Introduction
As a suspected carcinogenic agent and toxic pollutant, Cr(VI) poses a threat when present even at trace levels. Several electrochemical methods have been reported for the successful determination and quantification of Cr(VI),1-9 and these methods are of great interest due to the fact that they provide high sensitivity, portability, and the ability to distinguish Cr(VI) from Cr(III). An attractive method for analytical sensing is the use of sol-gel materials in conjunction with electrochemistry.10 We recently reported a method for the determination of Cr(VI) in solution using a pyridinium-functionalized sol-gel electrode.11 Unlike many sol-gel electrode films that are formed through either spin-coating or dip-coating processes, we have formed our sol-gel thin films using an electrodeposition process similar to that reported by Deepa and coworkers.12 In this process, a negative potential is applied at the electrode surface to generate hydroxide ions. When a sol solution is present, the OH-base catalyzes the hydrolysis and condensation of the sol at the electrode surface and produces a thin film. By incorporating precursors of varying functionality in the starting sol solution, films selective for a variety of analytes can be formed. In this research, we have used pyridinium-functionalized sol-gels for applications in Cr(VI) sensing, as previous studies have demonstrated the ability of pyridinium derivatives to form strong and stable complexes with Cr(VI) species.7,11,13
Experimental
All of the chemicals used in the study were purchased, except for the 4-[2- (trimethoxysilyl)ethyl]-pyridine, which was synthesized using a reported procedure.14 The electrodes were polished, sonnicated, and cleaned with piranha solution prior to their use. A CH Instruments model 400A potentiostat with electrochemcial quartz crystal microbalance (EQCM) capability and corresponding software were used for the electrochemical analyses.
For coating the electrodes, a sol solution typically consisting of 2 mL of 0.2 M KCl, 2 mL of EtOH, 250 μL of TMOS, and 250 μL of 4-[2-(trimethoxysilyl)ethyl]-pyridine was prepared and stirred thoroughly for several minutes to ensure a homogeneous mixture. After mixing, the working electrode, typically a glass-encased glassy carbon electrode, was exposed to the sol solution, and a potential of -0.9 V was applied for 60 s. This electrode was then rinsed with several aliquots of a 50:50 mixture of EtOH and DI water. It was then placed in an oven at 68 °C for 12 h and subsequently cured further at room temperature for an additional 12 h.
Results and Discussion
Scheme 1illustrates the electroanalysis process occurring at the functionalized electrode surface. When a film functionalized with 4-[2-(trimethoxysilyl)ethyl]-pyridine, or in some cases 2-[2-(trimethoxysilyl)ethyl]-pyridine, is exposed to a Cr(VI) solution, the Cr(VI) ions are preconcentrated at the electrode surface. This is due to the electrostatic interaction between the positively-charged pyridinium groups present in the film and the negatively-charged Cr(VI) anions. When a potential sweep is carried out, a peak occurs at approximately 0.17 V corresponding to the reduction of the preconcentrated Cr(VI) to Cr3+. This reduction converts the Cr anions to cations, regenerating the electrode surface for subsequent preconcentration and analysis.
Scheme 1.
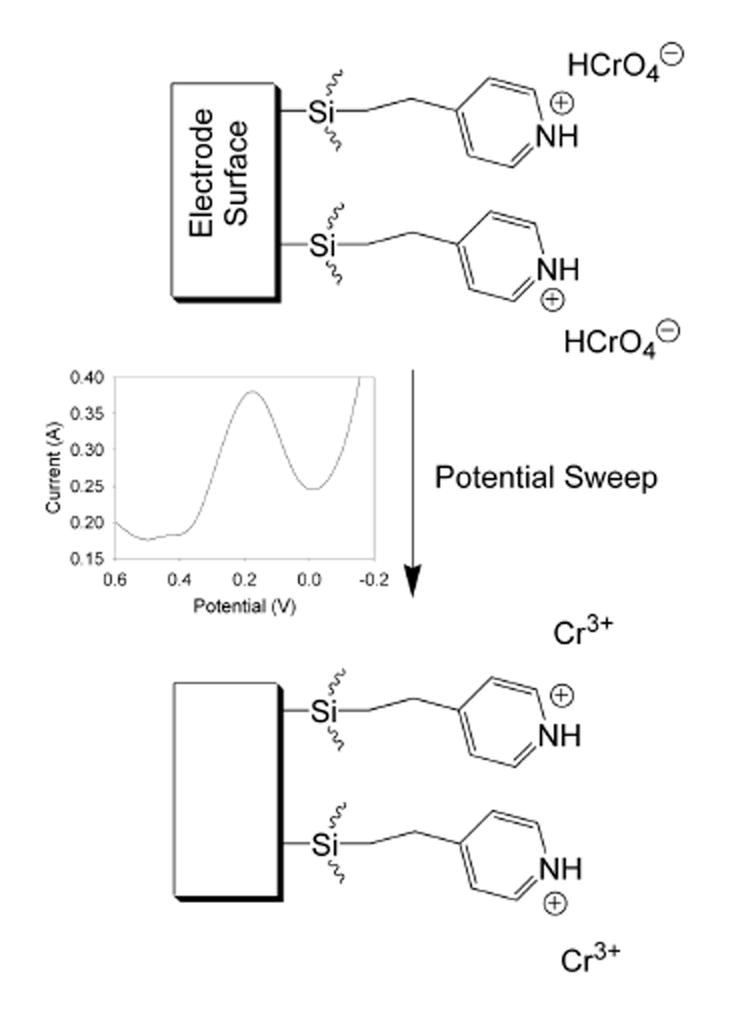
Schematic of the electroanalysis process at a 4-[2-(trimethoxysilyl)ethyl]-pyridine-functionalized sol-gel electrode. HCrO4− is used to represent the Cr(VI) anionic species.
Studies at Coated Glassy Carbon Electrodes
Fe(CN)64− was used as a redox probe to investigate the electrodeposited sol-gel films and their anion-exchange capabilities. It was chosen as a model analyte for its negative charge, the fact that it is a well-established redox system, and the fact that previous studies have demonstrated the use of anion-exchange selective layers for the partitioning of the Fe(CN)64−couple.11,15 Figure 1 shows the cyclic voltammograms obtained when a 4-[2-(trimethoxysilyl)ethyl]-pyridine-functionalized sol-gel electrode is used to analyze a 5 mM Fe(CN)64- solution. As time increases, the peak currents increase, indicating the time-dependent partitioning of Fe(CN)64- into the sol-gel film from the aqueous solution by the anion-exchange process. When the partitioned Fe(CN)64- permeates through the film to the electrode surface, it is oxidized to Fe(CN)63-. Eventually, the peak currents become fairly constant, signaling that the sol-gel film has become saturated with the analyte. This correlates well with previous reports showing the enhanced sensitivity achieved when anionic-exchange films are used for the determination of Fe(CN)64−.11,15 These results may suggest that the 4-[2-(trimethoxysilyl)ethyl]-pyridine-functionalized sol-gel films are adequate for the preconcentration and analysis of Cr(VI) anions, although the mechanisms of analyte transport for the two systems [mass transport for the irreversible Cr system, diffusion and charge-transport processes for the reversible Fe(CN)64− system] are greatly different.
Figure 1.
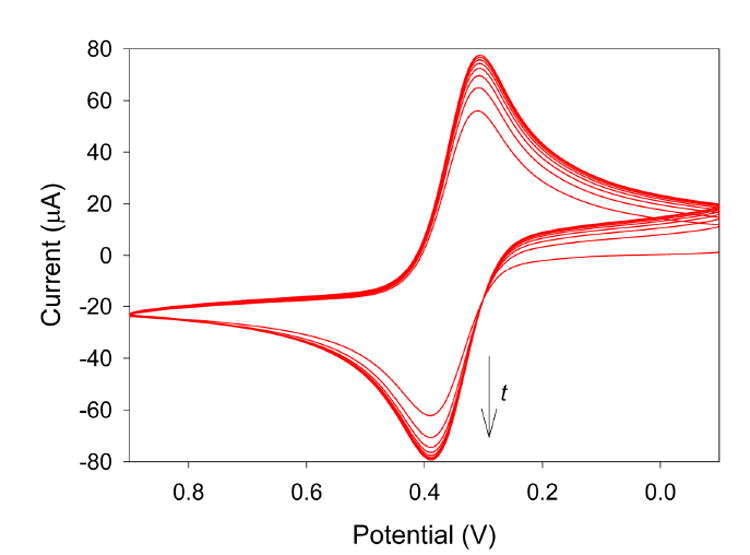
Cyclic voltammograms obtained of a solution consisting of 5 mM Fe(CN)64-, 0.1 M HCl, and 0.1 M KCl using a 4-[2-(trimethoxysilyl)ethyl]-pyridine-functionalized sol-gel electrode.
When Cr(VI) solutions are analyzed, a peak current is generated at approximately 0.17 V, corresponding to the reduction of Cr(VI) to Cr(III). Square wave voltammograms and a corresponding calibration plot are shown in Figures 2 and 3, respectively. As can be seen, there is a linear relationship (R2 = 0.997) between the Cr(VI) concentration and the peak current, and the limit of detection is approximately 1.0 ppb Cr(VI). This is an improvement over the limit of detection provided by the 2-[2-(trimethoxysilyl)ethyl]-pyridine-functionalized sol-gel electrode, previously determined to be approximately 4.6 ppb Cr(VI).11 This improvement correlates well with the report by Turyan and Mandler showing that 4-mercaptopyridine was able to preconcentrate Cr(VI) to a greater extent than 2-mercaptopyridine.7
Figure 2.
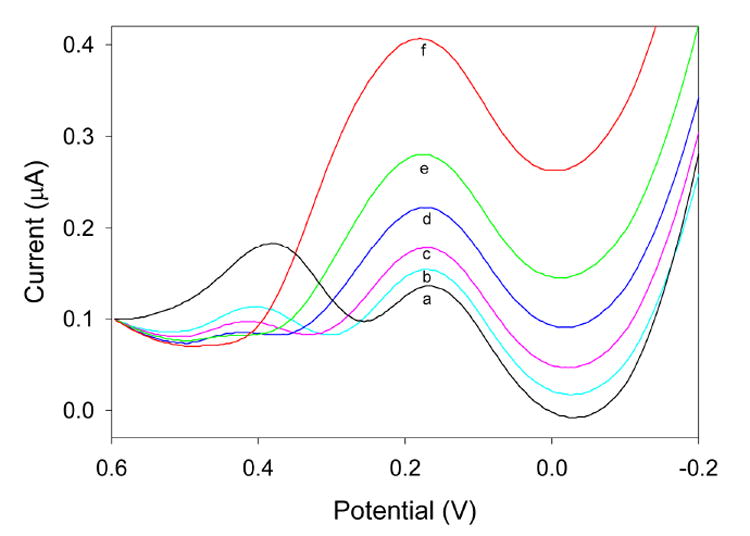
Square-wave voltammograms of various Cr(VI) concentrations collected at a 4-[2-(trimethoxysilyl)ethyl]-pyridine-functionalized sol-gel electrode: (a) 0 ppb; (b) 1.1 ppb; (c) 10.7 ppb; (d) 48.4 ppb; (e) 98.5 ppb; (f) 198 ppb.
Figure 3.
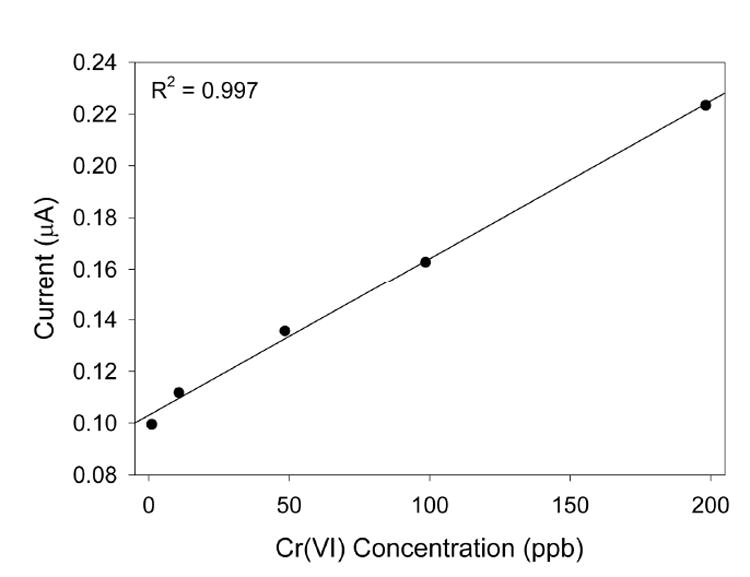
Calibration plot of the measurements in Figure 2.
Studies at Coated Gold Electrodes
In addition to glassy carbon electrodes, studies were carried out involving the electrodeposition of sol-gels onto the surfaces of gold electrodes. Collinson and coworkers have demonstrated that sol-gel films electrodeposited onto gold electrode surfaces show poor adhesion and tend to peel off easily.12 However, by using a binding agent, such as (3-mercaptopropyl) trimethoxysilane, the sol-gel coatings can be anchored to the electrode surfaces, improving their stability and durability. Scheme 2 depicts the use of this technique for the sol-gel coating of gold electrodes. Initially, a gold electrode is immersed in an ethanol solution of 21 mM (3-mercaptopropyl) trimethoxysilane for 10 minutes to form a self-assembled monolayer at the electrode surface. After rinsing the electrode with ethanol and water, it is then exposed to a sol solution and coated as discussed in the Experimental Section. Walcarius and Sibottier have used a similar procedure for the sol-gel coating of gold electrodes in their research.16
Scheme 2.
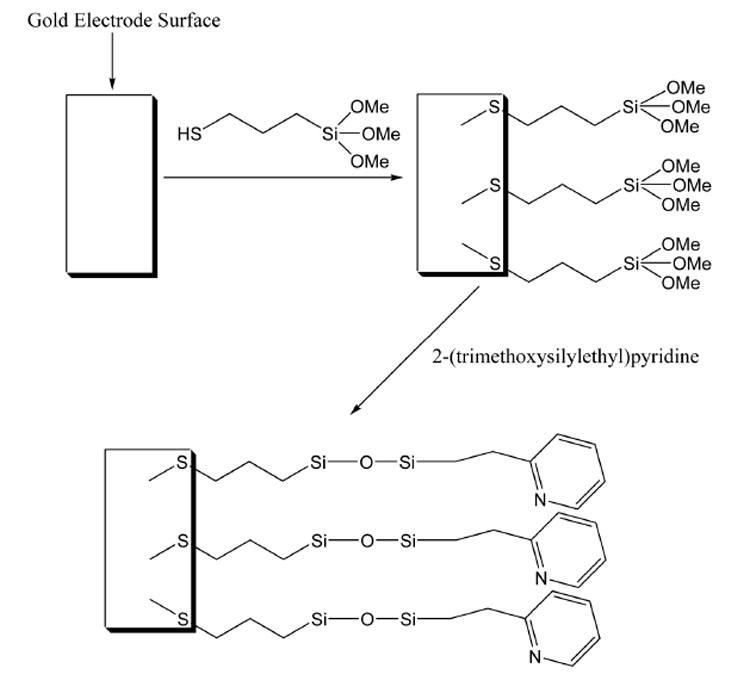
Schematic of sol-gel coating at a gold electrode.
Figure 4 shows cyclic voltammograms collected when a pyridium-functionalized sol-gel gold electrode is exposed to a solution consisting of 0.1 M HCl and 5 mM Fe(CN)64−. Initially, when the electrode is first exposed to the solution, peak currents occur due to the oxidation and reduction of the Fe(CN)64− couple, as was seen in Figure 1. These currents increase rather dramatically after the electrode has been exposed to the redox probe solution for 5 minutes. This is likely due to the fact that, during the five minutes, preconcentration of the negatively-charged analyte species in the positively-charged sol-gel film has taken place, resulting in an enhanced peak current.
Figure 4.
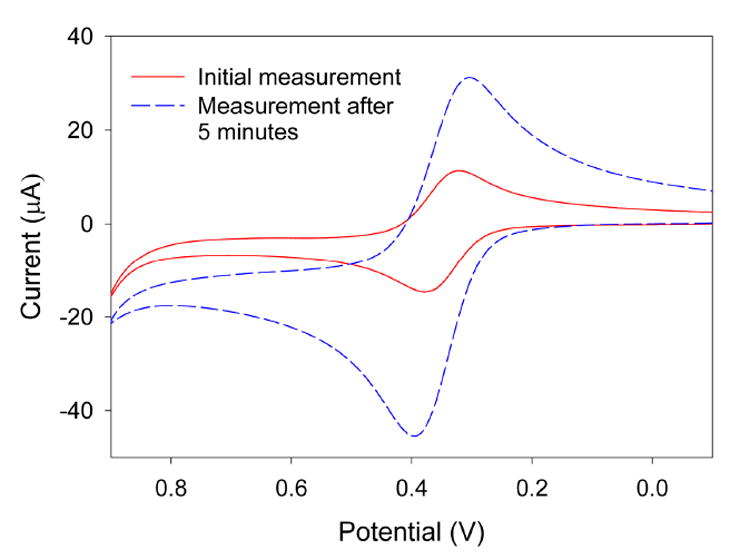
Cyclic voltammograms collected at a pyridinium-functionalized sol-gel gold electrode upon exposure to a solution consisting of 5 mM Fe(CN)64−, 0.1 M KCl, and 0.1 M HCl.
When the sol-gel modified gold electrodes were exposed to Cr(VI) solutions, they demonstrated a selectivity and sensitivity similar to that exhibited by the pyridinium-functionalized sol-gel glassy carbon electrodes. For example, when a sol-gel modified gold electrode was exposed to a solution consisting of 68 ppb Cr(VI), 0.1 M HCl, and 0.1 M KCl, a peak current corresponding to the reduction of Cr(VI) to Cr(III) was generated. This demonstrates that, when (3-mercaptopropyl) trimethoxysilane is employed as a binding agent, gold electrodes may be a viable alternative to glassy carbon electrodes for sol-gel electrodeposition applications.
Studies Involving Coated Indium-Tin Oxide (ITO) Electrodes
A possible use of the electrodeposited pyridinium-functionalized sol-gels is in spectroelectrochemical applications. Spectroelectrochemistry is typically carried out using optically transparent electrodes such as indium-tin oxide (ITO). When a functional film is coated onto the surface of such an electrode, three modes of selectivity (electrochemistry, spectroscopy, and selective partitioning) can take place, greatly enhancing the overall selectivity for the analyte of interest. Heineman, Seliskar, and coworkers have carried out much research in this area,15 and it should be possible to use electrodeposited sol-gels in such applications.
Polished float glass slides with indium-tin oxide coated on the surface (purchased from Delta Technologies, Ltd.) were cut into 1.5 cm × 1.0 cm pieces and washed thoroughly with DI water, acetone, and ethanol. Each slide was then coated using the procedure described in the Experimental Section, except the sol-gel deposition time used was 30 minutes instead of 60 seconds for each electrode. After curing, one of the pyridinium-functionalized sol-gel ITO electrodes was exposed for 10 minutes to a solution consisting of 19.7 ppm Cr(VI), 0.1 M HCl, and 0.1 M KCl. The electrode was then rinsed with DI water and transferred to pure electrolyte solution, and several CV scans were carried out. The results can be seen in Figure 5. Initially, a large peak current occurs at 0.43 V, corresponding to the reduction of a portion of the Cr(VI) partitioned in the film from the immersion of the electrode in the 19.7 ppm Cr(VI) solution. As time goes on and more voltammograms are carried out, the peak current decreases as more and more of the preconcentrated Cr(VI) is reduced to Cr(III). Eventually, the reduction peak becomes indistinguishable from the background, indicating that the majority of the Cr(VI) in the film has been removed.
Figure 5.
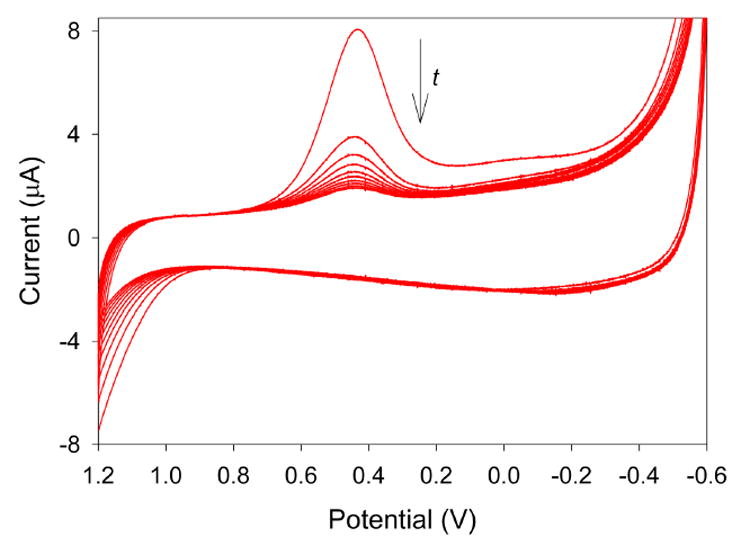
Cyclic voltammograms obtained in 0.1 M HCl/0.1 M KCl at a pyridinium-functionalized sol-gel ITO electrode after immersion in 19.7 ppm Cr(VI) for 10 minutes.
The successful electrochemical use of these electrodeposited films on ITO electrodes indicates their potential suitability for spectroelectrochemical applications. With the proper instrumentation, the pyridinium-functionalized sol-gel coated ITO electrodes in the current system could be coupled with transmission or attenuated total reflectance (ATR) spectroscopic techniques. Such a set-up would provide a multi-modal sensing system for Cr(VI).
Footnotes
A portion of this research was presented at the Pittcon 2006 National Meeting in Orlando, FL.
References
- 1.Cox JA, Kulesza PJ. Stripping voltammetry of chromium (VI) at a poly(4-vinylpyridine)-coated platinum electrode. Anal Chim Acta. 1983;154:71. [Google Scholar]
- 2.Paniagua AR, Vazquez MD, Tascon ML, Sanchez Batanero P. Determination of chromium(VI) and chromium(III) by using a diphenylcarbazide-modified carbon paste electrode. Electroanal. 1993;5:155. [Google Scholar]
- 3.Svancara I, Foret P, Vytras K. A study on the determination of chromium as chromate at a carbon paste electrode modified with surfactants. Talanta. 2004;64:844. doi: 10.1016/j.talanta.2004.03.062. [DOI] [PubMed] [Google Scholar]
- 4.Welch CM, Nekrassova O, Compton RG. Reduction of hexavalent chromium at solid electrodes in acidic media: reaction mechanism and analytical applications. Talanta. 2005;65:74. doi: 10.1016/j.talanta.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y-J, Huang H-J. Polyaniline-modified electrode-based FIA system for sub-ppb-level chromium (VI) analysis. Anal Chem. 2001;73:1377. [Google Scholar]
- 6.Lin L, Lawrence NS, Thongngamdee S, Wang J, Lin Y. Catalytic adsorptive stripping determination of trace chromium(VI) at the bismuth film electrode. Talanta. 2005;65:144. doi: 10.1016/j.talanta.2004.05.044. [DOI] [PubMed] [Google Scholar]
- 7.Turyan I, Mandler D. Selective determination of Cr(VI) by a self-assembled monolayer-based electrode. Anal Chem. 1997;69:894. doi: 10.1021/ac9607525. [DOI] [PubMed] [Google Scholar]
- 8.Ge H, Zhang J, Wallace GG. Use of overoxidized polypyrrole as a chromium(VI) sensor. Anal Lett. 1992;25:429. [Google Scholar]
- 9.Yong L, Armstrong KC, Dansby-Sparks RN, Carrington NA, Chambers JQ, Xue Z-L. Quantitative analysis of trace chromium in blood samples. Combination of the advanced oxidation process with catalytic adsorptive stripping voltammetry. Anal Chem. 2006;78:7582. doi: 10.1021/ac060707p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walcarius A, Mandler D, Cox JA, Collinson M, Lev O. Exciting new directions in the intersection of functionalized sol-gel materials with electrochemistry. J Mater Chem. 2005;15:3663. [Google Scholar]
- 11.Carrington NA, Yong L, Xue Z. Electrochemical deposition of sol-gel films for enhanced chromium(VI) determination in aqueous solutions. Anal Chim Acta. 2006;572:17. doi: 10.1016/j.aca.2006.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deepa PN, Kanungo M, Claycomb G, Sherwood PMA, Collinson MM. Electrochemically deposited sol-gel-derived silicate films as a viable alternative in thin-film design. Anal Chem. 2003;75:5399. doi: 10.1021/ac026459o. [DOI] [PubMed] [Google Scholar]
- 13.Carrington NA, Thomas GH, Rodman DL, Beach DB, Xue Z-L. Optical determination of Cr(VI) using regenerable, functionalized sol-gel monoliths. Anal Chim Acta. 2006 doi: 10.1016/j.aca.2006.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corriu RJP, Lancelle-Beltran E, Mehdi A, Reye C, Brandes S, Guilard R. Ordered mesoporous hybrid materials containing cobalt(II) Schiff base complex. J Mater Chem. 2002;12:1355. [Google Scholar]
- 15.Shi Y, Slaterbeck AF, Seliskar CJ, Heineman WR. Spectroelectrochemical sensing based on multimode selectivity simultaneously achievable in a single device. 1. Demonstration of concept with ferricyanide. Anal Chem. 1997;69:3679. doi: 10.1021/ac970520l. [DOI] [PubMed] [Google Scholar]
- 16.Walcarius A, Sibottier E. Electrochemically-induced deposition of amine-functionalized silica films on gold electrodes and application to Cu(II) detection in (hydro)alcoholic medium. Electroanal. 2005;17:1716. [Google Scholar]


