Abstract
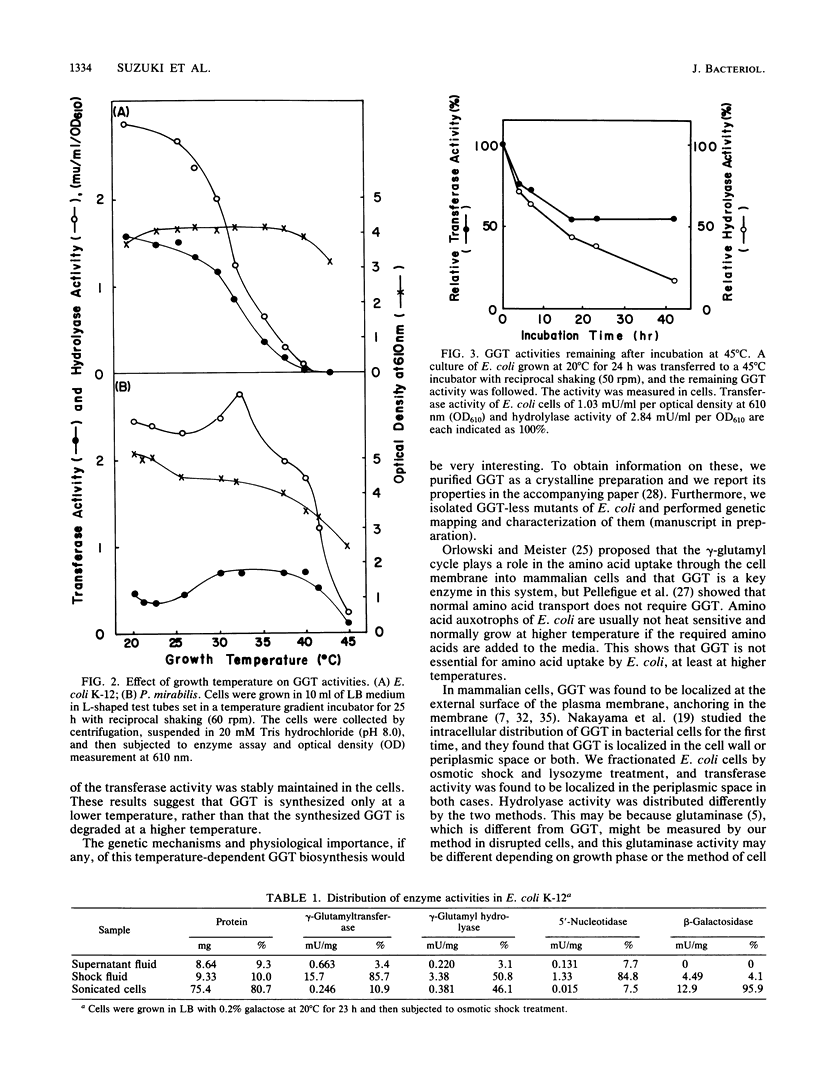
Escherichia coli cells showed maximum activity of gamma-glutamyltranspeptidase (EC 2.3.2.2) when they were grown at 20 degrees C, 14% of maximum activity at 37 degrees C, and none at 43 degrees C. The enzyme activity of intact cells grown at 20 degrees C was stably maintained after the temperature was changed to 45 degrees C. The activity increased during the exponential phase, and maximum activity was found at stationary phase. Its intracellular localization in the periplasmic space was confirmed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison R. D. gamma-Glutamyl transpeptidase: kinetics and mechanism. Methods Enzymol. 1985;113:419–437. doi: 10.1016/s0076-6879(85)13054-5. [DOI] [PubMed] [Google Scholar]
- Guyer M. S., Reed R. R., Steitz J. A., Low K. B. Identification of a sex-factor-affinity site in E. coli as gamma delta. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):135–140. doi: 10.1101/sqb.1981.045.01.022. [DOI] [PubMed] [Google Scholar]
- Inoue M., Kinne R., Tran T., Biempica L., Arias I. M. Rat liver canalicular membrane vesicles. Isolation and topological characterization. J Biol Chem. 1983 Apr 25;258(8):5183–5188. [PubMed] [Google Scholar]
- Kenny A. J., Booth A. G. Microvilli: their ultrastructure, enzymology and molecular organization. Essays Biochem. 1978;14:1–44. [PubMed] [Google Scholar]
- Kiuchi K., Kiuchi K., Nagatsu T., Togari A., Kumagai H. Highly sensitive assay for gamma-glutamyltranspeptidase activity by high-performance liquid chromatography with electrochemical detection. J Chromatogr. 1986 Apr 23;357(1):191–198. doi: 10.1016/s0021-9673(01)95820-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MALAMY M. H., HORECKER B. L. PURIFICATION AND CRYSTALLIZATION OF THE ALKALINE PHOSPHATASE OF ESCHERICHIA COLI. Biochemistry. 1964 Dec;3:1893–1897. doi: 10.1021/bi00900a018. [DOI] [PubMed] [Google Scholar]
- Milbauer R., Grossowicz N. Gamma-glutamyl transfer reactions in bacteria. J Gen Microbiol. 1965 Nov;41(2):185–194. doi: 10.1099/00221287-41-2-185. [DOI] [PubMed] [Google Scholar]
- Mizushima S., Yamada H. Isolation and characterization of two outer membrane preparations from Escherichia coli. Biochim Biophys Acta. 1975 Jan 14;375(1):44–53. doi: 10.1016/0005-2736(75)90071-1. [DOI] [PubMed] [Google Scholar]
- Mooz E. D., Wigglesworth L. Evidence for the gamma-glutamyl cycle in yeast. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1066–1072. doi: 10.1016/0006-291x(76)90304-1. [DOI] [PubMed] [Google Scholar]
- Nakayama R., Kumagai H., Tochikura T. Leakage of glutathione from bacterial cells caused by inhibition of gamma-glutamyltranspeptidase. Appl Environ Microbiol. 1984 Apr;47(4):653–657. doi: 10.1128/aem.47.4.653-657.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama R., Kumagai H., Tochikura T. Purification and properties of gamma-glutamyltranspeptidase from Proteus mirabilis. J Bacteriol. 1984 Oct;160(1):341–346. doi: 10.1128/jb.160.1.341-346.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama R., Kumagai H., Tochikura T. gamma-Glutamyltranspeptidase from Proteus mirabilis: localization and activation by phospholipids. J Bacteriol. 1984 Dec;160(3):1031–1036. doi: 10.1128/jb.160.3.1031-1036.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
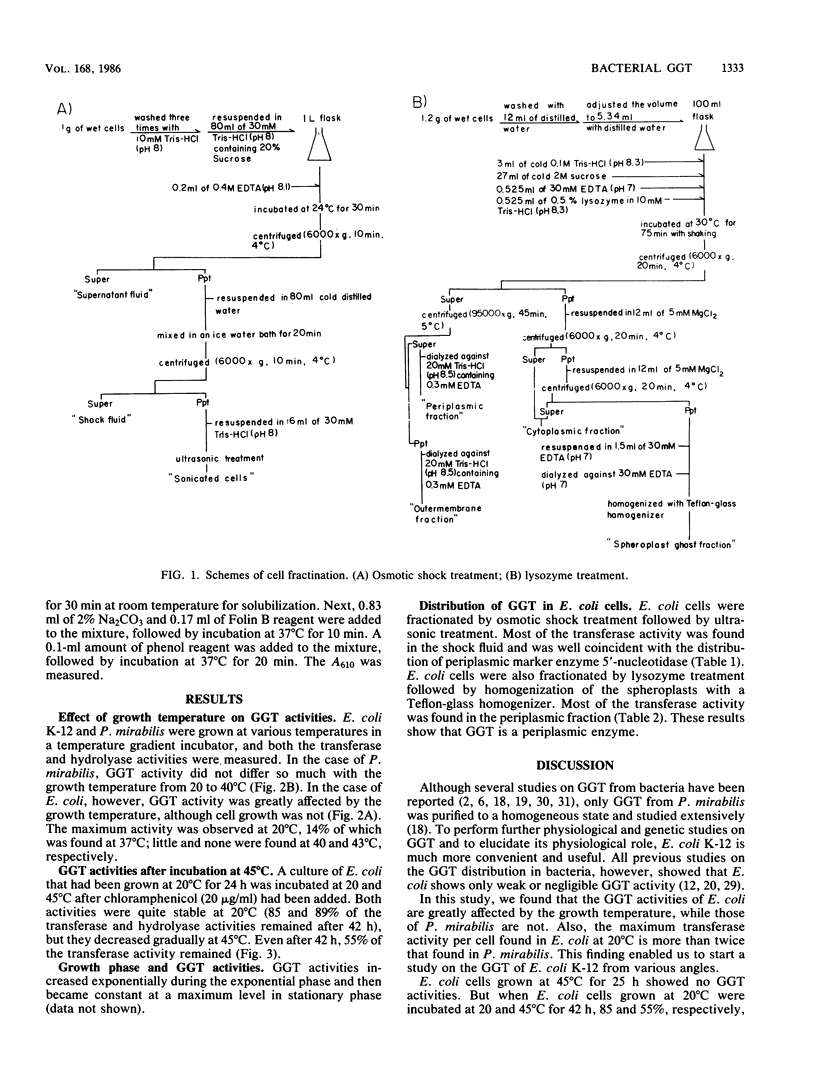
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Neu H. C. The 5'-nucleotidase of Escherichia coli. I. Purification and properties. J Biol Chem. 1967 Sep 10;242(17):3896–3904. [PubMed] [Google Scholar]
- ORLOWSKI M., MEISTER A. GAMMA-GLUTAMYL-P-NITROANILIDE: A NEW CONVENIENT SUBSTRATE FOR DETERMINATION AND STUDY OF L- AND D-GAMMA-GLUTAMYLTRANSPEPTIDASE ACTIVITIES. Biochim Biophys Acta. 1963 Aug 6;73:679–681. doi: 10.1016/0006-3002(63)90348-2. [DOI] [PubMed] [Google Scholar]
- ORLOWSKI M., MEISTER A. ISOLATION OF GAMMA-GLUTAMYL TRANSPEPTIDASE FROM HOG KIDNEY. J Biol Chem. 1965 Jan;240:338–347. [PubMed] [Google Scholar]
- Orlowski M., Meister A. The gamma-glutamyl cycle: a possible transport system for amino acids. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1248–1255. doi: 10.1073/pnas.67.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuji G. O. The pathways of the gamma-glutamyl cycle-mediated uptake of amino acids in yeast. FEBS Lett. 1979 Dec 1;108(1):240–242. doi: 10.1016/0014-5793(79)81219-3. [DOI] [PubMed] [Google Scholar]
- Pellefigue F., Butler J. D., Spielberg S. P., Hollenberg M. D., Goodman S. I., Schulman J. D. Normal amino acid uptake by cultured human fibroblasts does not require gamma-glutamyl transpeptidase. Biochem Biophys Res Commun. 1976 Dec 20;73(4):997–1002. doi: 10.1016/0006-291x(76)90221-7. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kumagai H., Tochikura T. gamma-Glutamyltranspeptidase from Escherichia coli K-12: purification and properties. J Bacteriol. 1986 Dec;168(3):1325–1331. doi: 10.1128/jb.168.3.1325-1331.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczuk A., Mulczyk M. Studies on gamma-glutamyl peptidase from Pseudomonas aeruginosa. Arch Immunol Ther Exp (Warsz) 1970;18(5):515–526. [PubMed] [Google Scholar]
- TALALAY P. S. Glutathione breakdown and transpeptidation reactions in Proteus vulgaris. Nature. 1954 Sep 11;174(4428):516–517. doi: 10.1038/174516b0. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Meister A. gamma-Glutamyl transpeptidase from kidney. Methods Enzymol. 1985;113:400–419. doi: 10.1016/s0076-6879(85)13053-3. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Meister A. gamma-Glutamyl transpeptidase: catalytic, structural and functional aspects. Mol Cell Biochem. 1981 Sep 25;39:357–368. doi: 10.1007/BF00232585. [DOI] [PubMed] [Google Scholar]
- Touster O., Aronson N. N., Jr, Dulaney J. T., Hendrickson H. Isolation of rat liver plasma membranes. Use of nucleotide pyrophosphatase and phosphodiesterase I as marker enzymes. J Cell Biol. 1970 Dec;47(3):604–618. doi: 10.1083/jcb.47.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao B., Curthoys N. P. The absolute asymmetry of orientation of gamma-glutamyltranspeptidase and aminopeptidase on the external surface of the rat renal brush border membrane. J Biol Chem. 1980 Aug 25;255(16):7708–7711. [PubMed] [Google Scholar]
- Widnell C. C., Unkeless J. C. Partial purification of a lipoprotein with 5'-nucleotidase activity from membranes of rat liver cells. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1050–1057. doi: 10.1073/pnas.61.3.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]



