Abstract
Objective:
To introduce new corneal high-speed, ultra–high-resolution optical coherence tomography (hsUHR-OCT) technology that improves the evaluation of complicated and uncomplicated cataract, corneal, and refractive surgical procedures.
Design:
This case series included a control subject and 9 eyes of 8 patients who had undergone phacoemulsification, Descemet membrane stripping endokeratoplasty, corneal implantation for keratoconus, and complicated and uncomplicated laser in situ keratomileusis. These eyes underwent imaging using a prototype ophthalmic hsUHR-OCT system. All the scans were compared with conventional slitlamp biomicroscopy.
Results:
Cross-sectional hsUHR-OCT imaging allowed in vivo differentiation of corneal layers and existing pathologic abnormalities at ultrahigh axial image resolution. These images illustrate the various incisional and refractive interfaces created with corneal procedures.
Conclusions:
The magnified view of the cornea using hsUHR-OCT is helpful in conceptualizing and understanding basic and complicated clinical pathologic features; hsUHR-OCT has the potential to become a powerful, noninvasive clinical corneal imaging modality that can enhance surgical management.
Trial Registration:
clinicaltrials.gov Identifier: NCT00343473
The cornea is routinely examined using slitlamp biomicroscopy at magnifications of ×10 to ×25 (and up to ×100) in the clinic setting. Several in vivo imaging devices are currently available that provide high magnification and detailed information regarding the ocular anterior segment. Confocal scanning laser microscopy has good transverse resolution capability1 but does not provide a cross-sectional view of the cornea with contiguous reference to neighboring corneal layers. Ultrasound biomicroscopy has good anterior segment penetration (4 mm) but requires an immersion bath and has an overall axial resolution of 20 to 60 μm.2 Commercially available time-domain anterior segment optical coherence tomographs (OCTs) (Optical Coherence Pachymetry; Heidelberg Engineering, Heidelberg, Germany; and Visante; Carl Zeiss Meditec Inc, Dublin, California) allow noncontact viewing of the cornea.3,4 Both OCT devices use a 1310-nm light source, resulting in an axial resolution of 11 and 18 μm, respectively.3,4 This wavelength allows better penetration into anterior structures, revealing structures internal to the sclera, including the angle.
The latest iteration of OCT technology uses Fourier domain (spectral) signal analysis. This OCT version acquires images with an axial resolution of 3.4 μm and increases scanning speed to 24000 A-scans per second compared with the 2000 A-scans per second with the commercially available technology.3 The diagnostic benefits of high-speed, ultra–high-resolution OCT (hsUHROCT) in glaucoma5 and in the retina6 have been reported. The aim of this hsUHR-OCT imaging case series is to examine the utility of hsUHR-OCT in the management of corneal surgical patients.
METHODS
PARTICIPANTS
Data on a variety of corneal abnormalities were retrospectively collected from a prospective study of hsUHR-OCT evaluation. All the participants underwent comprehensive anterior segment ocular examination by cornea specialists that defined the clinical diagnosis. Qualified individuals had good-quality corneal photographs and hsUHR-OCT scans acquired at the same visit. The study was approved by the University of Pittsburgh institutional review board/ethics committee and adhered to the Declaration of Helsinki and Health Insurance Portability and Accountability Act regulations. Informed consent was obtained from all of the participants.
INSTRUMENTATION
All of the participants underwent digital corneal photography using a camera (Nikon D1X; Nikon Corp, Tokyo, Japan) mounted on a slitlamp (Topcon 8Z; Topcon Medical Systems Inc, Paramus, New Jersey).
Detailed information on the hsUHR-OCT device has previously been published.7,8 Briefly, anterior segment hsUHR-OCT was based on Fourier domain (spectral) OCT technology. Infrared light is focused on the cornea using a condensing lens, and the back-reflected light from the cornea creates an interference pattern with light returning from a stationary reference arm. These interference data points are measured by means of low-coherence interferometry at a fast rate to yield high-resolution, cross-sectional tomographic corneal images. For this study, a prototype device of anterior segment hsUHR-OCT was used with a broadband (mean±SD) 840±50-nm super-luminescent diode laser that was projected onto the cornea at a distance of 25 mm. The optical power scanning across the eye was 750 μW, within the American National Standards Institute maximum permissible exposure limit for continuous exposure at that wavelength.9 The sample arm (camera head) was mounted on a standard slitlamp stand. Light reflected from the cornea was coupled with light from the reference arm, and the interference pattern was quantified by a spectrometer equipped with a linear charge-coupled device camera. The spectrogram was analyzed using a computer with advanced signal processing software and hardware.
Two scan patterns were used in the study. Raster scans of the cornea were acquired by an experienced operator (L.K.) and contained 180 consecutive frames covering a volume of 3×3×1.4 mm. Each frame contained 501 A-scan lines, and each A-scan contained 1024 points sampling reflectance in a 1.4-mm-thick window. Total acquisition time for raster scans was 3.8 seconds per scan. The second scan pattern consisted of 3 line scans, 2 vertical and 1 horizontal. Each 3-mm line scan contained 8000 A-scans, and the total scan time for all 3 line scans was 1 second.
Anterior segment imaging using a commercially available device (Visante) was also performed in a control subject. Visante and hsUHR-OCT images were acquired at the same visit. A high-resolution scan was acquired using Visante that was constructed from 4 repeated line scans, each with 512 A-scans, to cover a 10×3-mm window. The high-resolution scan time was 0.5 seconds.
RESULTS
Nine eyes of 8 patients who had undergone surgical cataract, corneal, or refractive procedures and a representative control subject were included in this case series.
CASE 1: CONTROL SUBJECT
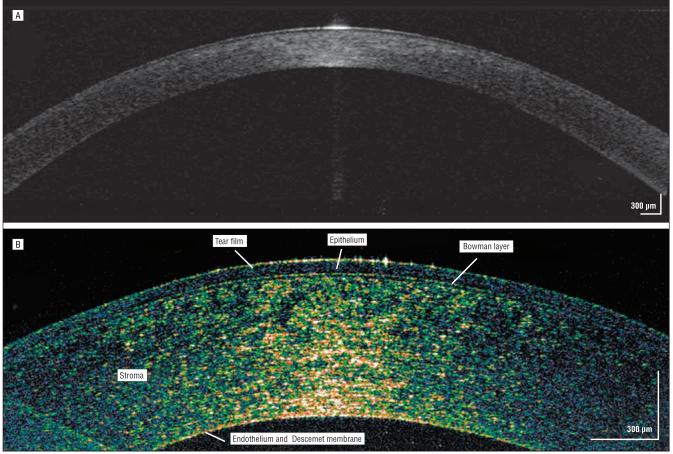
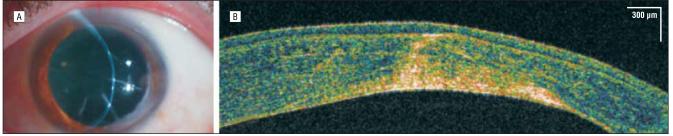
A healthy 45-year-old man with 20/20 uncorrected visual acuity had normal findings on clinical corneal examination, including keratometry, central corneal pachymetry, and corneal topography. Comparison of the conventional time-domain OCT (Visante) (Figure 1A) and hsUHR-OCT (Figure 1B) images showed a markedly enhanced view of the corneal structure and delineation of the various layers in the hsUHR-OCT image. This hsUHR-OCT image was used as a reference for interpreting the results of the other patients with corneal procedures or abnormalities.
Figure 1.
Control subject. Commercially available time-domain optical coherence tomography (A) and high-speed, ultra–high-resolution optical coherence tomography (B) images of a healthy human cornea in vivo. Note the high reflectivity centrally and especially at the tear-epithelial interface, where the light signal is greatest.
Three of the cornea's 5 individual layers were visualized as distinct entities: the epithelium, Bowman layer, and stroma. The Descemet membrane and the endothelial layer, for the most part, are seen as 1 complex. The centralmost aspect of the image shows more optical backscattered light than the adjacent cornea owing to the approximately tangential orientation of the tissue relative to the scanning beam. The central tear-epithelial interface has more reflectivity owing to the large difference in index of refraction between air and the tear film compared with differences between subsequent tissue layers.
CASE 2: UNCOMPLICATED AND COMPLICATED LASER IN SITU KERATOMILEUSIS
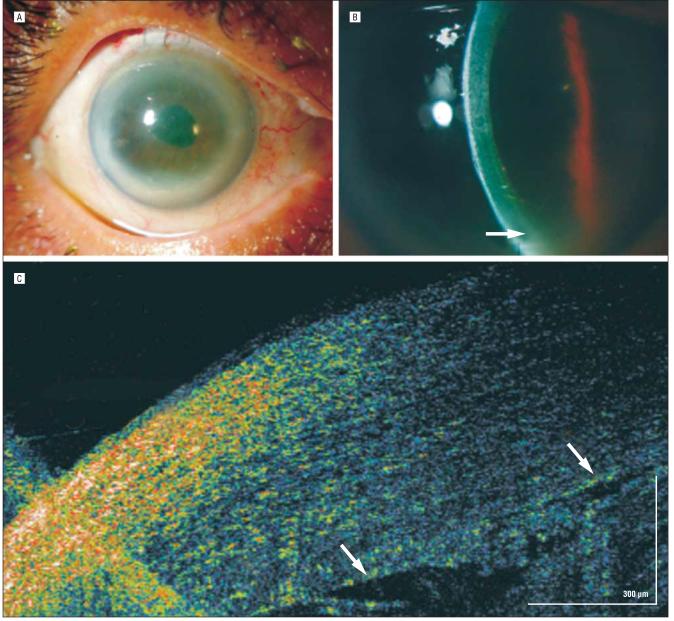
A 44-year-old man with best-corrected visual acuity of 20/15 OU who had undergone uncomplicated bilateral myopic laser in situ keratomileusis (LASIK) 3 years earlier sustained an injury during a hunting mishap that resulted in a thorn becoming embedded in his left cornea. The thorn was successfully removed, and the ensuing diffuse lamellar keratitis was resolved with medication. A few weeks later, a resultant epithelial ingrowth was removed surgically. Three weeks after the removal, a small iron foreign body from a welding accident lodged on the surface of the LASIK flap, inciting a second episode of diffuse lamellar keratitis (Figure 2A). The hsUHR-OCT image showed a highly reflective region underneath the overlying focal epithelial defect (Figure 2B). The adjacent LASIK interface was highly reflective owing to the inflammatory response (Figure 2C).
Figure 2.
Laser in situ keratomileusis (LASIK) trauma. A and B, A corneal epithelial defect at the 5-o'clock position with surrounding diffuse lamellar keratitis (DLK) of the inferotemporal flap. The arrow encompasses the epithelial defect where the foreign body was removed (seen as densely high reflectivity on high-speed, ultra–high-resolution optical coherence tomography [arrow]), surrounded by an area of dense DLK. C, Increased reflectivity of the temporal LASIK flap interface (right) adjacent to the most severe area of DLK. The nasal flap interface (left) is not as hyperreflective.
CASE 3: COMPLICATED LASIK
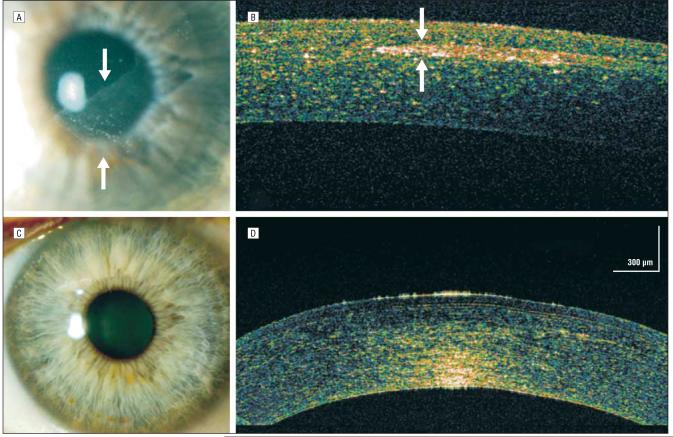
A 52-year-old man who had undergone bilateral myopic LASIK 5 years earlier returned for further myopic LASIK enhancement of his left eye. Five days after an uncomplicated procedure, the patient sustained eye trauma with resultant epithelial ingrowth (Figure 3A). The hsUHR-OCT image of the eye showed the resultant ingrowth as a highly reflective, well-circumscribed area in the stromal LASIK flap interface (Figure 3B). Eight weeks after surgical removal of the epithelial ingrowth, there was no further evidence of this finding either clinically or by means of hsUHR-OCT (Figure 3C and D).
Figure 3.
Complicated laser in situ keratomileusis (LASIK). A, Trauma to the LASIK flap resulted in a diaphanous sheet of epithelial ingrowth under the flap (arrows). B, Epithelial in-growth under a LASIK flap that appears as a space-occupying, high-reflectivity area in the stromal flap interface (arrows). C and D, Eight weeks after epithelial ingrowth removal, only residual high reflectivity is noted at the temporal extent of the LASIK flap interface, representing trace stromal scarring. (Note that the signal intensity is greater at the endothelial surface per the scanning setting for this case.)
CASE 4: LASIK FLUID CLEFT SYNDROME
A 54-year-old woman had undergone uncomplicated LASIK for bilateral −4.00-diopter spherical myopia 4 months earlier. She had episodic, recurring diffuse corneal haze in her left eye (visual acuity, 20/60) that was thought to be diffuse lamellar keratitis (Figure 4A). Lifting and irrigation of the LASIK flap 2 months earlier had not resolved the problem. The patient was referred to the University of Pittsburgh Medical Center Eye Center for further evaluation.
Figure 4.
Fluid cleft syndrome. A, Diffuse corneal haze secondary to endothelial cell deficiency. B, High-magnification slitamp biomicroscopy revealed fluid clefting in the laser in situ keratomileusis (LASIK) flap interface (arrows). C, Hyporeflective slit due to accumulation of aqueous fluid is seen on high-speed, ultra–high-resolution optical coherence tomography along the LASIK flap interface (arrows).
On slitlamp biomicroscopy (Figure 4B), there was diffuse corneal stromal haze and a subtle discontinuous fluid pocket at the level of the LASIK flap that was clearly observed on the hsUHR-OCT scan (Figure 4C). The finding of the fluid cleft between the LASIK flap and the stroma triggered a retrospective medical record review that revealed a discrepancy between the pre-LASIK pachymetry values of the 2 eyes (581 μm OD and 651 μm OS). The 70-μm difference in central corneal thickness between the patient's 2 eyes and the fluid cleft suggested that her persistent, recurring corneal stromal haze was primarily due to endothelial dysfunction after LASIK, manifesting as fluid cleft syndrome.10 Pre-LASIK and post-LASIK intraocular pressures were normal. She was administered a topical hyperosmotic agent and an aqueous suppressant to reduce endothelial stress, with moderate clearing of her corneal haze.
CASE 5: UNCOMPLICATED DESCEMET STRIPPING ENDOKERATOPLASTY
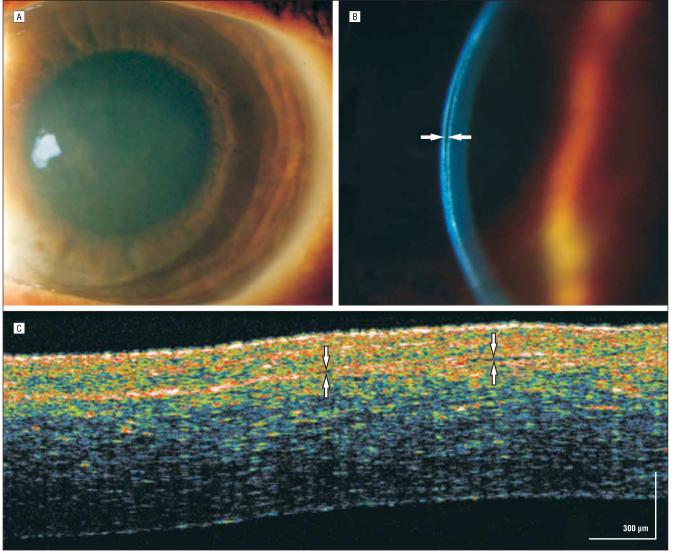
A 72-year-old woman experienced a reduction in visual acuity in her right eye secondary to Fuchs endothelial dystrophy. A Descemet stripping endokeratoplasty (DSEK) was performed (Figure 5A), and high slitlamp magnification showed a hyperreflective donor-host interface (Figure 5B). The hsUHR-OCT confirmed a well-delineated, highly reflective horizontal line representing the donor-host interface (Figure 5C). The patient's edematous anterior stroma appeared hyperreflective, possibly owing to edema and proximity to the entering light signal. The transplanted posterior graft (stroma–Descemet membrane–endothelial complex) was from an otherwise healthy 20-year-old cadaveric eye and appeared considerably less reflective in comparison. The donor Descemet membrane transplant was entirely adherent to the posterior corneal surface with the aid of an air bubble in the anterior chamber.
Figure 5.
Descemet membrane stripping endokeratoplasty. A, On postoperative day 1, 50% air bubble fill in the anterior chamber helps with adherence of the transplant. B, Magnified slitlamp view of the high-reflectivity donor-host interface (arrow). C, The interface between the recipient and donor corneas is marked by arrows. High-speed, ultra–high-resolution optical coherence tomography shows the strongest light signal approaching the epithelial surface. In addition to the proximity to the strongest light signal the patient's own corneal tissue is edematous and hyperreflective secondary to Fuchs endothelial dystrophy. The inner donor stromal–Descemet membrane–endothelial transplant is clearly seen as hyporeflective behind the hyperreflective donor-host interface. The donor and original corneas are seen in full adherence.
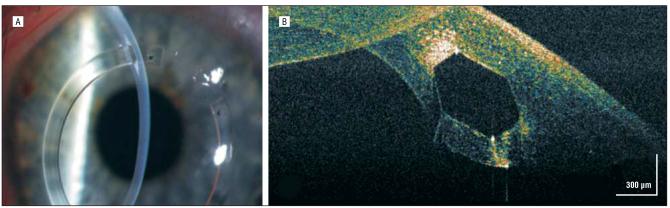
CASE 6: COMPLEX DSEK
An 81-year-old woman with Fuchs endothelial dystrophy underwent an otherwise uncomplicated DSEK, although an iridocorneal adhesion to a previous clear-corneal cataract wound was present temporally. On postoperative day 1, it was impossible to assess whether the transplant was fully adherent inferiorly on slitlamp examination because of the patient's dense corneal arcus (Figure 6A and B). The hsUHR-OCT image, unaffected by the dense corneal arcus, demonstrated the known iridocorneal adhesion and a hyporeflective space between the transplant interface, confirming that there was subtle nonadherence of the donor transplant inferiorly (Figure 6C).
Figure 6.
Iridocorneal touch. A, Dense corneal arcus obscuring slitlamp biomicroscopic details of a deep lamellar keratoplasty transplant in the inferior region. B, Magnified slitlamp view of the area of iridocorneal attachment (arrow) obscured under the corneal arcus. C, High-speed, ultra–high-resolution optical coherence tomography shows the area of the attachment (right) and the hyporeflective area between the donor-recipient interface (arrows).
CASE 7: KERATOCONUS AND CORNEAL IMPLANTS
A 42-year-old man with bilateral keratoconus underwent corneal implantation (Intacs; Addition Technology Inc, Des Plaines, Illinois) (Figure 7A). The hsUHR-OCT obtained 6 weeks after corneal implant placement confirms the intended placement at two-thirds corneal thickness (Figure 7B). The highly reflective stroma adjacent to the corneal implant channels is secondary to distortion of normal stromal intralamellar architecture (stress lines), with resultant increased light scattering.
Figure 7.
Intrastromal corneal implant A, Polymethyl methacrylate corneal implant ring placed at the corneal stroma. B, The hyporeflective space of the corneal implant ring appears in two-thirds corneal depth. Adjacent areas of high reflectivity correspond with “stretching” and altering normal corneal lamellar architecture and spacing. Folding of the cornea seen in the left upper-hand corner is a scanning artifact.
CASE 8: CORNEAL ECTASIA
A 59-year-old man had undergone radial keratotomy 14 years earlier. A subsequent LASIK procedure 7 years earlier for consecutive hyperopia ultimately resulted in corneal ectasia (Figure 8A). High, irregular astigmatism required either penetrating or deep anterior lamellar keratoplasty. The hsUHR-OCT was performed to ensure that a suggestive radial keratotomy scar was not actually full thickness, which would obviate the more desirable lamellar keratoplasty procedure (Figure 8B). The hsUHR-OCT revealed an intact Descemet membrane–endothelial complex. None of the radial keratotomy scars were found to be full thickness intraoperatively, and a deep lamellar keratoplasty was successfully performed.
Figure 8.
Corneal ectasia. A, Radial keratotomy followed by laser in situ keratomileusis with resultant corneal ectasia. B, The Descemet membrane–endothelial complex seems to be intact; therefore, a deep lamellar keratoplasty was performed successfully.
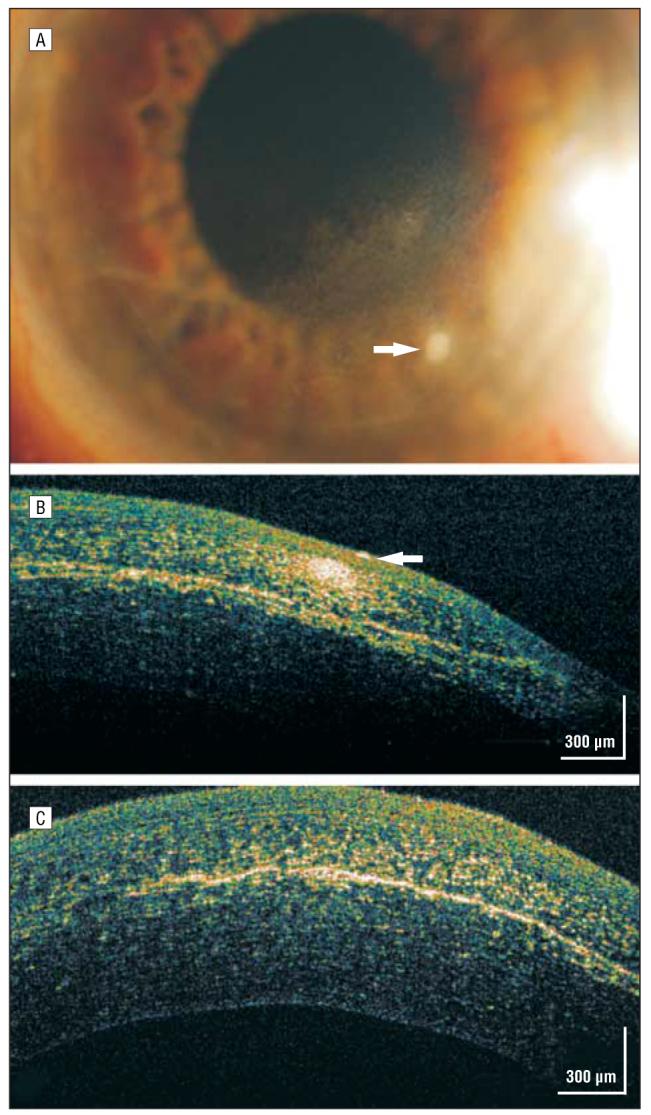
CASE 9: DESCEMET MEMBRANE DETACHMENT
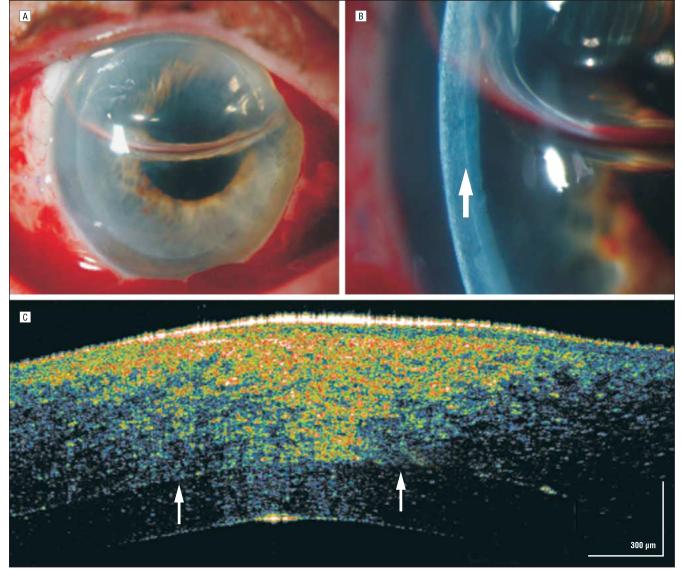
A 53-year-old man had 20/70 preoperative visual acuity in an eye with a long-standing traumatic cataract. He underwent phacoemulsification with posterior chamber intraocular lens implantation and capsular tension ring placement for zonular dehiscence. On the first postoperative day his vision was only hand motions, and slitlamp findings showed unexpected, dramatic 4+ microcystic corneal edema for which intensive topical corticosteroid treatment was initiated. On postoperative day 4 there was persistent microcystic corneal edema. He was referred to an experienced cornea/anterior segment surgeon who diagnosed possible endothelial failure and continued the intensive topical corticosteroid treatment with the addition of a hyperosmolar ointment.
One month later his vision had improved to 20/70, but he had persistent central bullous corneal edema (Figure 9A). Although a small Descemet membrane detachment was noted nasally (Figure 9B), no obvious Descemet membrane abnormality was noted more centrally on slitlamp examination. A hsUHR-OCT image showed a bullous epithelium (Figure 9C) and a detached and missing Descemet membrane centrally (Figure 9D and E). This patient's topical corticosteroid treatment was reduced, and he was referred for a definitive DSEK.
Figure 9.
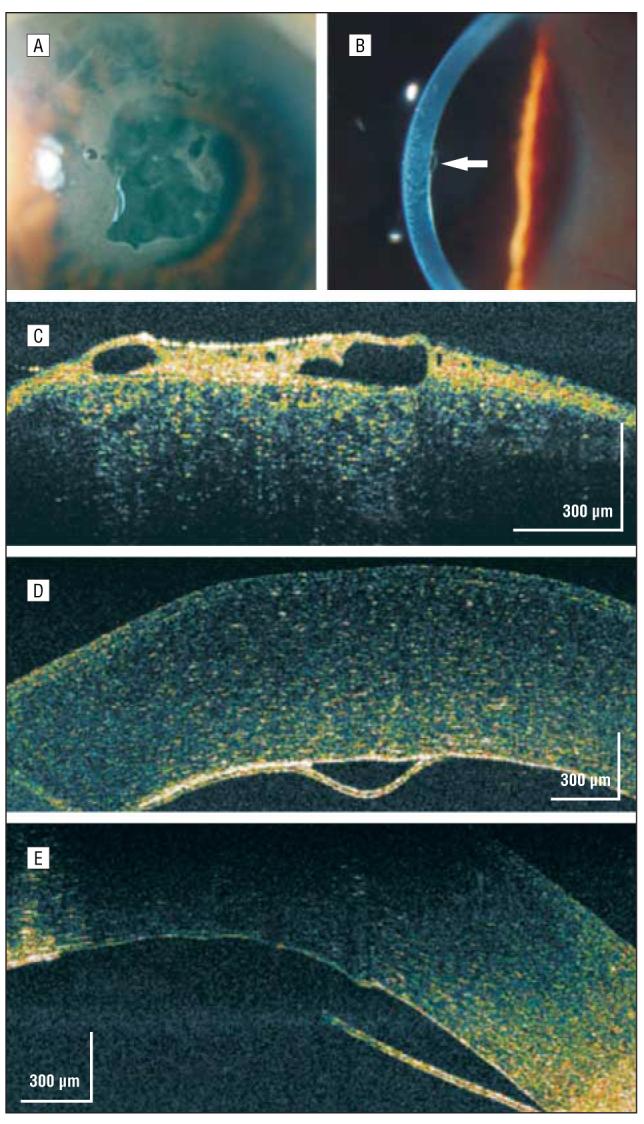
Descemet membrane detachment. A, Persistent central bullous corneal edema of unestablished etiology 1 month after phacoemulsification. B, On high-magnification slitlamp photography, a small nasal Descemet membrane detachment was noted (arrow). C, High-speed, ultra–high-resolution optical coherence tomography (hsUHR-OCT) shows a thickened epithelium with large hyporeflective corneal epithelial bullae. D, An hsUHR-OCT image highlights the small Descemet membrane separation adjacent to the missing Descemet membrane to the right. E, A more central view of the Descemet membrane detachment with the adjacent missing Descemet membrane consistent with the overlying central area of bullous epithelium (not shown).
COMMENT
In this study, we reported the hsUHR-OCT surgical corneal findings in cases in which the enhanced imaging capabilities provided by hsUHR-OCT affected clinical and surgical management compared with slitlamp examination alone. The hsUHR-OCT images confirmed slitlamp biomicroscopic findings and affected surgical and postoperative decision making.
The cornea, on average, is 540 μm thick and is made up of 5 layers.11 Each layer reflects light differently according to variable indices of refraction. Findings from time-based corneal OCT images were found to be in good agreement with those of histologic sections.12 The hsUHR-OCT case images delineated 4 layers of the cornea and showed good correlation with well-known histologic features. Unlike the commercially available anterior segment OCT, hsUHR-OCT does not compensate for refraction at the surface of the cornea, so there may also be geometric errors at the anterior and posterior surfaces. Nevertheless, findings from these hsUHR-OCT cornea images are in good agreement with anatomical corneal norms and provide substantially improved detailed information compared with the commercially available time-domain OCT images (Figure 1). The epithelium (approximately 50 μm), Bowman layer (approximately 10-15 μm), stroma (approximately 460 μm), and Descemet membrane (approximately 10 μm) were easily identifiable using hsUHR-OCT, whereas the endothelium usually appeared as a complex with the Descemet membrane. The endothelium, 5 to 6 μm thick, approaches the image resolution of the prototype hsUHR-OCT, which makes it difficult to differentiate as a separate entity. These findings seem to agree with those of a study by Kaluzny et al8 that examined various corneal abnormalities using hsUHR-OCT.
When the scanning beam is perpendicular to the tissue structures, reflectivity is very high. Tissue orientation combined with the large difference in refractive index between air and the tear film creates the high level of reflectivity observed centrally in the tear film.11 The varying nuclear to cytoplasmic ratio and shape of the superficial epithelial cells compared with the epithelial basal cells may also affect variable reflectivity in this layer.
An increase in light reflectivity in the hsUHR-OCT images corresponded to all surgical and refractive corneal incisions, scarring, dense inflammation, corneal edema, ectopic epithelial cells, and fibrosis. All of the cases illustrated the highly reflective interfaces of LASIK, DSEK, and corneal implantation procedures.
A decrease in light scattering or reflectivity was due to fluid in interface potential spaces, clear intrastromal appliances (corneal implants) (Figure 7B), bullous epithelial edema (Figure 9C), and shadowing. In the case of the interface fluid cleft syndrome, the fluid in the potential space appeared hyporeflective (Figure 4C). Although the finding of the fluid cleft could be viewed using the slitlamp, it was initially missed by an experienced refractive surgeon on multiple examinations. The hsUHR-OCT, which instigated retrospective pachymetric review, highlighted the importance of additional information provided by the device. If hsUHR-OCT had been performed early on, the patient most likely would not have undergone an unnecessary second procedure to lift the flap for presumed diffuse lamellar keratitis.
The hsUHR-OCT in this case study provided excellent visualization of various surgical and refractive corneal interfaces. Confluent abnormalities in refractive corneal interfaces (eg, epithelial in-growth after LASIK trauma) were clearly delineated (Figure 3B). The high-resolution capability of hsUHR-OCT was an advantage in case 4, the LASIK interface cleft, where the subtle fluid cleft between the LASIK flap and the underlying stroma was visualized and could be monitored (Figure 4C). Monitoring the light-scattering properties of certain corneal conditions will be useful in assessing disease progression or regression.8
In case 6, hsUHR-OCT imaging guided postoperative management. Because of the nonadherence of the inferior DSEK transplant (Figure 6C), which was otherwise not easily visualized behind the dense corneal arcus senilis, the patient was instructed to remain in a supine, chin-up position for 1 more day. By staying flat longer, the remaining air bubble positioned in the anterior chamber resulted in complete adhesion of the transplant during the next 24 hours. Similarly, in case 9, clinical and slitlamp examination findings inferred corneal endothelial dysfunction. The hsUHR-OCT image revealed a small nasal Descemet membrane detachment and a larger, central missing Descemet membrane that were not detected clinically (Figure 9D and E).
Although this prototype OCT technology has successfully captured high-quality corneal images, it does have limitations. Dense corneal opacities will result in shadowing of structures posterior to the lesion.
In conclusion, hsUHR-OCT improves visualization of structures and their relationship to incisional and refractive corneal procedures. The hsUHR-OCT images of corneal abnormalities enhanced slitlamp biomicroscopic examination. Corneal hsUHR-OCT imaging can be useful in the preoperative and postoperative clinical treatment of patients undergoing corneal and refractive surgery.
Acknowledgments
Funding/Support: Supported in part by grants RO1-EY013178-7, R01-EY011289-20, and P30-EY008098 from the National Institutes of Health; grants FA9550-040-1-0046 and FA9550-040-1-0011 from the Air Force Office of Scientific Research; grant BES-0522845 from the National Science Foundation; The Eye and Ear Foundation; and an unrestricted grant from Research to Prevent Blindness Inc.
Role of the Sponsor: The sponsors did not participate in the study at any stage other than providing the funding.
Footnotes
Financial Disclosure: None reported.
Previous Presentation: This study was presented in part at the Fourth Annual Meeting of the International Society for Imaging of the Eye; March 28, 2006; Fort Lauderdale, Florida.
REFERENCES
- 1.Masters BR, Thaer AA. Real-time scanning slit confocal microscopy of the in vivo human cornea. Appl Opt. 1994;33(4):695–701. doi: 10.1364/AO.33.000695. [DOI] [PubMed] [Google Scholar]
- 2.Hoerauf H, Wirbelauer C, Scholz C, et al. Slit-lamp–adapted optical coherence tomography of the anterior segment. Graefes Arch Clin Exp Ophthalmol. 2000;238(1):8–18. doi: 10.1007/s004170050002. [DOI] [PubMed] [Google Scholar]
- 3.Visante OCT. Carl Zeiss Meditec Inc; Dublin, California: 2006. (Publication 000000-1376-265). [Google Scholar]
- 4.Müller M, Hoerauf H, Geerling G, et al. Filtering bleb evaluation with slit-lamp–adapted 1310-nm optical coherence tomography. Curr Eye Res. 2006;31(11):909–915. doi: 10.1080/02713680600910528. [DOI] [PubMed] [Google Scholar]
- 5.Wollstein G, Paunescu LA, Ko TH, et al. Ultrahigh-resolution optical coherence tomography in glaucoma. Ophthalmology. 2005;112(2):229–237. doi: 10.1016/j.ophtha.2004.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ko TH, Fujimoto JG, Schuman JS, et al. Comparison of ultrahigh-and standard-resolution optical coherence tomography for imaging macular pathology. Ophthalmology. 2005;112(11):1922–1935. doi: 10.1016/j.ophtha.2005.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouma BE, Tearney GJ. Handbook of Optical Coherence Tomography. Informa Healthcare; New York, NY: 2001. [Google Scholar]
- 8.Kaluzny BJ, Kaluzny JJ, Szkulmowska A, et al. Spectral optical coherence tomography: a new imaging technique in contact lens practice. Ophthalmic Physiol Opt. 2006;26(2):127–132. doi: 10.1111/j.1475-1313.2006.00371.x. [DOI] [PubMed] [Google Scholar]
- 9.Z136 Committee, Laser Institute of America . American National Standards Institute for Safe Use of Lasers. American National Standards Institute; New York, NY: 1993. [Google Scholar]
- 10.Wirbelauer C, Pham DT. Imaging interface fluid after laser in situ keratomileusis with corneal optical coherence tomography. J Cataract Refract Surg. 2005;31(4):853–856. doi: 10.1016/j.jcrs.2004.08.045. [DOI] [PubMed] [Google Scholar]
- 11.Maurice DM. The structure and transparency of the cornea. J Physiol. 1957;136(2):263–268. doi: 10.1113/jphysiol.1957.sp005758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wirbelauer C, Winkler J, Bastian GO, et al. Histopathological correlation of corneal diseases with optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2002;240(9):727–734. doi: 10.1007/s00417-002-0518-3. [DOI] [PubMed] [Google Scholar]