Abstract
There is an increased interest in how lipids interact with each other, especially in the lateral separation of lipids into coexisting liquid phases as this is believed to be an attribute of raft formation in cell membranes. ToF-SIMS has shown itself to be an excellent tool for investigating cellular and model membrane systems and will be perhaps the most powerful one for investigating raft formation. Results from our laboratory show the capability of ToF-SIMS at identifying unequivocally the content of coexisting liquid lipid phases. Using supported lipid monolayers we find that the inclusion of dipalmitoylphosphatidylethanolamine (DPPE) to a homogeneous dipalmitoylphosphatidylcholine (DPPC)/cholesterol phase results in the formation of cholesterol-rich domains [A.G. Sostarecz, C.M. McQuaw, A.G. Ewing, N. Winograd, J. Am. Chem. Soc. 126 (2004) 13882]. Also, for DPPE/cholesterol systems a single homogeneous DPPE/cholesterol phase is formed at ~50 mol% cholesterol, whereas DPPC/cholesterol systems form a single phase at 30 mol% cholesterol [C.M. McQuaw, A. Sostarecz, L. Zheng, A.G. Ewing, N. Winograd, Langmuir 21 (2005) 807]. Currently we are exploring the incorporation of sphingomyelin into phospholipid–cholesterol mixtures in an effort to gain a better understanding of its role in raft formation.
Keywords: ToF-SIMS imaging, Lipid rafts, Cholesterol domains
1. Introduction
Recent studies suggest that lipids in the cellular membrane participate in transport and signaling within the cell via organized lipid regions termed ‘‘lipid rafts’’. Although, progress has been made in developing methods for detecting lipid rafts inside living cell membranes, characterizing these native structures is extremely difficult due to their debatable size and lifetimes, as well as the innate complexity of the cell membrane–eukaryotic cell membranes consist of up to 500 different lipid species [3]. The known behavior of lipid rafts in cell membranes is predominantly defined by the behavior of lipid mixtures in model systems [4], also monolayer and bilayer lipid phase behaviors have been found to depend similarly on lipid composition [5]. The lipids in these rafts are expected to include cholesterol and sphingolipids, aswell as phospholipids, and to be in a liquid-ordered (lo) phase that is characterized by tightly packed acyl chains and a high degree of lateral mobility [6]. The most common imaging methods to study lipid behavior are fluorescence microscopy and atomic force microscopy (AFM). These methods are able to identify the existence of different lipid phases but must surmise phase content via stepwise experiments with varying lipid composition or via the inclusion of fluorescent labels. Due to its chemical imaging capability we believe time-of-flight secondary ion mass spectrometry (ToF-SIMS) to be a much more powerful technique for investigating lipid raft formation.
Our research of binary and ternary lipid mixtures of dipalmitoylphosphatidylcholine (DPPC), dipalmitoylphosphatidylethanolamine (DPPE), and cholesterol give insight into the lateral organization of lipids in the phosphatidylethanolamine (PE)-rich inner leaflet of the cell membrane of which relatively little is known. Lipid raft formation in both membrane leaflets must be understood for a complete picture of transport and signaling in cells. In our PE-rich inner leaflet model we find that the inclusion of 40 mol% DPPE to single phase homogeneous 2:1 DPPC/cholesterol disrupts the interaction between cholesterol and DPPC resulting in the formation of cholesterol-rich domains [1]. Further investigation into the interaction of DPPE and cholesterol shows that a 2:1 DPPE/cholesterol model has two phases–acholesterol-rich phase and a DPPE-rich phase–and a single homogeneous phase is not formed until ~50 mol% cholesterol [2]. This differs from DPPC/cholesterol systems, which form a homogenous phase at 30 mol% cholesterol. To broaden our understanding of lipid organization we are increasing the complexity of our systems. We are now investigating the quintessential lipid raft model system [4] of phosphatidylcholine–glycerolphospholipids, sphingomyelin, and cholesterol. Currently, there is a debate about the location and interactions of sphingomyelin in this system, as well as its role in lipid raft organization. We believe that ToF-SIMS is the best method for determining unequivocally the location of sphingomyelin in these lipid raft model systems. These investigations are strong examples of the ability of ToF-SIMS to elucidate lipid raft behavior.
2. Materials and Methods
2.1. Sample preparation
The following were used without further purification: DPPC, DPPE, cholesterol (all purchased in powder form from Avanti Polar Lipids, Inc., Alabaster, AL), 16-mercaptohexadecanoic acid (Sigma–Aldrich Co., St. Louis, MO), methanol, and chloroform. The water used was purified by a Milli-Q system (Millipore, Burlington, MA) with a final resistivity of 18.2 MΩ cm and a total organic content <5 ppb.
Self-assembled monolayers (SAMs) on gold were used as Langmuir–Blodgett (LB) substrates. Single crystal (1 0 0) 3 in.silicon wafers were cut and piranha etched (3:1, H2SO4:H2O2) before further treatment. (Extreme caution must be exercised when using piranha etch. An explosion-proof hood should be used.) Hundred angstroms chromium followed by ~2000 Å Au was then deposited onto the clean silicon as described by Fisher et al. [7]. One millimolar solution 16-mercaptohexadecanoic acid in 2-propanol was used for self-assembly onto gold. All processes of self-assembly and LB film preparation were confirmed with a single wavelength (632.8 nm, 1 mm spot size, 70° incidence angle) Stokes ellipsometer LSE (Gaertner Scientific Co., Skokie, IL).
LB films were prepared using a Kibron μ Trough S-LB (Helsinki, Finland) with a subphase of ~60 mL purified room temperature water. All lipid mixtures were dissolved in 9:1 chloroform/methanol. At least 20 min was allotted after film application before compression to ensure complete solvent evaporation. Trough barriers were computer controlled to allow uniform compression and constant feedback when depositing monolayers. Surface pressure–area isotherms were taken at a rate of 7 Å2/molecule/min and films were deposited vertically onto SAM substrates at 3 mN/m upon first compression.
2.2. Sample analysis
Mass spectrometry was performed with an imaging ToF-SIMS (described in detail by Braun et al. [8]) equipped with a 15 keV Ga+ liquid metal ion gun. Spectra were acquired at room temperature with an ion dose <1012 ions/cm2. By rastering the ion beam across the surface a mass spectrum was acquired at each pixel thus generating a total ion image of the area being analyzed.
3. ToF-SIMS imaging of model membrane systems advances our view of lipid raft formation
Our investigations into lipid rafts begin with systems that mimic the inner leaflet of the cellular membrane because currently less is known about this half of the membrane. The lipids in our study are shown in Fig. 1 along with some of their +SIMS fragment ions. Cholesterol is included due to its believed essential participation in lipid raft formation. The most prevalent phospholipids in the inner leaflet are those with a PE-headgroup, therefore DPPE is selected to represent this class of phospholipid. We also include PC-phospholipids as they exist in the inner leaflet and by choosing DPPC we limit our investigation to the influence of phospholipid headgroups.
Fig. 1.
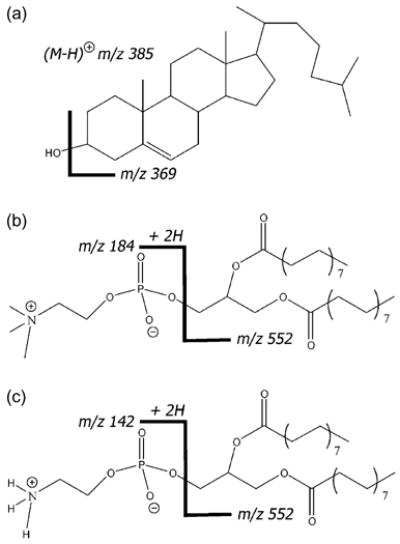
The molecular structures of (a) cholesterol, (b) DPPC, and (c) DPPE with +SIMS fragment ions labeled. Cholesterol has two main fragments at m/z 385 (M–H)+ and m/z 369 (M–OH)+. The DPPC headgroup fragment is at m/z 184, while the DPPE headgroup fragment is at m/z 142. Both phospholipids have the same tailgroups with m/z 552.
It is known that a binary system of 2:1 DPPC/cholesterol will result in a single homogeneous phase [6,9]. Our results show that a supported LB of this system has DPPC and cholesterol uniformly throughout the film [1]. When 40 mol% DPPE is added to the homogeneous 2:1 DPPC/cholesterol system, a heterogeneous film results [1]. Fig. 2 has a new view of three +SIMS ion images of this ternary DPPE/DPPC/cholesterol system. There is a cholesterol-rich phase with phospholipid signal observed throughout. DPPE disrupts the cholesterol–DPPC interactions and leads to the formation of cholesterol-rich domains. These results support the formation of cholesterol-rich lipid rafts in the PE-rich inner leaflet. In a single image, ToF-SIMS is capable of not only identifying multiple liquid phases, but identifying their lipid content.
Fig. 2.
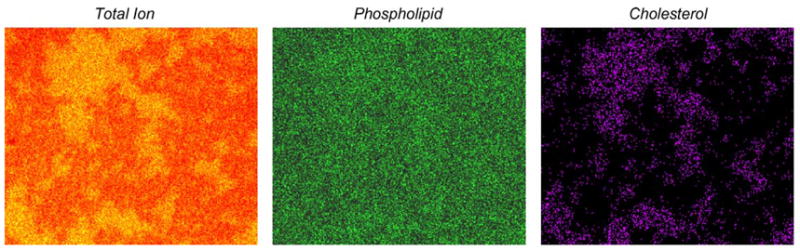
ToF-SIMS positive ion images of the 2:1 DPPC/cholesterol with 40 mol% DPPE film. The 15 keV Ga+ ion dose is <1012 ions/cm2, the field of view is 370 μm × 320 μm with 256 pixels × 222 pixels and there are 11 shots/pixel. The total ion image contains all ions within the m/z 1–1000 mass range. The phospholipid image is that of m/z 184 (DPPC headgroup) and m/z 552 (phospholipid tailgroup). The cholesterol image is that of m/z 369 and 385.
We continued to investigate the interaction of DPPE and cholesterol by looking at binary systems of the two lipids and find that the 2:1 DPPE/cholesterol LB film results in heterogeneity. A homogeneous single DPPE/cholesterol phase is not formed until ~50 mol% cholesterol [2]. Clearly, the interaction between DPPE and cholesterol is different than that of DPPC and cholesterol. The nature of the two different headgroups must be causal as DPPE and DPPC differ only by their headgroups. Both headgroups have a positive charge on the nitrogen (Fig. 1), but the DPPE headgroup has hydrogen atoms instead of methyl groups at the N-terminus. The DPPE molecules are capable of stronger inter-headgroup hydrogen bonding and electrostatic interactions than DPPC [9–11]. These strong DPPE–DPPE interactions lead to a minimization of cholesterol mixing, thus creating regions that are rich in cholesterol. This is a clear example of the influence of phospholipid headgroup on lipid interactions. By combining ToF-SIMS and AFM to study the DPPE/cholesterol binary systems we find that the thinner regions in the heterogeneous films are the cholesterol-rich phase. It is known that heterogeneous DPPC/cholesterol systems have a thicker cholesterol-rich phase [11,12], so perhaps the bilayer maintains a uniform thickness by coupling cholesterol-rich phases across the bilayer. Using ToF-SIMS in addition to AFM expands our resources and leads to a greater understanding of lipid rafts.
Ternary model membrane systems of phosphatidylcholine–glycerolphospholipids (PC), sphingomyelin, and cholesterol are considered the archetypical lipid raft mimic because sphingomyelin and cholesterol are the major constituents of detergent-insoluble fractions isolated from cell membranes [4]. One area of debate is the location and role of sphingomyelin in the formation of lipid rafts. ToF-SIMS is capable of chemically identifying the constituents of model membrane systems and we anticipate that it is the most appropriate tool for resolving this debate in the future.
4. Conclusions
These results emphasize the importance of ToF-SIMS imaging for investigating the mechanism of lipid raft formation. The ability of ToF-SIMS to laterally resolve chemical species demonstrates that cholesterol-rich domains form in a phosphatidylethanolamine-rich system. We believe that using the power of ToF-SIMS to chemically image surfaces our upcoming investigations of PC/sphingomyelin/cholesterol model membranes will lead to an answer to the question of the role and location of sphingomyelin in lipid rafts. We believe that future studies will further solidify the role of ToF-SIMS in lipid raft research.
Acknowledgments
Financial support is from the National Institutes of Health and the National Science Foundation.
References
- 1.Sostarecz AG, McQuaw CM, Ewing AG, Winograd N. J Am Chem Soc. 2004;126:13882. doi: 10.1021/ja0472127. [DOI] [PubMed] [Google Scholar]
- 2.McQuaw CM, Sostarecz A, Zheng L, Ewing AG, Winograd N. Langmuir. 2005;21:807. doi: 10.1021/la0479455. [DOI] [PubMed] [Google Scholar]
- 3.Mayor S, Rao M. Traffic (Oxford) 2004;5:231. doi: 10.1111/j.1600-0854.2004.00172.x. [DOI] [PubMed] [Google Scholar]
- 4.Edidin M. Annu Rev Biophys Biomol Struct. 2003;32:257. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- 5.Veatch SL, Keller SL. Phys Rev Lett. 2002;89:268101–1. doi: 10.1103/PhysRevLett.89.268101. [DOI] [PubMed] [Google Scholar]
- 6.Brown DA, London E. J Membr Biol. 1998;164:103. doi: 10.1007/s002329900397. [DOI] [PubMed] [Google Scholar]
- 7.Fisher GL, Hooper AE, Opila RL, Allara DL, Winograd N. J Phys Chem B. 2000;104:3267. [Google Scholar]
- 8.Braun RM, Blenkinsopp P, Mullock SJ, Corlett C, Willey KF, Vickerman JC, Winograd N. Rapid Commun Mass Spectrom. 1998;12:1246. doi: 10.1002/(SICI)1097-0231(19980930)12:18<1246::AID-RCM316>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 9.Yuan C, Johnston LJ. Biophys J. 2000;79:2768. doi: 10.1016/S0006-3495(00)76516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ohvo-Rekila H, Ramstedt B, Leppimaki P, Slotte JP. Prog Lipid Res. 2002;41:66. doi: 10.1016/s0163-7827(01)00020-0. [DOI] [PubMed] [Google Scholar]
- 10.McMullen TPW, Lewis RNAH, McElhaney RN. Biochim Biophys Acta. 1999;1416:119. doi: 10.1016/s0005-2736(98)00214-4. [DOI] [PubMed] [Google Scholar]
- 11.Kim K, Kim C, Byun Y. Langmuir. 2001;17:5066. [Google Scholar]
- 12.Yuan C, Johnston LJ. J Microsc. 2002;205:136. doi: 10.1046/j.0022-2720.2001.00982.x. [DOI] [PubMed] [Google Scholar]


