Abstract
Macrophage migration inhibitory factor (MIF), a proinflammatory mediator, has been shown to be elevated following heart surgery in adults and may be associated with several postoperative complications, including cardiac and pulmonary dysfunction. In this study, we aimed to measure perioperative plasma MIF, interleukin (IL)-8, and free T4 in 20 children age <4 years undergoing surgical repair of congenital heart lesions with left ventricular volume overload, and to determine whether the response of these mediators determined postoperative outcomes. Plasma samples were obtained preoperatively, immediately on arrival in the pediatric intensive care unit (PICU), and at 12, 24, and 48 h. Patients were continuously monitored in the PICU, and data were recorded daily for therapeutic and monitoring procedures that reflected the invasiveness, intensity, and complexity of care rendered (therapeutic interventional scoring system, TISS). Preoperative plasma MIF, IL-8, and free T4 were not different from age-matched healthy children. However, plasma MIF and IL-8 increased significantly 2 h after completion of cardiopulmonary bypass, and then normalized within 24 h. Peak postoperative levels of MIF (48 ± 24 ng/mL) and IL-8 (79 ± 57 pg/mL) correlated significantly with duration of cardiopulmonary bypass. The magnitude of the postoperative increase in plasma MIF was associated with increased number of days required for mechanical ventilation (r = 0.553; P = 0.012), and peak plasma IL-8 correlated significantly with the fraction of inhaled oxygen (FiO2) required immediately after surgery (r = 0.510; P = 0.02). Higher circulating MIF levels correlated significantly with increased inotropic support requirements on the second postoperative day, whereas higher postoperative IL-8 levels were associated with higher TISS scores, suggesting increased need for postoperative medical care. These data suggest a potential negative effect of high circulating levels of MIF in the immediate postoperative period on respiratory and cardiovascular functions, and support the development of therapeutic strategies targeting MIF function in this clinical setting.
INTRODUCTION
Heart surgery with cardiopulmonary bypass (CPB) induces a complex inflammatory reaction that may result in multi-organ dysfunction including cardiac contractile depression (1,2). Several factors can trigger the systemic inflammatory response, including cardiac ischemia-reperfusion injury, activation of leukocytes with bioactive surfaces, endotoxemia, use of cardioplegia, and surgical trauma (3). This inflammatory process involves the activation of lymphocytes, monocytes/macrophages, endothelial cells, and cardiac myocytes that can express and secrete proinflammatory cytokines, including tumor necrosis factor α (TNFα), interleukin (IL)-1, IL-6, and IL-8, and anti-inflammatory cytokines [IL-4, IL-10, transforming growth factor β (TGFβ)] (4,5).
Macrophage migration inhibitory factor (MIF) has been identified as a central mediator of the innate immune response in patients with autoimmune disorders, severe sepsis, and respiratory distress syndrome (6,7). Similar to other cytokines, MIF is present in the myocardium (8) and has been shown to be secreted in response to oxidative stress as would occur during myocardial infarction or with ischemia/reperfusion injury of the heart during CPB (9,10). In adult patients experiencing acute myocardial infarction, serum MIF levels were shown to be significantly elevated within the first 30 h (11). Several studies of adult patients undergoing surgery with cardiopulmonary bypass have reported elevated circulating levels of MIF immediately after surgery that were associated with mild pulmonary dysfunction and multiple organ dysfunction (12–14). Garner et al. (8) reported that in animal models of sepsis, cardiac-derived MIF could act as a myocardial depressant factor, and that perfusion of the isolated heart with recombinant MIF led to decreased systolic and diastolic function. In our studies of polymicrobial sepsis in rats, elevated levels of circulating MIF were associated with a significant impairment in cardiac contractile function, and this effect could be attenuated by inhibition of MIF activity (15,16). Similarly, Chagnon et al. (17) reported studies of experimental sepsis showing that inhibition of MIF using neutralizing antibodies could effectively block endotoxin-induced myocardial dysfunction.
We have previously reported that pediatric cardiac surgery involving CPB induced proinflammatory cytokine production that correlated with postoperative morbidity and cardiopulmonary dysfunction (18) and caused an impairment of the hypothalamic-pituitary-thyroid axis as measured by reduction in plasma thyroid hormone levels (19–21). The objectives of this study were to measure circulating levels of MIF before surgery and after cardiopulmonary bypass in children and to correlate these finding with intra-operative variables and postoperative outcomes. Because inflammatory processes activated by cardiopulmonary bypass can contribute significantly to postoperative morbidity, these studies take on significance in delineating potential targets for therapeutic intervention.
MATERIALS AND METHODS
Study Inclusion Criteria and Diagnostic Classifications
This prospective study was conducted at Schneider Children’s Hospital during the period January 2005 to July 2005 and was approved by the Institutional Review Board of the North Shore Long Island Jewish Health System. Written informed consent was obtained from parents or guardians before enrollment. We enrolled 24 patients <4 years old with congenital heart defects known to cause left ventricular volume overload who were scheduled for complete surgical repair requiring cardiopulmonary bypass. The lesions consisted of ventricular septal defect (VSD), VSD with patent ductus arteriosus (PDA), and complete common atrioventricular canal (CCAVC) with or without PDA (Table 1). Patients with isolated PDA, single ventricle physiology, and obstructive lesions such as aortic stenosis or coarctation of the aorta were excluded from the study.
Table 1.
Patient (n = 20) characteristics and intra- and postoperative variables and outcomes.
| Mean ± SD | Median | Minimum-maximum | |
|---|---|---|---|
| Age, months | 9.0 ± 11.3 | 3.5 | 1–43.5 |
| Weight, kg | 6.3 ± 4.1 | 4.4 | 2.5–19.3 |
| Weight-for-age percentile | 12 ± 15 | <3 | <3–50 |
| Ejection fraction, % | 37 ± 4 | 37 | 30–42 |
| Intraoperative data | |||
| Bypass time, min | 94 ± 35 | 84 | 45–177 |
| Cross-clamp time, min | 59 ± 29 | 52 | 25–136 |
| Postoperative outcomes | |||
| Intensive care unit stay, days | 5.1 ± 3.9 | 4 | 2–18 |
| Mechanical ventilation, days | 1.3 ± 0.9 | 1 | 0–4 |
| TISS score, cumulative | 71 ± 20 | 69 | 32–102 |
| Inotropic score, cumulative | 6.7 ± 8.3 | 4.6 | 0–30 |
Patient n = 20, 10 girls and 10 boys. TISS and inotropic score are cumulative scores from POD1, POD2, and POD3. Anatomical defects (n): ventricular septal defect (VSD) 11, VSD/patent ductus arteriosus (PDA) 3, complete common atrioventricular canal (CAVC) 3, CAVC/PDA 1, VSD/ASD/PDA 1, double outlet right ventricle (DORV)/VSD/PDA 1.
Age-matched healthy children were recruited into the study during their well-care visits at Schneider Children’s Hospital to provide normative blood cytokine values. Written informed consent was obtained before obtaining blood samples. Any history of acute or chronic illness or any prior cardiac disease or clinical evidence of congenital heart disease precluded participation in this study.
Surgery and Anesthesia
Surgical procedures including cardiopulmonary bypass and perioperative anesthesia followed standard practices (18). Aprotinin was administered to all patients as an initial loading dose (240 mg/m2) after anesthesia induction, followed by infusion of 56 mg/m2/h and an equivalent dose in the bypass prime. Antibiotic prophylaxis was given at the induction of anesthesia and at the end of the surgical procedure. Postoperative patient management in the pediatric intensive care unit (PICU) was based on standard institutional protocol.
Data Collection and Measures of Clinical Outcomes
Preoperative peripheral venous blood samples were obtained from enrolled patients during outpatient presurgical testing. After surgery, patients were admitted to the PICU, and blood samples were drawn from central intravenous catheters 2, 12, 24, and 48 h after CPB. Blood samples were processed immediately by centrifugation at room temperature, and plasma was stored at −20ºC until all samples could be analyzed simultaneously.
Patients were monitored continuously in the PICU, and clinical data were recorded daily, including quantities of vasodilator and inotropic drugs, blood gas and lactate analyses, oxygen requirement, urine output, blood pressures, cardiac rhythm, and heart rates. These values were among 76 different therapeutic and monitoring procedures used to assess the overall degree of postoperative care as calculated by the therapeutic interventional scoring system (TISS) (22). TISS scores were calculated for every 24-h period in the PICU. Inotropic scores were obtained at 24, 48, and 72 h after CPB using a modification of the calculation described by Wernovsky et al. (23) as follows: dopamine + dobutamine + [(epinephrine + norepinephrine) × 100]. The daily inotropic score was calculated by obtaining the total amount of inotropic drug administered in a 24-h period and expressed as micrograms per kilogram per minute. Consistent care management was maintained in the PICU by following institutional standards of postoperative care. Decisions of medical management were based on clinical assessment of the patient made by an experienced intensive care physician. To limit investigator bias, the PICU team was unaware of the study end points and the data analysis. Blood samples were analyzed at the conclusion of the study, and TISS scores were calculated by one individual who was unaware of the blood test results.
Cytokine Analysis
Plasma IL-8 and MIF were quantified by solid-phase 96-well microplate ELISA using commercially available kits (R&D Systems, Minneapolis, MN, USA). IL-8 and MIF assays used 50 and 10 μL plasma, respectively, and concentrations were determined using a standard curve generated by regression analysis with BioLinx 2.20 software (Dynatech Laboratories). Concentration of free l-thyroxine (fT4) in plasma (50 μL) was measured by radioimmunoassay (RIA) using the GammaCoat [125I] Free T4 (2-step) radioimmunoassay kit from DiaSorin (Stillwater, MN, USA), following the manufacturer’s instructions.
Statistical Analysis
Repeated-measures analysis of variance (RMANOVA) was performed to determine if the plasma levels of MIF, IL-8, and fT4 varied significantly over time in the pre- and postoperative period. Bonferroni-corrected pairwise comparisons were carried out to determine which time points differed from one another. It was determined that the log transformation of MIF, IL-8, and fT4 conformed to the standard ANOVA assumptions. Accordingly, all analyses were conducted using the log-transformed data, but the summaries and graphs are presented in their original untransformed units of measurement. Strength of correlations between plasma markers (maximum MIF and IL-8 or minimum fT4) and each of the clinical outcome measures (bypass time, cross-clamp time, cumulative TISS score, etc.) were assessed using Spearman correlations. In addition, correlations between pairs of measurements collected serially (such as MIF and IL-8, MIF and fT4, IL-8 and fT4) were assessed using the method of Bland and Altman (24). Statistical analysis was performed with SAS software (Cary, NC, USA). Results are expressed as mean ± SD, along with median, minimum, and maximum values.
RESULTS
Study Patients and Perioperative Data
Demographics and perioperative data of 20 patients are summarized in Table 1. Four of the 24 patients enrolled in the study presented with pulmonary complications and were excluded from the data analysis. The median age of the patients in this study was 3.5 months, with mean age of 9 ± 11.3 months, and mean body weight of 6.3 ± 4.1 kg and equal sex distribution. The primary pathology leading to left ventricular volume overload in this patient group was ventricular septal defect, with a smaller number having complete common atrioventricular canal with or without patent ductus arteriosus. The mean duration of cardiopulmonary bypass (CPB) was 94 ± 35 min with cross-clamp time of 59 ± 29 min. Postoperative data indicate that as a group, these patients recovered relatively rapidly from surgery, requiring 1.3 ± 0.9 days of mechanical ventilation support with an average stay of 5.1 ± 3.9 days in the intensive care unit. The cumulative TISS and inotropic scores of 71 ± 20 and 6.7 ± 8.3, respectively, represent the sum of 24-h scores calculated for the initial 72-h period after CPB surgery (Table 1).
All patients admitted to the study were in heart failure before surgery as indicated by their low weight-for-age percentiles, with the median value for the group being less than the 3rd percentile (Table 1). The mean value for ejection fraction before surgery was 37% ± 4%, range 30% to 42% (Table 1).
Preoperative Plasma Cytokine Concentrations
Concentrations of MIF in the presurgical plasma samples of the 20 patients were not significantly different from those of the healthy children, 23 ± 10 versus 20 ± 5 ng/mL, respectively (Figure 1 and Table 2). Presurgical plasma IL-8 and fT4 concentrations were also not different between the study group and the healthy subjects.
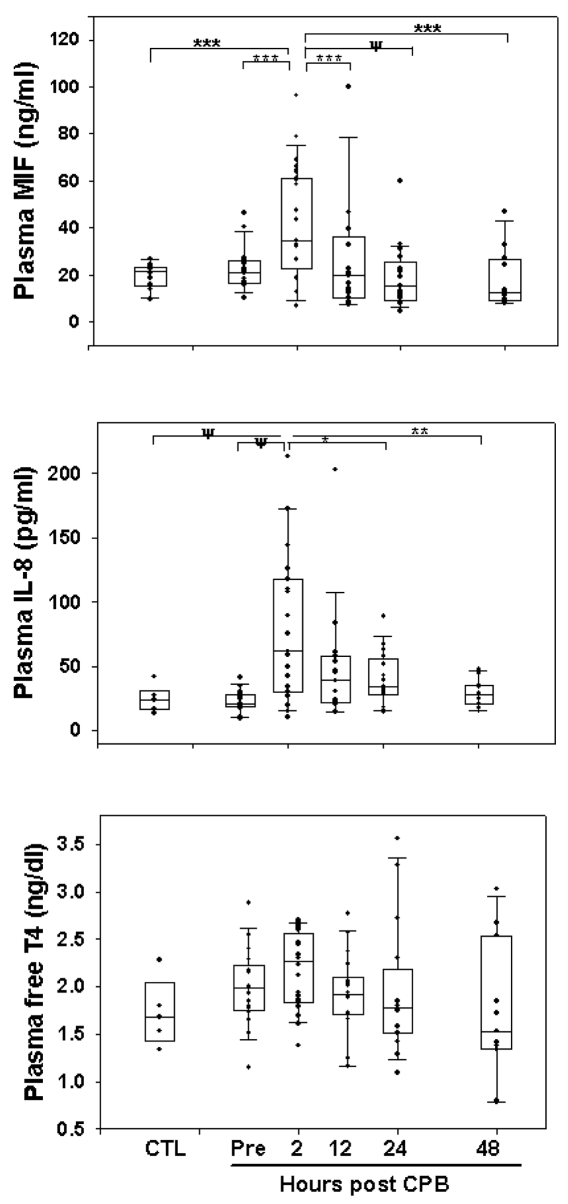
Figure 1.
Circulating MIF, IL-8, and fT4 in control (CTL) healthy children and in pediatric patients before and after CPB surgery. Box plot encompasses 25th to 75th percentiles, the horizontal line is the median value, and the caps represent 10th and 90th percentiles. Significant differences between time points analyzed by RM-ANOVA are indicated: ψP = 0.0001; ***P = 0.001; **P = 0.005; *P = 0.02.
Table 2.
Plasma concentrations of cytokines in healthy controls and patients before and after surgery.
| Time point | MIF, ng/mL | IL-8, pg/mL | fT4, ng/dL |
|---|---|---|---|
| Healthy controls | 20 ± 5 (22; 10–27) | 24 ± 10 (24; 13–42) | 1.7 ± 0.4 (1.7; 1.3–2.3) |
| Patients | |||
| Before surgery | 23 ± 10 (20; 10–46) | 23 ± 8 (20; 9–40) | 2.0 ± 0.4 (2.0; 1.6–2.9) |
| 2 h after surgery | 48 ± 24 (48; 7–96) | 79 ± 57 (62; 10–213) | 2.2 ± 0.4 (2.3; 1.4–2.7) |
| 12 h after surgery | 23 ± 21 (16; 7–100) | 48 ± 44 (39; 14–203) | 1.9 ± 0.4 (1.9; 1.2–2.8) |
| 24 h after surgery | 19 ± 13 (14; 5–60) | 40 ± 21 (34; 14–89) | 1.9 ± 0.7 (1.8; 1.1–3.6) |
| 48 h after surgery | 18 ± 12 (13; 8–47) | 29 ± 10 (28; 15–47) | 1.7 ± 0.7 (1.5; 0.8–3.0) |
Data are mean ± SD (median; minimum-maximum).
Four patients initially admitted into the study had presurgical plasma MIF levels 3- to 5-fold higher than the mean MIF value (23 ± 10 ng/mL) of the remaining 20 patients. Our previous experimental studies had shown that lung-derived MIF could contribute to circulating MIF levels as a result of pulmonary dysfunction (16). In contrast, presurgical plasma IL-8 and fT4 levels in these patients were normal. These patients had increased pulmonary dysfunction as a result of a combination of the following additional conditions: pulmonary edema due to mitral stenosis, discontinuous left pulmonary artery with large VSD, additional large ASD resulting in right ventricle volume overload possibly contributing to increased pulmonary overcirculation. One patient scheduled for surgical repair of a large VSD presented with RUL pneumonia. These four patients were 2 to 3 months of age, with two patients below the 3rd percentile and two below the 10th percentile of weight-for-age, suggesting significant heart failure. These patients were not included in the study analysis of the remaining 20 patients because the focus of this investigation was to determine the effect of cardiopulmonary bypass per se on the MIF response.
Cytokine and fT4 Responses to Cardiopulmonary Bypass Surgery
Figure 1 shows plasma levels of proinflammatory mediators, IL-8 and MIF, and free T4 in control subjects and in patients before surgery and at various time points after corrective heart surgery. Plasma MIF increased significantly (P < 0.001) within the first 2 h after cardiopulmonary bypass (48 ± 24 ng/mL), and then returned to presurgical levels by 12 h post-CPB (23 ± 21 ng/mL vs. presurgery 23 ± 10 pg/mL). Table 2 lists the circulating cytokine values before and after surgery as mean ± SD and the median with minimum and maximum values for each time point.
Plasma IL-8 concentrations increased significantly (P < 0.0001) by 2 h after completion of CPB (79 ± 57 ng/mL), with normalization within 24 h after surgery (40 ± 21 pg/mL) (Figure 1; Table 2). This IL-8 response to CPB was similar to our previously published study in pediatric patients undergoing cardiopulmonary bypass surgery (18). There was significant within-patient positive correlation between plasma MIF and IL-8 levels (r = 0.57, P < 0.001), suggesting that CPB resulted in a significant induction of these inflammatory mediators with similar temporal resolution after surgery.
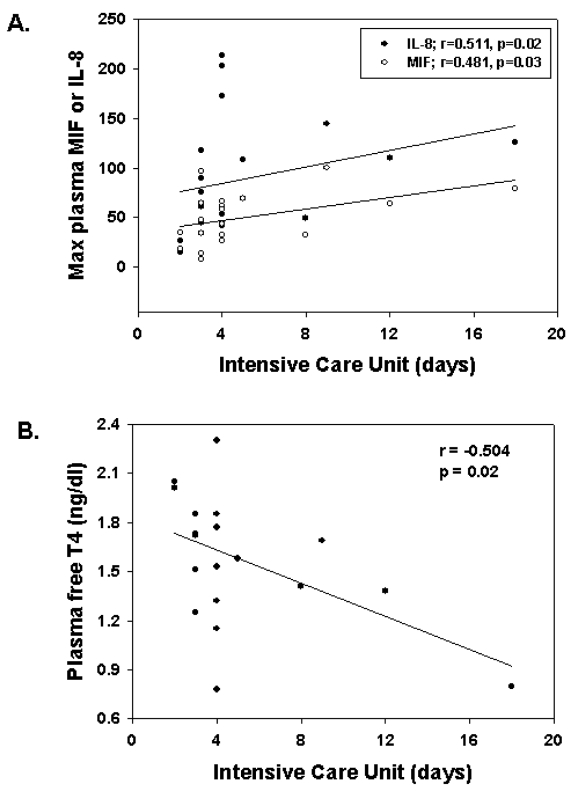
Plasma free T4 concentrations remained essentially unchanged over the time course studied and did not correlate with plasma MIF or IL-8 levels in response to CPB surgery (Figure 1). However, those patients who remained in the ICU for the longest period of time had significantly lower plasma fT4 levels (r = −0.504, P = 0.023) (Table 3).
Table 3.
Correlation analysis between maximum circulating postoperative cytokine concentrations or minimum plasma free T4 levels and postoperative data.
| Inotropic score
|
||||||
|---|---|---|---|---|---|---|
| Ventilation duration, days | PICU stay, days | POD1 | POD2 | FiO2 at 2 h | TISS on POD2 | |
| MIFMAX | ||||||
| r | 0.553 | 0.481 | 0.451 | |||
| P | 0.012 | 0.032 | NS | 0.046 | NS | NS |
| IL-8MAX | ||||||
| r | 0.491 | 0.511 | 0.510 | 0.510 | ||
| P | 0.028 | 0.021 | NS | NS | 0.022 | 0.022 |
| fT4MIN | ||||||
| r | −0.504 | −0.523 | −0.495 | |||
| P | NS | 0.023 | 0.018 | 0.027 | NS | NS |
NS, not significant.
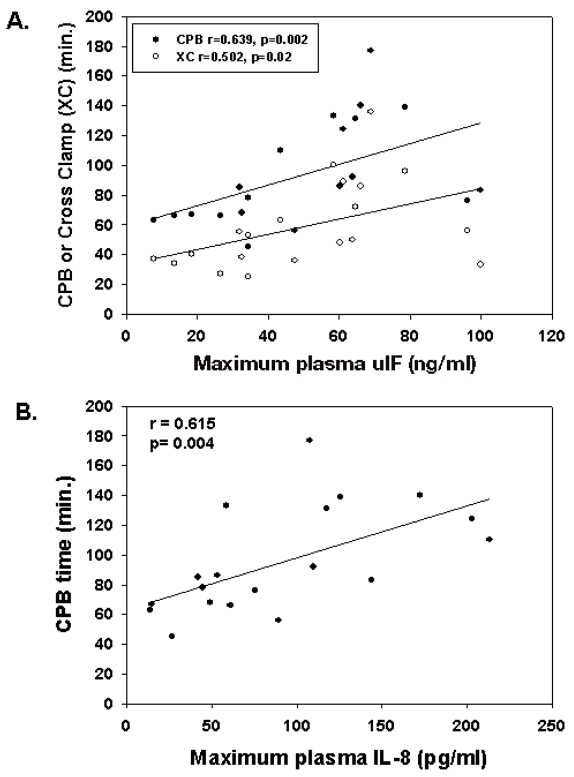
The time period of cardiopulmonary bypass and the total cross-clamp time (blood flow arrest) correlated significantly with the maximum plasma MIF levels attained after termination of CPB (Figure 2A). Similarly, plasma IL-8 concentrations immediately after CPB correlated positively with length of CPB (Figure 2B), suggesting that the cardiopulmonary bypass procedure per se was likely responsible for the magnitude of the inflammatory response as measured by these two mediators. We also found that lower body weight was associated with higher plasma IL-8 levels (r = −0.493, P = 0.03) after surgery, potentially owing to significantly longer CPB times indicative of more complex surgical procedures. Presurgical plasma IL-8 levels were not significantly higher in patients with lower body weight.
Figure 2.
Intraoperative parameters correlate with postoperative plasma MIF and IL-8. Maximum plasma MIF (A) and IL-8 (B) attained at 2 or 12 h after CPB correlated with CPB time or cross-clamp time.
Cytokine Responses Predict Clinical Outcomes
The inflammatory mediators correlated with postoperative outcomes, and these are summarized in Table 3. The maximum circulating MIF and IL-8 levels that were measured within the 2- to 12-h period after surgery were associated with the length of stay in the PICU (Figure 3A), and the decrease in plasma free T4 levels observed during the 12 to 48 h after surgery correlated significantly with longer stays in the PICU (Figure 3B). The magnitude of the postoperative increase in plasma MIF and IL-8 levels was associated with an increased number of days for which mechanical ventilation was required (r = 0.553, P = 0.012, and r = 0.491, P = 0.028, respectively) (Table 3). In addition, the increase in plasma IL-8 at 2 h after cessation of CPB showed a significant positive correlation (r = 0.510; P = 0.02) with the fraction of inhaled oxygen (FiO2) required immediately (2 h) after surgery (Table 3).
Figure 3.
Circulating mediator responses correlate with length of stay in the intensive care unit. Maximum postoperative plasma MIF or IL-8 levels (A) correlate positively with number of days in the ICU, whereas the minimum circulating level of postoperative plasma free T4 (B) shows a significant negative correlation with ICU days.
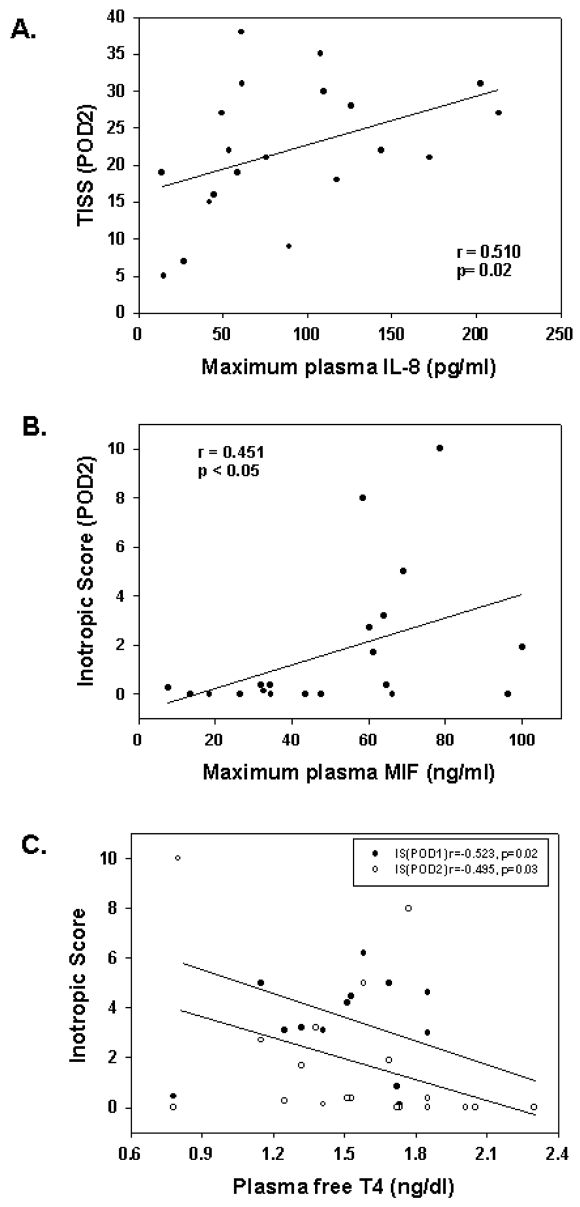
The TISS provides a measure of the overall level of medical care during each 24-h period postoperatively. The maximum circulating level of IL-8 measured immediately after surgery correlated significantly with the magnitude of therapeutic intervention during the second postoperative day (POD2) as measured by the TISS score (r = 0.510, P = 0.02) (Figure 4A; Table 3).
Figure 4.
Postoperative plasma cytokine and fT4 levels correlate with outcome measures. (A) Maximum plasma IL-8 measured at 2 or 12 h after CPB correlated with the TISS score measured during POD2. (B) Plasma MIF correlated with inotropic score measured during POD2. (C) The minimum plasma level of free T4 measured during the postoperative period correlated inversely with inotropic scores measured on POD1 and POD2.
The inotrope requirements to maintain cardiac output after surgery correlated significantly with postoperative changes in circulating levels of MIF and free T4 (Table 3). The maximum plasma MIF measured immediately after surgery at 2 or 12 h was associated with the amount of inotrope support during the POD2, with higher circulating MIF correlating significantly with increased inotropic score (Figure 4B). Furthermore, lower postoperative plasma fT4 levels correlated significantly with increased inotropic scores measured on both POD1 and POD2 (Figure 4C).
DISCUSSION
The present study, to the best of our knowledge, is the first to measure circulating MIF in pediatric patients undergoing surgical repair of cardiac lesions involving left ventricular volume overload. Young children and infants are known to be at increased risk of developing inflammatory complications after cardiopulmonary bypass surgery that can result in multisystem organ dysfunction (1,25–27). The salient observations of the present study are (1) plasma MIF increased after cessation of CPB and returned to presurgery values within 12 h; (2) postoperative peak plasma MIF and IL-8 levels correlated with the complexity of surgical repair as assessed by cardiopulmonary bypass (CPB) time and aortic cross-clamp time; and (3) postoperative peak plasma MIF and IL-8 levels correlated positively with length of stay in the PICU and with intensity of postoperative medical care as assessed by the TISS score and inotropic score. Results also support our previously published reports showing that thyroid hormone metabolism was altered in children undergoing cardiac surgery (19,20). In the present study, plasma free T4 was inversely correlated with inotropic score and length of stay in the PICU.
Studies in adults undergoing heart surgery have shown that plasma MIF levels peaked after termination of CPB, and although lower by 3 h after surgery, levels remained significantly elevated from presurgery values (13,14). Therefore, peak plasma MIF values measured 2 h after CPB in the present study may actually be lower than if measured immediately after CPB. Similar to adults (14), our study with children shows that postoperative peak plasma MIF correlated with the length of CPB and aortic cross-clamp time, suggesting that surgical repair or CPB per se induced MIF production. The source of circulating MIF in this surgical setting is not known, although activation of the hypothalamic-pituitary-adrenal axis has been suggested (28,29). MIF is also produced by numerous tissues including the myocardium and secreted from circulating macrophages and neutrophils (8,11,30). MIF has been shown to cause myocardial contractile depression, and inactivation of MIF by chemical inhibitors or neutralizing antibodies have improved cardiac function in experimental animal models of sepsis (8,16,17). In adult patients undergoing cardiac surgery, de Mendonca-Filho et al. (13) showed that peak MIF levels after CPB surgery correlated significantly with circulatory dysfunction (defined as norepinephrine >1.0 μg/kg/min). In the present study with children, we have similarly shown that peak circulating MIF after CPB was associated with increased need for inotropic support during the second postoperative day, suggesting that MIF may have negative effects on cardiac function.
MIF has also been shown to play a significant role in mediating inflammatory lung injury in acute respiratory distress syndrome (31). An adverse effect of MIF on lung function is supported by a report of adults undergoing cardiopulmonary artery bypass grafting in which the postoperative peak MIF levels showed a significant inverse association with the PaO2/FiO2 (partial arterial oxygen tension to fraction of inspired oxygen ratio) and a direct correlation with the duration of mechanical ventilation (12). Our results support these observations, with postoperative maximum circulating MIF levels correlating significantly with duration of mechanical ventilation.
The duration of stay in the intensive care unit was associated with maximum postoperative MIF and IL-8 levels, and it was inversely correlated with plasma free T4 levels. Our previously published studies in adults and children have reported impaired thyroid hormone metabolism after cardiac surgery with CPB, and that treatment with tri-iodothyronine (T3) improved outcomes as assessed by inotropic scores and TISS scores (20,32,33). In the present study, postoperative free T4 was inversely correlated with inotropic scores measured on postoperative days 1 and 2, and therefore, supports our previous studies. Only peak postoperative IL-8 levels correlated significantly with the TISS score, a measure of the intensity of medical management of the patient in the ICU. The use of therapeutic indexes and clinical scoring systems to assess cardiovascular and pulmonary functions immediately after surgery are clear limitations of the present study, because access for direct measurement of cardiac output is limited in very young patients and is not routinely done in the intensive care unit.
It is well appreciated that inflammatory mediators are activated in adults with heart failure, and that these molecules play a role in disease progression in this setting (34). Specifically, TNFα, IL-1β, and IL-6 have been shown to be expressed in direct relation to worsening New York Heart Association functional classification of heart failure. This association has not been reported in very young pediatric patients with significant heart failure. In the present study, we did not observe increased circulating IL-8 or MIF in the patients before surgery, as might be expected based on the degree of cardiac dysfunction. Analysis of other proinflammatory mediators in these children may provide a more complete picture of the inflammatory process in this setting, and may determine whether surgical correction of the lesions attenuates the inflammatory response.
In conclusion, the present study in children supports the findings of recent investigations in adults undergoing cardiac surgery, and further recognizes the potential negative role of MIF as an inflammatory mediator activated in response to cardiopulmonary bypass. In this controlled clinical setting, strategies to target MIF may provide therapeutic benefit to the pediatric patient at risk of developing postoperative complications as a result of cardiopulmonary bypass.
Footnotes
Online address: http://www.molmed.org
REFERENCES
- 1.Casey WF, et al. Circulating endotoxin and tumor necrosis factor during pediatric cardiac surgery. Crit Care Med. 1992;20:1090–6. doi: 10.1097/00003246-199208000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Hirai S. Systemic inflammatory response syndrome after cardiac surgery under cardiopulmonary bypass. Ann Thorac Cardiovasc Surg. 2003;9:365–70. [PubMed] [Google Scholar]
- 3.Varan B, Tokel K, Mercan S, Donmez A, Aslamaci S. Systemic inflammatory response related to cardiopulmonary bypass and its modification by methyl prednisolone: high dose versus low dose. Pediatr Cardiol. 2002;23:437–41. doi: 10.1007/s00246-002-0118-3. [DOI] [PubMed] [Google Scholar]
- 4.Kawamura T, Wakusawa R, Okada K, Inada S. Elevation of cytokines during open heart surgery with cardiopulmonary bypass: participation of interleukin 8 and 6 in reperfusion injury. Can J Anaesth. 1993;40:1016–21. doi: 10.1007/BF03009470. [DOI] [PubMed] [Google Scholar]
- 5.Sablotzki A, et al. Alterations of the cytokine network in patients undergoing cardiopulmonary bypass. Perfusion. 1997;12:393–403. doi: 10.1177/026765919701200608. [DOI] [PubMed] [Google Scholar]
- 6.Gando S, Nishihira J, Kobayashi S, Morimoto Y, Nanzaki S, Kemmotsu O. Macrophage migration inhibitory factor is a critical mediator of systemic inflammatory response syndrome. Intensive Care Med. 2001;27:1187–93. doi: 10.1007/s001340000818. [DOI] [PubMed] [Google Scholar]
- 7.Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garner LB, et al. Macrophage migration inhibitory factor is a cardiac-derived myocardial depressant factor. Am J Physiol. 2003;285:H2500–9. doi: 10.1152/ajpheart.00432.2003. [DOI] [PubMed] [Google Scholar]
- 9.Fukuzawa J, et al. Contribution of macrophage migration inhibitory factor to extracellular signal-regulated kinase activation by oxidative stress in cardiomyocytes. J Biol Chem. 2002;277:24889–95. doi: 10.1074/jbc.M112054200. [DOI] [PubMed] [Google Scholar]
- 10.Yu C-M, Lai KW-H, Chen Y-X, Huang X-R, Lan HY. Expression of macrophage migration inhibitory factor in acute ischemic myocardial injury. J Histochem Cytochem. 2003;51:625–31. doi: 10.1177/002215540305100508. [DOI] [PubMed] [Google Scholar]
- 11.Takahasi M, et al. Macrophage migration inhibitory factor as a redox-sensitive cytokine in cardiac myocytes. Cardiovasc Res. 2001;52:438–45. doi: 10.1016/s0008-6363(01)00408-4. [DOI] [PubMed] [Google Scholar]
- 12.de Mendonca-Filho HTF, Gomes RV, de Almeida Campos LA, Castro-Faria-Neto HC, Tura B, Nunes EM, Castro-Faria-Neto HC. Circulating levels of macrophage migration inhibitory factor are associated with mild pulmonary dysfunction after cardiopulmonary bypass. Shock. 2004;22:533–7. doi: 10.1097/01.shk.0000142817.84070.df. [DOI] [PubMed] [Google Scholar]
- 13.de Mendonca-Filho HTF, Pereira KC, Fontes M, de Souza Aranha Vieira DA, de Mendonca MLA, de Almeida Campos LA, Castro-Faria-Neto HC. Circulating inflammatory mediators and organ dysfunction after cardiovascular surgery with cardiopulmonary bypass: a prospective observational study. Crit Care. 2006;10:46. doi: 10.1186/cc4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gando S, Nishihira J, Kemmotsu O, Kobayashi S, Morimoto Y, Matsui Y, Yasuda K. An increase in macrophage migration inhibitory factor release in patients with cardiopulmonary bypass surgery. Surg Today. 2000;30:689–94. doi: 10.1007/s005950050041. [DOI] [PubMed] [Google Scholar]
- 15.Lin X, Sakuragi T, Metz CN, Ojamaa K, Wang P, Skopicki H, Al-Abed Y, Miller EJ. Macrophage migration inhibitory factor within the alveolar spaces induces changes in the heart during late experimental sepsis. Shock. 2005;24:556–563. doi: 10.1097/01.shk.0000183238.70374.a8. [DOI] [PubMed] [Google Scholar]
- 16.Sakuragi T, Lin X, Metz CN, Ojamaa K, Kohn N, Al-Abed Y, Miller EJ. Lung-derived macrophage migration inhibitory factor in sepsis induces cardio-circulatory depression. Surg Infect. 2007;8:29–40. doi: 10.1089/sur.2006.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chagnon F, Metz CN, Bucala R, Lesur O. Endotoxin-induced myocardial dysfunction: effects of macrophage migration inhibitory factor neutralization. Circ Res. 2005;96:1095–1102. doi: 10.1161/01.RES.0000168327.22888.4d. [DOI] [PubMed] [Google Scholar]
- 18.Madhok AB, Ojamaa K, Viraga H, Parnell VA, Pahwa S, Chowdhury D. Cytokine response in children undergoing surgery for congenital heart disease. Pediatr Cardiol. 2006;27:408–13. doi: 10.1007/s00246-006-0934-y. [DOI] [PubMed] [Google Scholar]
- 19.Chowdhury D, Parnell VA, Ojamaa K, Boxer R, Cooper R, Klein I. Usefulness of tri-iodothyronine (T3) treatment after surgery for complex congenital heart disease in infants and children. Am J Cardiol. 1999;84:34–36. doi: 10.1016/s0002-9149(99)00513-5. [DOI] [PubMed] [Google Scholar]
- 20.Chowdhury D, Ojamaa K, Parnell VA, McMahon CK, Sison CP, Klein I. A prospective randomized clinical study of thyroid hormone treatment after operations for complex congenital heart disease. J Thorac Cardiovasc Surg. 2001;122:1023–5. doi: 10.1067/mtc.2001.116192. [DOI] [PubMed] [Google Scholar]
- 21.McMahon CK, Klein I, Ojamaa K. Interleukin-6 and thyroid hormone metabolism in pediatric cardiac surgery patients. Thyroid. 2003;13:301–4. doi: 10.1089/105072503321582123. [DOI] [PubMed] [Google Scholar]
- 22.Keene AR, Cullen DJ. Therapeutic intervention scoring system: update 1983. Crit Care Med. 1983;11:1–3. doi: 10.1097/00003246-198301000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Wernovsky G, Wypij D, Jonas RA, et al. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants: a comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation. 1995;92:2226–35. doi: 10.1161/01.cir.92.8.2226. [DOI] [PubMed] [Google Scholar]
- 24.Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: part I, correlation within subjects. Br Med J. 1995;310:446. doi: 10.1136/bmj.310.6977.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butler J, et al. Acute-phase responses to cardiopulmonary bypass in children weighing less than 10 kilograms. Ann Thorac Surg. 1996;62:538–42. [PubMed] [Google Scholar]
- 26.Casey LC. Role of cytokines in the pathogenesis of cardio-pulmonary-induced multisystem organ failure. Ann Thorac Surg. 1993;56:S92–6. doi: 10.1016/0003-4975(93)91143-b. [DOI] [PubMed] [Google Scholar]
- 27.Gessler P, Pfenninger J, Pfammatter JP, Carrel T, Dahinden C. Inflammatory response of neutrophil granulocytes and monocytes after cardiopulmonary bypass in pediatric cardiac surgery. Intensive Care Med. 2002;28(12):1786–91. doi: 10.1007/s00134-002-1525-x. [DOI] [PubMed] [Google Scholar]
- 28.Bernhagen J, et al. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365:756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- 29.Calandra T, et al. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995;377:68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- 30.Calandra T, Echtenacher B, Roy DL, Pugin J, Metz CN, Hultner L, Heumann D, Mannel D, Bucala R, Glauser MP. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med. 2000;6:164–70. doi: 10.1038/72262. [DOI] [PubMed] [Google Scholar]
- 31.Lai KN, Leung JC, Metz CN, Lai FM, Bucala R, Lan HY. Role for macrophage migration inhibitory factor in acute respiratory distress syndrome. J Pathol. 2003;199:496–508. doi: 10.1002/path.1291. [DOI] [PubMed] [Google Scholar]
- 32.Klemperer JD, Klein I, Gomez M, Helm RE, Ojamaa K, Thomas SJ, Isom OW, Krieger K. Effects of thyroid hormone supplementation in cardiac surgery. N Engl J Med. 1995;333:1522–7. doi: 10.1056/NEJM199512073332302. [DOI] [PubMed] [Google Scholar]
- 33.Klemperer JD, Klein I, Ojamaa K, Helm R, Gomez M, Isom W, Krieger K. Triiodothyronine decreases atrial fibrillation after coronary artery bypass surgery. Ann Thoracic Surg. 1996;61:1323–1329. doi: 10.1016/0003-4975(96)00102-6. [DOI] [PubMed] [Google Scholar]
- 34.Mann DL. Inflammatory mediators and the failing heart: past, present and the foreseeable future. Circ Res. 2002;91:988–998. doi: 10.1161/01.res.0000043825.01705.1b. [DOI] [PubMed] [Google Scholar]