Abstract
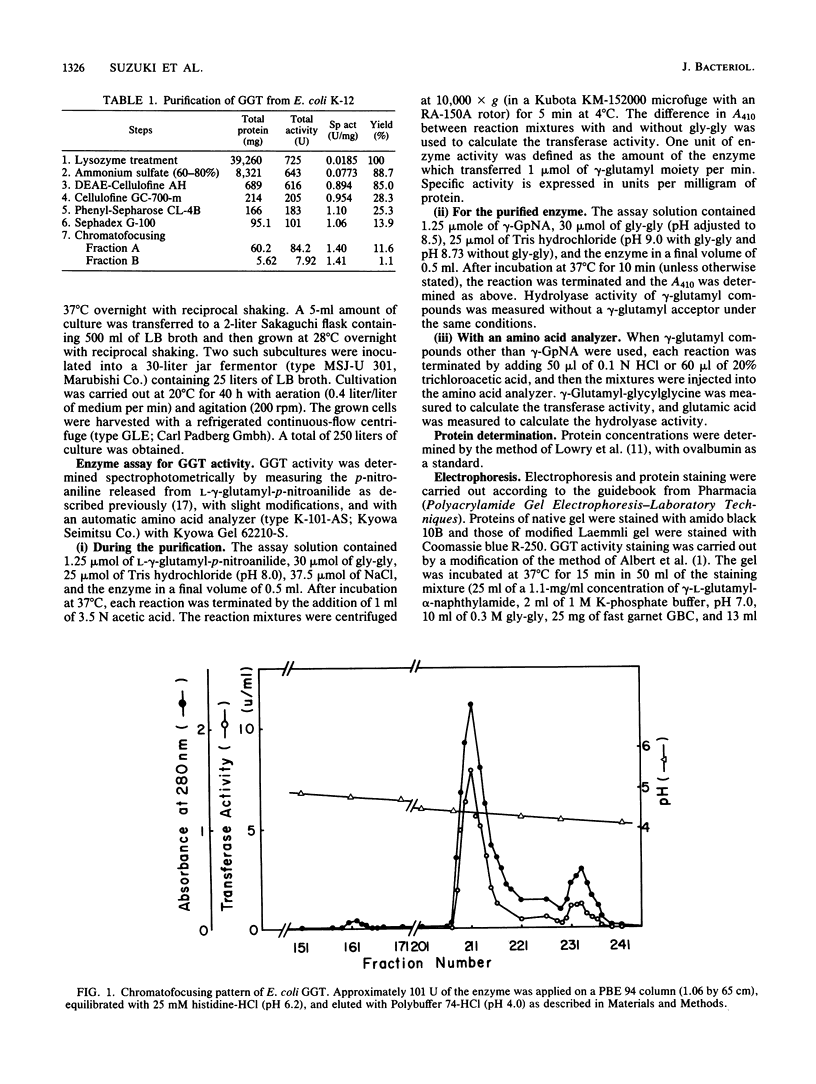
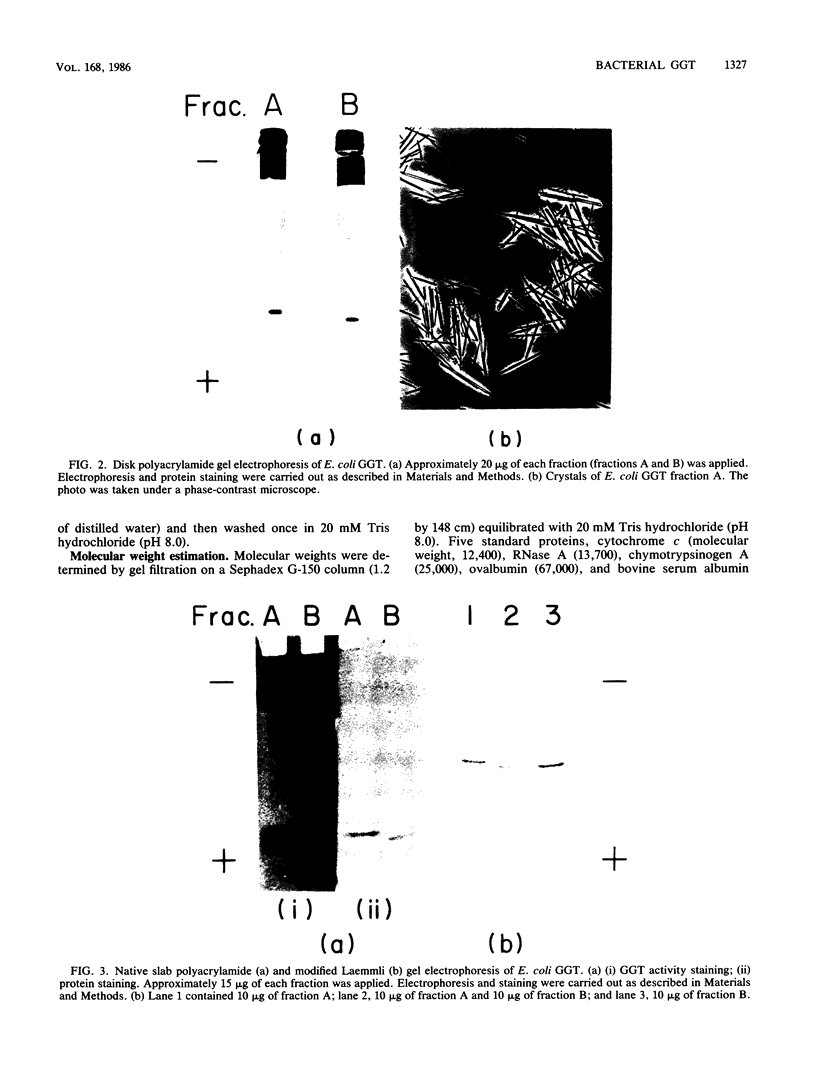
gamma-Glutamyltranspeptidase (GGT) (EC 2.3.2.2) was purified from the periplasmic fraction of Escherichia coli K-12 to electrophoretic homogeneity. The final purification step, chromatofocusing, gave two protein peaks showing GGT activity (fractions A and B). The major heavy fraction (fraction A) consisted of two different subunits, with molecular weights of 39,200 and 22,000. The minor light fraction (fraction B) consisted of those with molecular weights of 38,600 and 22,000. Fraction A catalyzes the hydrolysis and transpeptidation of all gamma-glutamyl compounds tested, but it prefers basic amino acids and aromatic amino acids as acceptors. The apparent Km values for glutathione and gamma-glutamyl-p-nitroanilide as gamma-glutamyl donors in the transpeptidation reaction were both 35 microM, and those for glycylglycine and L-arginine as acceptors were 0.59 and 0.21 M, respectively. The enzyme was inhibited by some amino acids and by protease inhibitors and affinity-labeling reagents for GGT. The temperature stability of the purified GGT supports our hypothesis that E. coli GGT is synthesized only at lower temperature rather than that the synthesized GGT is degraded or inactivated at higher temperature.
Full text
PDF
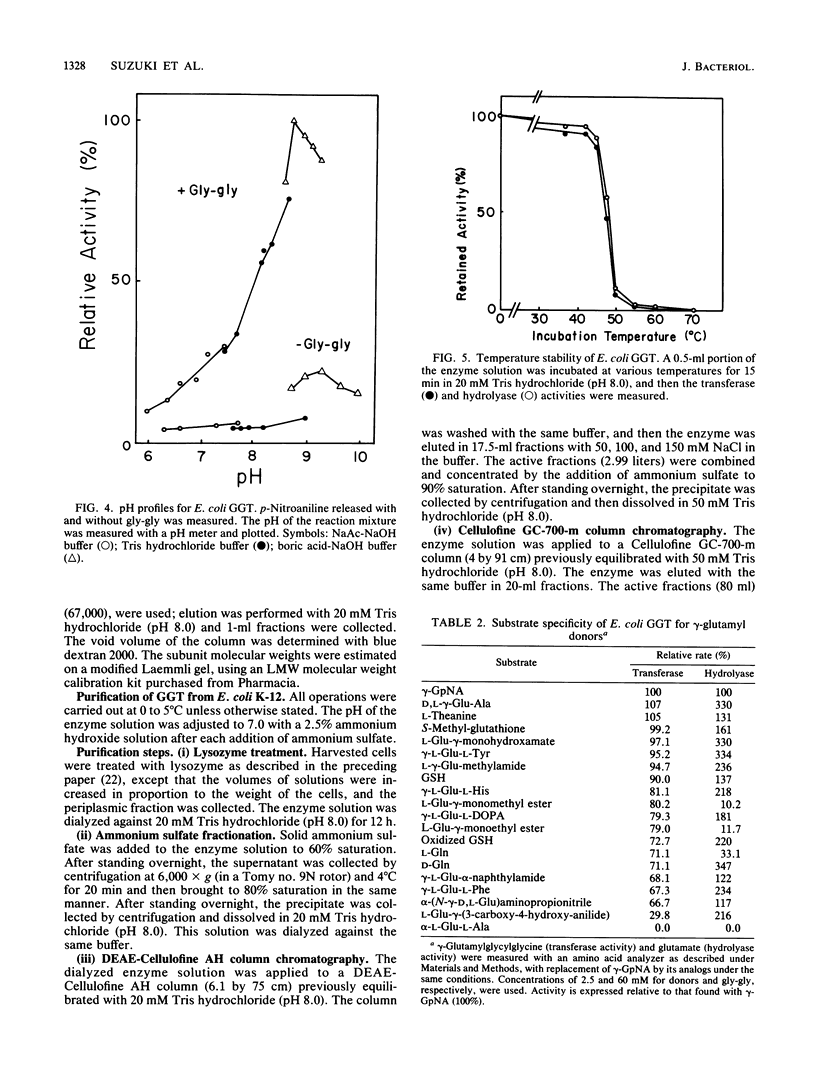



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERT Z., ORLOWSKI M., SZEWCZUK A. Histochemical demonstration of gamma-glutamyl transpeptidase. Nature. 1961 Aug 19;191:767–768. doi: 10.1038/191767a0. [DOI] [PubMed] [Google Scholar]
- Allison R. D. gamma-Glutamyl transpeptidase: kinetics and mechanism. Methods Enzymol. 1985;113:419–437. doi: 10.1016/s0076-6879(85)13054-5. [DOI] [PubMed] [Google Scholar]
- Boyland E., Chasseaud L. F. The role of glutathione and glutathione S-transferases in mercapturic acid biosynthesis. Adv Enzymol Relat Areas Mol Biol. 1969;32:173–219. doi: 10.1002/9780470122778.ch5. [DOI] [PubMed] [Google Scholar]
- Gardell S. J., Tate S. S. Latent proteinase activity of gamma-glutamyl transpeptidase light subunit. J Biol Chem. 1979 Jun 25;254(12):4942–4945. [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Translocation of intracellular glutathione to membrane-bound gamma-glutamyl transpeptidase as a discrete step in the gamma-glutamyl cycle: glutathionuria after inhibition of transpeptidase. Proc Natl Acad Sci U S A. 1979 Jan;76(1):268–272. doi: 10.1073/pnas.76.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Novogrodsky A., Meister A. Translocation of glutathione from lymphoid cells that have markedly different gamma-glutamyl transpeptidase activities. Proc Natl Acad Sci U S A. 1979 May;76(5):2249–2252. doi: 10.1073/pnas.76.5.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Horiuchi S., Morino Y. gamma-Glutamyl transpeptidase in rat ascites tumor cell LY-5. Lack of functional correlation of its catalytic activity with the amino acid transport. Eur J Biochem. 1977 Sep;78(2):609–615. doi: 10.1111/j.1432-1033.1977.tb11774.x. [DOI] [PubMed] [Google Scholar]
- Inoue M., Kinne R., Tran T., Biempica L., Arias I. M. Rat liver canalicular membrane vesicles. Isolation and topological characterization. J Biol Chem. 1983 Apr 25;258(8):5183–5188. [PubMed] [Google Scholar]
- Kozak E. M., Tate S. S. Interaction of the antitumor drug, L-(alpha S, 5S)-alpha-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid (AT-125) with renal brush border membranes. Specific labeling of gamma-glutamyl transpeptidase. FEBS Lett. 1980 Dec 29;122(2):175–178. doi: 10.1016/0014-5793(80)80431-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Nakayama R., Kumagai H., Tochikura T. Purification and properties of gamma-glutamyltranspeptidase from Proteus mirabilis. J Bacteriol. 1984 Oct;160(1):341–346. doi: 10.1128/jb.160.1.341-346.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORLOWSKI M., MEISTER A. GAMMA-GLUTAMYL-P-NITROANILIDE: A NEW CONVENIENT SUBSTRATE FOR DETERMINATION AND STUDY OF L- AND D-GAMMA-GLUTAMYLTRANSPEPTIDASE ACTIVITIES. Biochim Biophys Acta. 1963 Aug 6;73:679–681. doi: 10.1016/0006-3002(63)90348-2. [DOI] [PubMed] [Google Scholar]
- Okajima K., Inoue M., Morino Y. Topology and some properties of the renal brush border membrane-bound peptidase(s) participating in the metabolism of S-carbamidomethyl glutathione. Biochim Biophys Acta. 1981 Jul 17;675(3-4):379–385. doi: 10.1016/0304-4165(81)90029-5. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Meister A. The gamma-glutamyl cycle: a possible transport system for amino acids. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1248–1255. doi: 10.1073/pnas.67.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellefigue F., Butler J. D., Spielberg S. P., Hollenberg M. D., Goodman S. I., Schulman J. D. Normal amino acid uptake by cultured human fibroblasts does not require gamma-glutamyl transpeptidase. Biochem Biophys Res Commun. 1976 Dec 20;73(4):997–1002. doi: 10.1016/0006-291x(76)90221-7. [DOI] [PubMed] [Google Scholar]
- Robins R. J., Davies D. D. The role of glutathione in amino-acid absorption. Lack of correlation between glutathione turnover and amino-acid absorption by the yeast Candida utilis. Biochem J. 1981 Jan 15;194(1):63–70. doi: 10.1042/bj1940063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman J. D., Goodman S. I., Mace J. W., Patrick A. D., Tietze F., Butler E. J. Glutathionuria: inborn error of metabolism due to tissue deficiency of gamma-glutamyl transpeptidase. Biochem Biophys Res Commun. 1975 Jul 8;65(1):68–74. doi: 10.1016/s0006-291x(75)80062-3. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kumagai H., Tochikura T. gamma-Glutamyltranspeptidase from Escherichia coli K-12: formation and localization. J Bacteriol. 1986 Dec;168(3):1332–1335. doi: 10.1128/jb.168.3.1332-1335.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate S. S., Meister A. Affinity labeling of gamma-glutamyl transpeptidase and location of the gamma-glutamyl binding site on the light subunit. Proc Natl Acad Sci U S A. 1977 Mar;74(3):931–935. doi: 10.1073/pnas.74.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate S. S., Meister A. gamma-Glutamyl transpeptidase from kidney. Methods Enzymol. 1985;113:400–419. doi: 10.1016/s0076-6879(85)13053-3. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Meister A. gamma-Glutamyl transpeptidase: catalytic, structural and functional aspects. Mol Cell Biochem. 1981 Sep 25;39:357–368. doi: 10.1007/BF00232585. [DOI] [PubMed] [Google Scholar]
- Tsao B., Curthoys N. P. The absolute asymmetry of orientation of gamma-glutamyltranspeptidase and aminopeptidase on the external surface of the rat renal brush border membrane. J Biol Chem. 1980 Aug 25;255(16):7708–7711. [PubMed] [Google Scholar]