Abstract
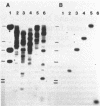
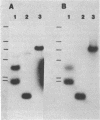
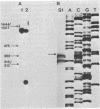
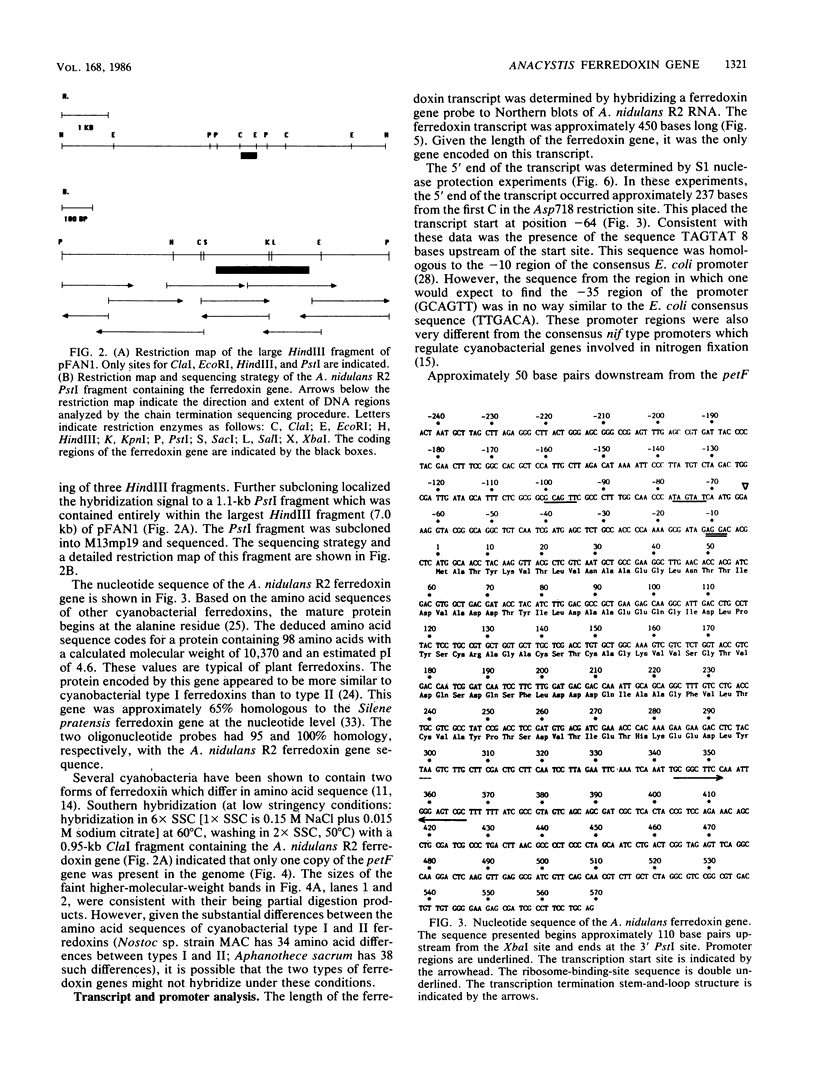
Two mixed oligonucleotide probes derived from conserved regions of the Synechocystis sp. strain PCC 6714 ferredoxin amino acid sequence were utilized to isolate an Anacystis nidulans R2 clone containing the ferredoxin I gene. Nucleotide sequence analysis revealed a 297-base-pair (bp) open reading frame with a deduced amino acid sequence having high homology to other cyanobacterial ferredoxins. Assuming proteolytic cleavage of the initial methionine residue, the molecular weight of the mature A. nidulans R2 ferredoxin was 10,370. The initial methionine residue was preceded by a probable ribosome-binding site sequence, AGGA. Northern hybridization analysis with the cloned ferredoxin gene indicated an RNA transcript of approximately 450 bp. S1 nuclease mapping localized the transcription start site to a position 64 bases upstream from the initial methionine residue. The nucleotide sequence 14 to 8 bp preceding the transcription start site resembled a typical Escherichia coli promoter, but the sequence in the -35 region did not. Southern hybridization detected only a single copy of the ferredoxin sequence in the A. nidulans R2 genome.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borbely G., Simoncsits A. 3'-Terminal conserved loops of 16S rRNAs from the cyanobacterium Synechococcus AN PCC 6301 and maize chloroplast differ only in two bases. Biochem Biophys Res Commun. 1981 Aug 14;101(3):846–852. doi: 10.1016/0006-291x(81)91827-1. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B. The ferredoxin/thioredoxin system: a key element in the regulatory function of light in photosynthesis. Bioscience. 1984 Jun;34(6):378–383. [PubMed] [Google Scholar]
- Cammack R., Rao K. K., Hall D. O. Metalloproteins in the evolution of photosynthesis. Biosystems. 1981;14(1):57–80. doi: 10.1016/0303-2647(81)90022-8. [DOI] [PubMed] [Google Scholar]
- Conley P. B., Lemaux P. G., Grossman A. R. Cyanobacterial light-harvesting complex subunits encoded in two red light-induced transcripts. Science. 1985 Nov 1;230(4725):550–553. doi: 10.1126/science.3931221. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Hase T., Inoue K., Matsubara H., Williams M. M., Rogers L. J. Amino acid sequence of Synechocystis 6714 ferredoxin: a unique structural feature of unicellular blue-green algal ferredoxin. J Biochem. 1982 Nov;92(5):1357–1362. doi: 10.1093/oxfordjournals.jbchem.a134059. [DOI] [PubMed] [Google Scholar]
- Hase T., Matsubara H., Hutber G. N., Rogers L. J. Amino acid sequences of Nostoc strain MAC ferredoxins I and II. J Biochem. 1982 Nov;92(5):1347–1355. doi: 10.1093/oxfordjournals.jbchem.a134058. [DOI] [PubMed] [Google Scholar]
- Hase T., Wada K., Matsubara H. Amino acid sequence of the major component of Aphanothece sacrum ferredoxin. J Biochem. 1976 Feb;79(2):329–343. doi: 10.1093/oxfordjournals.jbchem.a131076. [DOI] [PubMed] [Google Scholar]
- Hase T., Wada K., Matsubara H. Horsetail (Equisetum arvense) ferredoxins I and II Amino acid sequences and gene duplication. J Biochem. 1977 Jul;82(1):277–286. doi: 10.1093/oxfordjournals.jbchem.a131680. [DOI] [PubMed] [Google Scholar]
- Hase T., Wakabayashi S., Wada K., Matsubara H. Amino acid sequence of Aphanothece sacrum Ferredoxin II (minor component). Structural characteristics and evolutionary implications. J Biochem. 1978 Mar;83(3):761–770. doi: 10.1093/oxfordjournals.jbchem.a131970. [DOI] [PubMed] [Google Scholar]
- Hutson K. G., Rogers L. J., Haslett B. G., Boulter D., Cammack R. Comparative studies on two ferredoxins from the cyanobacterium Nostoc strain MAC. Biochem J. 1978 Jun 15;172(3):465–477. doi: 10.1042/bj1720465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea P. J., Miflin B. J. Alternative route for nitrogen assimilation in higher plants. Nature. 1974 Oct 18;251(5476):614–616. doi: 10.1038/251614a0. [DOI] [PubMed] [Google Scholar]
- Manzano C., Candau P., Gomez-Moreno C., Relimpio A. M., Losada M. Ferredoxin-dependent photosynthetic reduction of nitrate and nitrite by particles of Anacystis nidulans. Mol Cell Biochem. 1976 Feb 25;10(3):161–169. doi: 10.1007/BF01731687. [DOI] [PubMed] [Google Scholar]
- Nierzwicki-Bauer S. A., Curtis S. E., Haselkorn R. Cotranscription of genes encoding the small and large subunits of ribulose-1,5-bisphosphate carboxylase in the cyanobacterium Anabaena 7120. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5961–5965. doi: 10.1073/pnas.81.19.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pustell J., Kafatos F. C. A convenient and adaptable package of computer programs for DNA and protein sequence management, analysis and homology determination. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):643–655. doi: 10.1093/nar/12.1part2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Trebst A. The mechanism of photosynthetic sulfate reduction by isolated chloroplasts. Biochim Biophys Acta. 1969 Aug 5;180(3):529–535. doi: 10.1016/0005-2728(69)90031-0. [DOI] [PubMed] [Google Scholar]
- Shin M., Sukenobu M., Oshino R., Kitazume Y. Two plant-type ferredoxins from a blue-green alga, Nostoc verrucosum. Biochim Biophys Acta. 1977 Apr 11;460(1):85–93. doi: 10.1016/0005-2728(77)90154-2. [DOI] [PubMed] [Google Scholar]
- Smeekens S., van Binsbergen J., Weisbeek P. The plant ferredoxin precursor: nucleotide sequence of a full length cDNA clone. Nucleic Acids Res. 1985 May 10;13(9):3179–3194. doi: 10.1093/nar/13.9.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. V., Noy R. J., Evans M. C. Physiological electron donor systems to the nitrogenase of the blue-green alga Anabaena cylindrica. Biochim Biophys Acta. 1971 Nov 2;253(1):104–109. doi: 10.1016/0005-2728(71)90238-6. [DOI] [PubMed] [Google Scholar]
- Tandeau de Marsac N., Borrias W. E., Kuhlemeier C. J., Castets A. M., van Arkel G. A., van den Hondel C. A. A new approach for molecular cloning in cyanobacteria: cloning of an Anacystis nidulans met gene using a Tn901-induced mutant. Gene. 1982 Nov;20(1):111–119. doi: 10.1016/0378-1119(82)90092-0. [DOI] [PubMed] [Google Scholar]
- Tandeau de Marsac N., Mazel D., Bryant D. A., Houmard J. Molecular cloning and nucleotide sequence of a developmentally regulated gene from the cyanobacterium Calothrix PCC 7601: a gas vesicle protein gene. Nucleic Acids Res. 1985 Oct 25;13(20):7223–7236. doi: 10.1093/nar/13.20.7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Tomioka N., Sugiura M. The complete nucleotide sequence of a 16S ribosomal RNA gene from a blue-green alga, Anacystis nidulans. Mol Gen Genet. 1983;191(1):46–50. doi: 10.1007/BF00330888. [DOI] [PubMed] [Google Scholar]