CTL from naive T cells and can stimulate CD4+ T cells to specific antigens in an MHC class I- and II-restricted manner (7). We have shown that whole tumor lysates can serve as effective immunogens to stimulate CD4+ T cell reactivity in vitro when processed and presented by murine Langerhans cells or splenic DC (11, 12). These CD4+ T cells, taken from tumor-immune mice, predominantly recognized unshared tumor-associated antigens. In these earlier studies, the fact that MHC class II-bearing Langerhans cells or splenic DC pulsed with tumor lysate-stimulated CD4+ T cells was of particular interest because in several tumor models antitumor CD4+ T cells have proven capable of mediating tumor rejection or conferring protective immunity (17, 24, 25). Moreover, the predominant immune T cell subset that specifically could traffic to and accumulate in murine sarcomas upon adoptive transfer i.v. has been shown to be CD4+ (17, 25).
In the current study, we demonstrated that bone marrow-derived, cytokine-driven DC pulsed with tumor lysates could generate both tumor-specific cytolytic and proliferative T cells from naive splenocytes in vitro. The successful generation of CTL is in contrast to our earlier studies using Langerhans cells and splenic-derived DC in which tumor-specific CD8+ T cell reactivity (as measured by proliferation) was not evoked after pulsing of these APC with tumor lysates (11, 12). However, in direct comparative studies between spleen-derived vs. cytokine-driven, bone marrow-derived DC, we have recently noted (R.C.F., J. J. Osterholzer, J. A. Fuller, E. K. Thomas, P. J. Geraghty, and J.J.M., unpublished work) enhanced phagocytosis of dextran particles and heightened stimulation of primary allogeneic mixed lymphocyte reactions by the latter compared with the former source of DC. The CTL response generated by MCA-207 tumor lysate-pulsed DC was shown to be MHC class I-restricted in antibody blocking experiments (Fig. 3). This finding is in agreement with earlier studies by others demonstrating that MHC class I presentation of exogenous, soluble antigens indeed can be achieved by professional APC both in vitro and in vivo (26).
Treatment of mice with s.c. injections of tumor lysate-pulsed DC could elicit effective immune priming, which also resulted in regression of established pulmonary metastases (Fig. 7). These in vivo antitumor effects were observed in two different strains of mice (B6 and BALB/c) against two histologically distinct syngeneic tumors (i.e., MCA-207 sarcoma and MT-901 breast carcinoma). We have reported (27) that vaccination with DC pulsed with a whole cell lysate likewise could mediate effective immune priming of naive mice to reject a subsequent lethal challenge dose of a subline of the B16 melanoma, denoted D5. This melanoma subline had been shown to be virtually nonimmunogenic by its complete inability to effectively immunize mice even when admixed with an optimum dose of the nonspecific immune adjuvant, Cryptosporidium parvum. By FACS analysis, this tumor also expressed few to no detectable surface MHC class I molecules and no detectable MHC class II molecules. Recent studies in some murine models have argued that the induction of antigen-specific immune tolerance is an early event in the course of tumor progression, which can inhibit immunotherapy (28). However, in our study the fact that immunization with tumor lysate-pulsed DC effectively could mediate the regression of pulmonary metastases (Fig. 7) either argues against early tolerance induction in this model or, alternatively, further supports a role of DC, other than a thymic-derived one (29), in breaking tolerance (30).
The absence of cross-protection to syngeneic MCA-induced sarcomas in mice has long indicated that recognition of tumor-specific transplantation antigens often plays the central role in the rejection of these tumors. Such rejections depend on both CD4+ and CD8+ effector T cells (ref. 24; Figs. 5 and 7). DC pulsed with tumor-associated peptides or model “tumor” proteins have shown therapeutic efficacy in vivo (9). The use of tumor lysate as the source of antigen(s), however, offers the advantage of potentially providing to the DC multiple tumor-associated antigens in the form of both helper- and CTL-defined epitopes for presentation to T cells, which could overcome tumor evasion by stimulating both arms of the cellular immune response. One potential disadvantage of this approach, however, is the possible induction of autoimmune reactivity to self or to normal tissue antigens present in the tumor lysate as a consequence of processing by potent antigen-presenting DC. It has been shown in melanoma (31), for example, that normal tissue antigens can be targets for cancer immunotherapy, leading in some instances to vitiligo in the treated patients. Although the potential exists for eliciting autoimmunity by immunization with tumor lysate-pulsed DC, our in vitro studies of CTL and proliferative T cell responses as well as in vivo immune priming and treatment of visceral metastases from murine sarcomas and breast carcinoma [as well as melanoma; (27)] have not shown to date the development of patterns of autoimmunity. Moreover, recent studies have demonstrated that expression of some self antigens can serve as targets for CTL-mediated destruction of tumors in the absence of any demonstrable damage to normal tissue (32).
In the MCA-207 tumor model, in vitro stimulation of naive splenocytes by tumor lysate-pulsed DC also resulted in the generation of specific GM-CSF producing T cells (Fig. 4). This finding is of particular interest because previous studies in mice and in humans have suggested a correlation between tumor-specific GM-CSF secretion by TIL or tumor-draining lymph node cells and their antitumor therapeutic efficacy in vivo (33). Primed T cells have the capacity to release cytokines such as TNF-α, INF-γ, and GM-CSF, which can lead to the recruitment and activation of other lymphocytes, monocytes, neutrophils, and DC. Unlike most CD8+ T cells, however, CD4+ TH1 cells also can produce IL-2, which can provide critical signaling to other effector cells and can help in the generation of optimal CD8+ T cell cytotoxic responses. Our previous work in the mouse had shown that both lytic and nonlytic CD8+ TIL could mediate substantial antitumor effects in vivo against established visceral metastases on adoptive transfer (23), which correlated with specific cytokine secretion in vitro and in vivo. In the current study, tumor lysate-pulsed DC efficiently could generate T cells with two separate critical functions, namely tumor-specific cytotoxicity and GM-CSF production. In vivo, s.c. administration of tumor lysate-pulsed DC resulted in a potent antitumor effect, which presumably is related to the triggering of these two distinct T cell functions.
DC can be obtained in large numbers from peripheral blood leukaphereses by culturing progenitor cells in GM-CSF, TNF-α, and/or IL-4 (21, 34). The establishment of such DC cultures from cancer patients has raised the important possibility of using these cells now as immunotherapeutic agents for the treatment of a variety of human tumors. DC-based tumor vaccines are now entering the clinical arena for the treatment of patients with cancer (35). Our data have shown that relatively crude membrane preparations of murine tumor cells will suffice as sources of tumor antigen(s), and they suggest that tumor lysate-pulsed DC may be of value in the treatment of human cancer as well. We recently initiated a phase I clinical trial at our institution of autologous tumor lysate-pulsed DC in pediatric and adult patients with advanced solid tumors.
Acknowledgments
We thank Dr. Elaine Thomas and Kathleen Picha of Immunex Corporation, Dr. Martin Giedlin of Chiron Corporation, and Dr. Satwant Narula of Schering-Plough Research Institute for providing recombinant mGM-CSF, hIL-2, and mIL-4, respectively. This work was supported by grants from the National Cancer Institute/National Institutes of Health (1 R01 CA71669 and 5 P01 CA59327); from the Department of Defense/United States Army (DAMD17-96-1-6103 and DAAG55-97-1-0239); and by gifts from C. J. and E. C. Aschauer and Abbott Laboratories.
ABBREVIATIONS
- TIL
tumor-infiltrating lymphocytes
- CTL
cytotoxic T lymphocyte
- DC
dendritic cells
- APC
antigen-presenting cells
- CM
complete medium
- MCA
3-methylcholanthrene
- MHC
major histocompatibility complex
- FACS
fluorescence-activated cell sorter
- CSF
colony-stimulating factor
- IL
interleukin
- MT
mammary tumor
References
- 1.Rosenberg S A. In: Biologic Therapy of Cancer. DeVita V T Jr, Hellman S, Rosenberg S A, editors. Philadelphia: Lippincott; 1994. pp. 487–507. [Google Scholar]
- 2.Hwu P, Shafer G E, Treisman J, Schindler D G, Gross G, Cowherd R, Rosenberg S A. J Exp Med. 1993;178:361–366. doi: 10.1084/jem.178.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg S A. Immunol Today. 1997;18:175–182. doi: 10.1016/s0167-5699(97)84664-6. [DOI] [PubMed] [Google Scholar]
- 4.Tepper R I, Mulé J J. Hum Gene Ther. 1994;5:153–164. doi: 10.1089/hum.1994.5.2-153. [DOI] [PubMed] [Google Scholar]
- 5.Kerr W G, Mulé J J. J Leukocyte Biol. 1994;56:210–214. doi: 10.1002/jlb.56.2.210. [DOI] [PubMed] [Google Scholar]
- 6.Ochoa A C, Longo D L. In: Important Advances in Oncology 1995. DeVita V T Jr, Hellman S, Rosenberg S A, editors. Philadelphia: Lippincott; 1995. pp. 43–57. [Google Scholar]
- 7.Steinman R. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 8.Takamizawa M, Rivas A, Fagnoni F, Benike C, Kosek J, Hyakawa H, Engleman E. J Immunol. 1997;158:2134–2142. [PubMed] [Google Scholar]
- 9.Stingl G, Bergstresser P R. Immunol Today. 1995;16:330–333. doi: 10.1016/0167-5699(95)80148-0. [DOI] [PubMed] [Google Scholar]
- 10.Shuler G, Steinman R M. J Exp Med. 1997;186:1183–1187. doi: 10.1084/jem.186.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen P J, Cohen P A, Rosenberg S A, Katz S I, Mulé J J. Eur J Immunol. 1994;24:315–319. doi: 10.1002/eji.1830240206. [DOI] [PubMed] [Google Scholar]
- 12.Cohen P A, Cohen P J, Rosenberg S A, Mulé J J. Cancer Res. 1994;54:1055–1058. [PubMed] [Google Scholar]
- 13.Bevan M J. J Exp Med. 1976;143:1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahin U, Tureci O, Schmitt H, Cochlovirus B, Johannes T, Schmits R, Stenner F, Luo G, Schobert I, Pfreundschuh M. Proc Natl Acad Sci USA. 1995;92:11810–11813. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toes R E M, Blom R J J, Offringa R, Kast W M, Melief C J M. J Immunol. 1996;156:3911–3918. [PubMed] [Google Scholar]
- 16.Topalian S L, Rivoltini L, Mancini M, Markus N R, Robbins P F, Kawakami Y, Rosenberg S A. Proc Natl Acad Sci USA. 1994;91:9461–9465. doi: 10.1073/pnas.91.20.9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulé J J, Hellstrom I, Hellstrom K E. Am J Pathol. 1982;107:142–149. [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell M S, Harel W, Kan-Mitchell J, LeMay L G, Goedegebuure P, Huang X Q, Hofman F, Groshen S. Ann N Y Acad Sci. 1993;690:153–166. doi: 10.1111/j.1749-6632.1993.tb44005.x. [DOI] [PubMed] [Google Scholar]
- 19.Mulé J J, Shu S, Schwarz S L, Rosenberg S A. Science. 1984;225:1487–1489. doi: 10.1126/science.6332379. [DOI] [PubMed] [Google Scholar]
- 20.Margaretten N C, Witschi H. Cancer Res. 1988;48:2779–2786. [PubMed] [Google Scholar]
- 21.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman R. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jicha D L, Mulé J J, Rosenberg S A. J Exp Med. 1991;174:1511–1515. doi: 10.1084/jem.174.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barth R J, Mulé J J, Spiess P J, Rosenberg S A. J Exp Med. 1991;173:647–658. doi: 10.1084/jem.173.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chou T, Shu S. J Immunol. 1987;139:2103–2109. [PubMed] [Google Scholar]
- 25.Mulé J J, Jones F R, Hellstrom I, Hellstrom K E. J Immunol. 1979;123:600–607. [PubMed] [Google Scholar]
- 26.Rock K L, Gamble S, Rothstein L. Science. 1990;249:918–922. doi: 10.1126/science.2392683. [DOI] [PubMed] [Google Scholar]
- 27.Geraghty P J, Fields R C, Mulé J J. Surg Forum. 1996;47:459–461. [Google Scholar]
- 28.Sotomayor E M, Borrello I, Staveley-O’Carroll K, Montgomery J, Fein S, Hwang L, Levitsky H I, Fuchs E. Blood. 1997;90:458a. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kronin V, Suss G, Winkel K, Shortman K. Adv Exp Med Biol. 1997;417:239–248. doi: 10.1007/978-1-4757-9966-8_40. [DOI] [PubMed] [Google Scholar]
- 30.Ridge J P, Fuchs E J, Matzinger P. Science. 1996;271:1723–1726. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg S A, White D E. J Immunother. 1996;19:81–84. [PubMed] [Google Scholar]
- 32.Vierboom M P M, Nijman H W, Offringa R, van der Voort E I H, van Hall T, van den Broek L, Fleuren G J, Kenemans P, Kast W M, Melief C J M. J Exp Med. 1997;186:695–704. doi: 10.1084/jem.186.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aruga A, Aruga E, Cameron M J, Chang A E. J Leukocyte Biol. 1997;61:507–516. doi: 10.1002/jlb.61.4.507. [DOI] [PubMed] [Google Scholar]
- 34.Hart D N C. Blood. 1997;90:3245–3287. [PubMed] [Google Scholar]
- 35.Hsu F J, Benike C, Fagnoni F, Liles T M, Czerwinski D, Taidi B, Engleman E G, Levy R. Nat Med. 1996;2:52–56. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]