Abstract
The cytoplasmic domains of integrins are essential for cell adhesion. We report identification of a novel protein, ICAP-1 (integrin cytoplasmic domain– associated protein-1), which binds to the β 1 integrin cytoplasmic domain. The interaction between ICAP-1 and β1 integrins is highly specific, as demonstrated by the lack of interaction between ICAP-1 and the cytoplasmic domains of other β integrins, and requires a conserved and functionally important NPXY sequence motif found in the COOH-terminal region of the β1 integrin cytoplasmic domain. Mutational studies reveal that Asn and Tyr of the NPXY motif and a Val residue located NH2-terminal to this motif are critical for the ICAP-1 binding. Two isoforms of ICAP-1, a 200–amino acid protein (ICAP-1α) and a shorter 150–amino acid protein (ICAP-1β), derived from alternatively spliced mRNA, are expressed in most cells. ICAP-1α is a phosphoprotein and the extent of its phosphorylation is regulated by the cell–matrix interaction. First, an enhancement of ICAP-1α phosphorylation is observed when cells were plated on fibronectin-coated but not on nonspecific poly-l-lysine–coated surface. Second, the expression of a constitutively activated RhoA protein that disrupts the cell–matrix interaction results in dephosphorylation of ICAP-1α. The regulation of ICAP-1α phosphorylation by the cell–matrix interaction suggests an important role of ICAP-1 during integrin-dependent cell adhesion.
Integrins comprise a family of heterodimeric cell adhesion receptors responsible for attachment of cells to the extracellular matrix or to specific cell surface counterreceptors (Hynes, 1992). Each subunit consists of a large extracellular domain that participates in the ligand recognition, a transmembrane region, and a short cytoplasmic domain. In adherent cells, the ligand binding induces recruitment of integrins to the focal adhesion plaques or focal contacts, where actin cytoskeletons converge onto the site of cell–extracellular matrix contact (for review see Burridge and Chrzanowska-Wodnicka, 1996). Studies have shown that the integrin-dependent cell adhesion can be regulated either by direct affinity modulation of integrins (Bennett and Vilaire, 1979; Altieri and Edgington, 1988; Faull et al., 1993; Stewart et al., 1996) or by clustering of integrins, which requires cytoskeletal rearrangement (Hermanoswki-Vosatka et al., 1988; Haverstick et al., 1992; van Kooyk et al., 1994; Stewart et al., 1996). Either through recruitment of regulatory proteins such as adaptor protein Shc or focal adhesion kinase (FAK)1 to the focal contacts or by inducing reorganization of actin cytoskeleton, integrins function as transmembrane receptors for extracellular signals and participate in the activation of cytoplasmic signaling cascade (for review see Schwartz et al., 1995). The dependence of cell proliferation, prevention of apoptosis, and cell differentiation on the cell–matrix interaction mediated by integrins illustrates the importance of this adhesion-dependent cell signaling.
Although the cytoplasmic domains of integrins lack any known enzymatic activity or sequence motif involved in protein–protein interaction, studies have shown that the short cytoplasmic tails of α or β subunits are important for the regulation of integrin affinity and cytoskeletal interaction (Sastry and Horwitz, 1993; Schwartz et al., 1995). The cytoplasmic domains of different β subunits are similar in size and sequence. Mutational analysis of the cytoplasmic domain of integrin β1 has identified three regions that are important for the recruitment of integrins to the focal contacts (Marcantonio et al., 1990; Reszka et al., 1992). The first region, located in the membrane-proximal region, is rich in charged residues and predicted to form an α-helical structure. The second and third region consist of short sequences Asn-Pro-X-Tyr (NPXY). The NPXY motif was initially recognized as a sequence motif required for receptor-mediated endocytosis (Chen et al., 1990) and represents a unique structural motif capable of generating a reverse turn in solution (Bansal and Gierasch, 1991). The two tandem NPXY motifs of the integrins are situated in the membrane-distal region that is known to undergo alternative splicing (Languino and Ruoslahti, 1992; Zhidkova et al., 1995). Naturally occurring splicing variants of the β1 integrin lacking the NPXY motifs do not localize to the focal contacts (Balzac et al., 1993). The first NPXY motif (membrane-proximal), in addition to playing a role in integrin– cytoskeleton interaction (Reszka et al., 1992; Ylanne et al., 1995), is also involved in affinity regulation of integrins (O'Toole et al., 1995) and integrin-dependent endocytic processes (Van Nhieu et al., 1996). Mutational studies have shown that the second NPXY motif (membrane-distal), like the first NPXY motif, is important for the focal contact localization of β1 integrins (Reszka et al., 1992) and cell adhesion by the β2 integrins (Hibbs et al., 1991; Peter and O'Toole, 1995).
How the integrin β subunit cytoplasmic domain participates in the regulation of cell–matrix interaction has not been resolved. The initial molecular models for the adhesion-dependent recruitment of integrins to the focal contacts were based on the observation that talin (Horwitz et al., 1986) and α-actinin (Otey et al., 1990) bind to the β1 integrins. As both of these proteins can bind actin, either directly as in α-actinin or through interaction with vinculin as in talin, the proposed function of talin and α-actinin in linking integrins to the cytoskeletal structures remains an attractive model. Other proteins that have been shown to bind β1 integrins include FAK (Schaller et al., 1995), paxillin (Schaller et al., 1995), and a Ser/Thr kinase (ILK-1) (Hannigan et al., 1996). Of these proteins, FAK and ILK-1, because of their ability to affect cell adhesion and cell spreading, represent potential regulators of the integrin–matrix interaction.
A second unresolved issue is whether the adhesive function and cytoskeletal interaction of different integrins are regulated by a common mechanism or by similar but distinct processes. Despite the remarkable similarities in the amino acid sequences of different integrin β subunit cytoplasmic domains, each β subunit displays distinct differences in its ability to localize to the focal contacts (Wayner et al., 1991; LaFlamme et al., 1994), to induce tyrosine phosphorylation of cytoplasmic proteins upon surface clustering (Freedman et al., 1993), and to participate in gene induction (Yurochko et al., 1992). In particular, a direct comparison of β1 and β5 cytoplasmic domains using a chimeric β1–β5 construct where the cytoplasmic domain of β1 was replaced with that of β5 has demonstrated that the β5 cytoplasmic domain, unlike the β1 counterpart, does not efficiently direct integrins to the focal contact or promote cell proliferation (Pasqualini and Hemler, 1994). Recently identified β3-endonexin (Shattil et al., 1995) and cytohesin-1 (Kolanus et al., 1996), which display restricted binding to the β3 and β2 cytoplasmic domain, respectively, suggest the presence of proteins that can discriminate the subtle differences in the amino acid sequence of different β subunits. These β subunit cytoplasmic domain–specific binding proteins may allow specific regulation of individual β subunits.
In the present study, we report a novel polypeptide named ICAP-1α (integrin cytoplasmic domain–associated protein-1) that binds to the β1 integrin cytoplasmic domain. The interaction, which can be demonstrated both in vitro and in vivo, is specific for the β1 integrins and requires the Asn and Tyr residues of the membrane-distal NPXY motif. The ability of ICAP-1α to interact only with the β1 cytoplasmic domain is attributed to an additional requirement of Val residue NH2-terminal to the NPXY. The functional role of ICAP-1 in cell adhesion is suggested by the observation that ICAP-1α is a phosphoprotein and that the degree of phosphorylation is regulated by integrin-dependent, cell–matrix interaction.
Materials and Methods
Cell Lines and Antibodies
293, HeLa, Jurkat, K562, and Cos-7 cells were obtained from American Type Culture Collection (Rockville, MD). SaOS, Rat-1, NIH3T3, 2f-TGH (human fibroblast cells), and UTA-6 (Englert et al., 1995) were gifts from C. Sawyers (University of California, Los Angeles, CA [UCLA]), K. Shuai (UCLA), and D. Haber (Massachusetts General Hospital, Boston, MA).
Cells expressing constitutively activated RhoA were generated using UTA-6 (Englert et al., 1995), a derivative of U2OS cells (osteosarcoma cell line) expressing tetracycline-repressible transactivator (Gossen and Bujard, 1992). An NH2-terminal FLAG epitope–tagged RhoA(Q63L) (Coso et al., 1995) was cloned into pTPH-1 (Gossen and Bujard, 1992). The expression construct was introduced into UTA-6 cells using a Ca2PO4-precipitation method (Ausubel et al., 1994) and hygromycin- resistant clones were isolated in the presence of 1 μg/ml tetracycline.
An anti-β1 integrin mAb producing hybridoma cell line, TS2/16, was generously provided by M. Hemler (Dana Farber Cancer Institute, Boston, MA). A mouse hybridoma cell line TS1/18 producing anti-β1 integrin mAb were obtained from ATCC. Hybridoma cell lines were cultured in DME + 10% CPSR-3 (Sigma Chemical Co., St. Louis, MO). mAb from tissue culture supernatant was purified on protein A–Sepharose (Pharmacia LKB Biotechnology, Inc., Piscataway, NJ).
Polyclonal rabbit antisera against the α subunit of LFA-1 (αLβ2) were prepared by immunizing rabbits with bacterially expressed glutathione-S-transferase (GST) fusion protein containing the entire 58–amino acid (aa) cytoplasmic domain (aa 1,088–1,145). For the generation of rabbit antisera against ICAP-1, the entire ICAP-1α coding sequences were cloned in pET16b (Novagen Inc., Madison, WI) and expressed as a histidine-tagged protein in Escherichia coli DE21 (Studier and Moffatt, 1986). His-tagged ICAP-1α was purified on nickel charged column (Novagen Inc.) under denaturing conditions following the manufacturer's recommendations and used as the immunogen.
Yeast Genetic Screening
The yeast genetic screening for the isolation of proteins interacting with the cytoplasmic domain of β1 integrin was carried out essentially as described previously (Gyuris et al., 1993). COOH-terminal 21 aa (GENPIYKSAVTTVVNPXYEGK) of the β1 subunit was cloned in frame into LexA coding sequence to generate a “bait” plasmid pNlex-β1cyto. The resulting Lex-β1cyto fusion protein was able to bind LexA operator in yeast, but displayed no basal transcriptional activity. A yeast expression library was generated from oligo dT-primed cDNA from JY cell (human B cell line) mRNA. The cDNA was cloned unidirectionally into the EcoRI/ XhoI sites of a yeast expression vector pJG4-5. This cDNA library that had the complexity of >106 was amplified once and used to transform a yeast strain EGY48 harboring pNlex-β1cyto and JK103 lacZ reporter plasmid. Approximately 2 × 106 independent yeast transformants were pooled and subjected to selection as described. Eight positive clones obtained all had identical cDNA insert. Plasmid DNA from one isolate (Clone E16-1) was rescued using E. coli KC8 (Gyuris et al., 1993) and amplified for further analysis. All bait constructs containing various integrin cytoplasmic domains or β1 integrin cytoplasmic domain mutants were tested for proper expression in a “suppression assay” in yeast using a reporter construct JK101 (Gyuris et al., 1993). β-galactosidase activity measurement was carried out as described previously (Ausubel, 1994).
Northern Blot Analysis and cDNA Cloning
The cDNA insert from Clone E16-1 was used as the probe to screen a multiple tissue mRNA blot (Clontech, Palo Alto, CA). A full-length cDNA was isolated from a HeLa cell cDNA library (a gift from K. Shuai, UCLA) using Clone E16-1 as the probe. An expressed sequence tag (EST) clone corresponding to ICAP-1β was obtained from the IMAGE Consortium (these sequence data are available EMBL/GenBank/DDBJ under accession number T69975). The presence of cDNA corresponding to ICAP-1β was independently confirmed by PCR (40 cycles: 94°C for 45 s; 55°C for 45 s; 72°C for 30 s) using primers A3 (5′-CCCAGCAAGATGGAAAGTTGCC-3′) and B2 (5′-GATCAGCATTTTACACAATCCA-3′) flanking the deleted sequences, which generated a 459- (ICAP-1α) or a shorter 308-bp fragment (ICAP-1β).
In Vitro Interaction Assay
For in vitro GST “pull-down” experiments, the COOH-terminal cytoplasmic domains of β1 subunit (see above), β2 subunit (DNPLFKSATTTVMNPKFAES), and αL (KVGFFKRNLKEKMEAGRGVPNGIPAEDSEQLASGQEAGDPGCLKPLHEKDSESGGGD) were individually ex-pressed in bacteria as GST fusion proteins. The EcoRI/XhoI insert of Clone E16-1 encoding ICAP-1α (aa 54–200) was cloned into the EcoRI/ XhoI site of an in vitro transcription vector pCITE-3a (Novagen, Inc.). The resulting pCITE-ICAP1α plasmid was used as the template in cotranscription/translation reaction using T7 RNA polymerase and rabbit reticulocyte lysate (Promega Corp., Madison, WI) to generate [35S]methionine-labeled polypeptides. An equal amount of labeled polypeptides was added to ∼2 μg of GST fusion proteins bound on glutathione-Sepharose beads (Pharmacia LKB Biotechnology Inc.) and incubated overnight at 4°C in NET (25 mM Tris-HCl, pH 7.6, 100 mM NaCl, 3 mM EDTA) containing 1 mM DTT, 1% BSA, and 0.1% Triton X-100. Beads were washed twice in the binding buffer and twice in 0.05% Triton X-100 in NET. Bound proteins were eluted by boiling in SDS sample buffer and analyzed by SDS gel electrophoresis.
Eukaryotic Expression Plasmids
The coding sequences of human αL integrin (CD11a) and human β2 (CD18) from the expression vectors previously described (Hibbs et al., 1991) were cloned into the XbaI site of the eukaryotic expression vector pcDNA3 (Invitrogen Inc., Madison, WI). To construct a hybrid β2.1 subunit, two PCR-generated fragments corresponding to the amino acids 634–749 of β2 and amino acids 778–798 of β1 were ligated together using a XcmI site that was introduced during the PCR. This fragment was cloned into the BstBI and NotI site of pcDNA3/β2 to generate pcDNA/β2.1. The sequence of the cytoplasmic domain in this hybrid β2.1 subunit consists of NH3-KALIHLSDLREYRRFEKEKLKSQWNGENPIYKSAVTTVV- NPXYEGK-COOH.
The full-length ICAP-1α cDNA was cloned into the EcoRV site of pcDNA3 to generate pcDNA/ICAP-1α. To generate the full-length ICAP-1β coding sequence, the 5′ half of the above PCR fragment and the 3′ half of a second PCR fragment amplified from T69975 clone (see above) were ligated in frame using a unique HpaII restriction site. The ligation product was cloned into the EcoRV site of pcDNA3 to produce pcDNA/ICAP-1β. The GST-tagged ICAP-1 constructs were generated using eukaryotic expression vector pEBG. The coding sequence of the partial ICAP-1α (aa 54–200) was derived from Clone E16-1.
Eukaryotic Expression and In Vivo Interaction Assay
5–10 μg of plasmid DNA was transfected into 293T cells by using a Ca2PO4 precipitation method (Ausubel et al., 1994). 48 h after transfection, cells were lysed in TBSM (25 mM Tris-HCl, pH 7.6, 150 mM NaCl, and 2 mM MgCl2) containing 0.5% NP-40, leupeptin, aprotinin, and PMSF. Detergent insoluble materials were removed by centrifugation at 12,000 g for 15 min. 500 μg of cleared lysates were mixed with an equal volume of TBSM to reduce the final detergent concentration to 0.25% NP-40 and incubated with glutathione–Sepharose beads for 3 h at 4°C. Beads were washed with TBSM + 0.25% NP-40 once, TBSM + 0.1% NP-40 twice, and TBSM alone twice. Coprecipitation of β1 integrins with bound GST-ICAP1 was determined on a Western analysis using mAb TS2/16 (anti-β1 integrin).
In Vitro Phosphatase Assay
50 μg NP-40 detergent lysate in 40 μl of 25 mM Tris-HCl, pH 8.0, 50 mM NaCl, and 10 mM MgCl2, was incubated at 30°C for 30 min, either in the presence or absence of phosphatase inhibitors (1 mM NaVO3 and 0.5 μM calyculin A). The reaction was terminated by adding SDS sample buffer and boiling for 5 min. 15 μg of lysates were analyzed on Western blot using anti-ICAP1 antibody.
Adhesion Assay
Cell adhesion was carried out using six-well tissue culture plates. Each 35-mm well was coated with 1 ml of fibronectin (20 μg/ml) or poly-l-lysine (PLK) (50 μg/ml) in TBS (25 mM Tris-HCl, pH 8.0, 150 mM NaCl) overnight at 37°C. The coated wells were subsequently blocked with 1% BSA (Faction V; GIBCO BRL, Gaithersburg, MD) in TBS before the addition of cells. Adhesion was carried out using 5 × 105 cells per well at 37°C for 15–30 min and bound cells were lysed in 0.2% SDS in TE (25 mM Tris-HCl, pH 8.0, 1 mM EDTA).
Results
Cloning of ICAP-1
A genetic screening based on the protein–protein interaction in yeast (Gyuris et al., 1993) was used to identify polypeptides that interact with the β1 integrin cytoplasmic domain. Of the 47 aa that comprise the entire β1 integrin cytoplasmic domain, the COOH-terminal 21 aa were cloned in frame into the DNA-binding domain of bacterial protein LexA. Mutational studies and analysis of naturally occurring splicing variants lacking this 21-aa region have shown that this region is involved in the localization of β1 integrins to the focal contacts and proper adhesive function of integrins (Balzac et al., 1993; Meredith et al., 1995).
Using the COOH-terminal 21 aa of integrin β1 as the bait, a ∼1.1-kb partial cDNA was isolated in a yeast two-hybrid screening of a human B cell cDNA library. This partial cDNA insert was used to obtain a full-length cDNA encoding a polypeptide of 200 aa. In a yeast two-hybrid assay, both the original partial cDNA (aa 154–200) and the full-length cDNA exhibited comparable interaction with the β1 integrin cytoplasmic domain but not with unrelated baits (Table I). In addition, the partial or full-length cDNA clones interacted with baits containing either the β1 integrin COOH-terminal 21 aa or a longer 47 aa that comprise the complete cytoplasmic domain. The interaction was specific towards only the β1 integrin as neither the partial nor the full-length cDNA clone interacted with the β2, β3, or β5 integrin cytoplasmic domain. The isolated clone hereon will be referred as ICAP-1α.
Table I.
ICAP1: Integrin Cytoplasmic Domain Interaction in Yeast Genetic Screen
| Baits | Sequence* | Interaction‡ | β-gal. Activity§ | |||
|---|---|---|---|---|---|---|
| β1cyto | KLLMIIHDRREFAKFEKEKMNAKWDTGENPIYKSAVTTVVNPKYEGK | + | ND | |||
| β2cyto | KALIHLSDLREYRRFEKEKLKSQWNN DNPLFKSATTTVMNPKFAES | − | <1.0 | |||
| β1(e16) | GENPIYKSAVTTVVNPKYEGK | + | 149.5 ± 18.3 | |||
| β2(e16) | DNPLFKSATTTVMNPKFAES | − | <1.0 | |||
| β3(e16) | ANNPLYKEATSTFTNITYRGT | − | <1.0 | |||
| β5(e16) | ASNPLYRKPISTHTVDFTFNKSYNGTVD | − | 2.41 ± 3.0 | |||
| αL | KVGFFKRNLKEKMEAGRGVPNGIPAEDSEQLASGQEAGDPGCLKPLHEKDSESGGGD | − | <1.0 | |||
| α2 | KVGFFKRKYEKMTKNPDEIDETTELSS | − | ND |
The conserved NPXY motifs of integrin β chains are indicated in bold letters. β1 cyto, complete cytoplasmic domain of integrin β1. β1(E16), COOH-terminal 21 aa of integrin β1. αL, complete cytoplasmic domain of integrin αL.
Positive interaction denotes growth of blue colonies on X-gal indicator plates lacking leucine.
β-galactosidase activity was derived from a minimum four independent measurements.
The deduced amino acid sequence of ICAP-1α was unrevealing except for the preponderance (especially in the NH2-terminal region) of Ser and Thr, several of which represented potential phosphorylation sites (Fig. 1 A). In particular, Ser20, Ser46, and Ser197 represent potential phosphorylation sites by protein kinase C (Woodgett et al., 1986), and Ser10 is present in sequence context favorable for phosphorylation by cyclic nucleotide dependent protein kinases (Glass et al., 1986). The initiation codon ATG in the sequence was preceded by an in frame termination codon and was present in correct sequence context for translational initiation (Kozak, 1992). Although clones corresponding to the ICAP-1α cDNA were represented in the National Center for Biotechnology Information (NCBI) EST database, analyses of the NCBI Non-Redundant database using the BLAST Enhanced Alignment Utility algorithm failed to identify any significant similarities to known proteins.
Figure 1.
ICAP-1α is a novel serine/threonine-rich 20-kD protein. (A) Sequence of ICAP-1. The translated amino acid sequence of ICAP-1α is shown. 50 amino acids absent in ICAP-1β are indicated in underlined italics. The nucleotide sequence data are available from GenBank/EMBL/DDBJ under accession number AF012023. (B) Northern blot analysis of ICAP-1α. Northern blots of transcripts from various human tissues (Clontech) were hybridized with ICAP-1α cDNA probe. The 0.9-kb mRNA is expressed in all eight tissue samples. The minor 1.3-kb mRNA, derived from the use of the downstream polyadenylation site, is also expressed in varying amount in all eight samples. (C) Western blot analysis of ICAP-1. ICAP-1α and ICAP-1β were detected in the total cell lysate (5 μg) from 293T cells using polyclonal anti-ICAP-1 antibody (lane 1). The assignments of ICAP-1α and ICAP-1β were confirmed by transiently expressing ICAP-1α (lane 2) and ICAP-1β cDNA (lane 3) in 293T cells. (D) The full-length 200-aa ICAP-1α and an alternatively spliced ICAP-1β lacking internal 50 aa are shown in a schematic diagram.
In Northern analyses, ICAP-1α transcripts of 0.9 and 1.3 kb in size were detected in mRNA isolated from several tissues (Fig. 1 B). The β1 integrins are ubiquitously expressed and ICAP-1, as a β1 integrin–binding protein, is expected to have a similar broad expression in various tissues and cell lines. The differences in the size of two transcripts probably reflect the usage of two different polyadenylation sites observed during cDNA sequence analysis.
In Western analyses using polyclonal anti-ICAP1 antibodies, two distinct polypeptides of 20 and 16 kd were detected (Fig. 1 C, lane 1). In hypotonic cell lysis, ICAP-1α fractionates in the cytosolic fraction suggesting ICAP-1α is a cytoplasmic protein (data not shown). Expression of ICAP-1α cDNA in 293T cells produced the exact 20-kD polypeptides (Fig. 1 C, lane 2). The shorter 16-kD species likely represent ICAP-1β, an alternatively spliced variant of ICAP-1α lacking internal 50 aa (Fig. 1 D). A cDNA corresponding to ICAP-1β mRNA was recognized during the database search. The presence of ICAP-1β cDNA in several human cDNA libraries was subsequently confirmed by PCR analyses (data not shown). The expression of ICAP-1β cDNA in 293T cells, as expected, produced the exact 16-kD polypeptide that comigrated with the endogenous ICAP-1β polypeptides (Fig. 1 C, lane 3). Interestingly, ICAP-1β did not interact with the integrin β1 cytoplasmic domain in a yeast two-hybrid assay (data not shown).
β1 Integrin Cytoplasmic Domain Restricted Binding of ICAP-1α
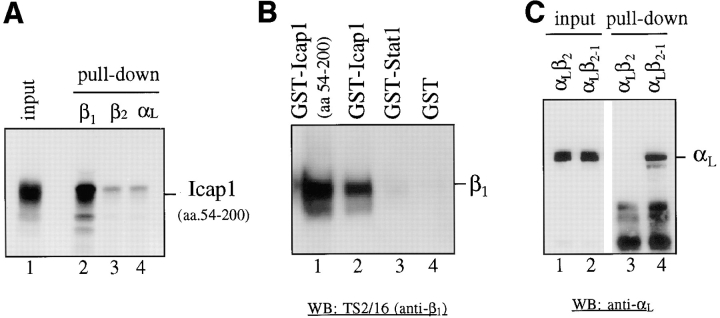
To verify the specific interaction between ICAP-1α and the β1 integrin cytoplasmic domain seen in the yeast genetic screening, in vitro–translated ICAP-1α was incubated with various integrin cytoplasmic domains expressed in bacteria as GST fusion proteins (Fig. 2 A). After incubation, GST fusion proteins were isolated on glutathione–Sepharose beads and the bound ICAP-1α polypeptides were analyzed by a SDS gel electrophoresis. As in a yeast two-hybrid assay, ICAP-1α was bound only to the GST-β1 fusion protein and not to the GST-β2 or GST-αL fusion proteins.
Figure 2.
In vitro and in vivo interaction between ICAP-1α and β1 integrins. (A) Interaction between GST-β1 and ICAP-1α in vitro. ICAP-1α (aa 54–200) was synthesized in vitro using reticulocyte lysate (lane 1) and incubated with 2 μg of bacterially expressed GST fusion proteins containing the cytoplasmic domains of integrin β1 (lane 2), β2 (lane 3), and αL (lane 4). (B) Interaction between GST-ICAP1α and β1 integrins in vivo. ICAP-1α (aa 54– 200) (lane 1) and a full-length ICAP1α (lane 2) were expressed as GST fusion protein in 293T cells using eukaryotic expression vector pEBG. For controls, GST-Stat1 (lane 3) and GST (lane 4) were used. Coprecipitation of the endogenous β1 integrins with the GST fusion proteins were determined on a Western blot using TS2/16 (anti–human β1 integrin antibody). (C) Restricted binding specificity of ICAP1α. GST-ICAP1α was expressed in 293T cells along with expression constructs for αLβ2 or αLβ2-1 (chimeric β2 subunit that has the COOH-terminal 20 aa replaced with the COOH-terminal 21 aa of β1 integrin). Coprecipitation of transfected integrins with the GST-ICAP1α was determined on a Western blot using anti-αL antibody. Lanes 1 and 2, total lysates of cells expressing GST-ICAP1α and αLβ2 (lane 1) and αLβ2-1 (lane 2). Lanes 3 and 4, the same samples as in lanes 1 and 2, respectively, after GST “pull-down.”
Reciprocal GST pull-down experiments were used to demonstrate the interaction between ICAP-1α and endogenous β1 integrins in vivo. GST epitope-tagged ICAP-1α was transiently expressed in 293T cells. Glutathione– Sepharose beads were used to purified GST-ICAP1α and copurification of endogenous β1 integrins was tested on a Western analysis using anti-β1 antibody. Confirming the results of the yeast two-hybrid assays and in vitro binding studies, β1 integrins copurified with GST-ICAP1α, but not with unrelated GST fusion proteins or GST alone (Fig. 2 B).
Finally, to demonstrate that ICAP-1α is a specific β1 integrin binding protein in vivo, GST epitope-tagged ICAP-1α was coexpressed in 293T cells with the integrin β2 subunit or a chimeric β2 subunit (β2.1) constructed by replacing the COOH-terminal 20 aa region of the β2 subunit with the corresponding sequences of the β1 subunit. The expression of β2 integrins is limited to cells of hematopoietic origin and 293T cells completely lack expression of β2 integrins (Liu, J., and D. Chang, unpublished observation). To ensure a proper cell surface expression, the αL subunit capable of forming stable heterodimer with the β2 subunit was also expressed. A comparable surface expression of αLβ2 and αLβ2.1 was seen on FACS® analysis (data not shown). After precipitation of GST-ICAP1α on glutathione–Sepharose beads, copurification of β2 integrin was indirectly tested in Western analysis using anti-αL antibody. In GST pull-down experiments, as predicted from the yeast two-hybrid assays and in vitro binding assays, only αLβ2.1 and not αLβ2 copurified with ICAP-1α (Fig. 2 C). These findings demonstrate the ability of ICAP-1α to interact with integrin αβ heterodimer in a β1 cytoplasmic domain-dependent fashion.
Conserved NPXY Motif of Integrin β1 Cytoplasmic Domain is Required for the ICAP-1α Binding
ICAP-1α interacts with the β1 integrins through the COOH-terminal 21 aa region. Naturally occurring, alternatively spliced variants of β1 integrins, which lack this region or mutant β1 integrins containing amino acid substitutions at either of the two conserved NPXY motifs do not localize to the focal contacts (Reszka et al., 1992; Balzac et al., 1993). Furthermore, mutations in the analogous region of β2 or β3 integrins affect the leukocyte integrin αLβ2 (LFA-1)-dependent cell adhesion to intercellular cell adhesion molecules-1 or the affinity regulation of the platelet integrin αIIbβ3 (Hibbs et al., 1991; O'Toole et al., 1995; Peter and O'Toole, 1995).
The exact ICAP-1α binding site within the β1 integrin cytoplasmic domain was further delineated by testing the interaction between ICAP-1α and truncated β1 integrin cytoplasmic domain (Table II). NH2-terminal deletions up to 8 aa did not affect the interaction with ICAP-1α. A 3-aa deletion in the COOH terminus, however, completely abolished the interaction. Therefore, a minimum binding site for ICAP-1α on the β1 integrin cytoplasmic must consist of this 13-aa region that included one of the two NPXY motifs. The NPXY motif is required for the interaction as amino acid substitutions at the conserved Asn to Glu or Ala abolished the binding. Similarly, a substitution of Tyr to Ala disrupted the ICAP-1α binding whereas a more conservative replacement of Tyr to Phe had no effect.
Table II.
ICAP1: β1 Subunit Deletion Mutant Interaction
| Baits | Sequence* | Interaction‡ | ||
|---|---|---|---|---|
| β1(e16) | GENPIYKSAVTTVVNPKYEGK | + | ||
| β1 nΔ-3 | IYKSAVTTVVNPKYEGK | + | ||
| β1 nΔ-8 | AVTTVVNPKYEGK | + | ||
| β1 cΔ-3 | GENPIYKSAVTTVVNPKY | − | ||
| β1 egk→ aes | GENPIYKSAVTTVVNPKY AES | + |
The conserved NPXY motifs of integrin β chain are indicated in bold letters.
Positive interaction denotes growth of blue colonies on X-gal indicator plates lacking leucine.
In yeast interaction and in vitro binding assays, ICAP-1α failed to interact with the β2 integrin cytoplasmic domain, which is closely related to the β1 integrin cytoplasmic domain in sequence (Table III). The molecular basis of this highly discriminatory binding specificity of ICAP-1α was investigated by progressively mutating the β2 integrin cytoplasmic domain. There are two conserved and four nonconserved amino acid differences between the β1 and β2 integrins within the minimum 13 aa ICAP-1α binding region. A replacement of three consecutive nonconserved residues found in the COOH terminus of β2 integrin from Ala-Glu-Ser to Glu-Gly-Lys found in the β1 subunit did not allow the ICAP-1α binding. Interestingly, a single amino acid replacement at the −11 position from Thr to Val allowed the mutant β2 integrin to interact with ICAP-1α, demonstrating that in addition to the NPXY motif, Val at the −11 position is essential for the interaction between β1 integrins and ICAP-1α.
Table III.
ICAP1: β1 and β2 Subunit Point Mutant Interaction
| Baits | Sequence* | Interaction‡ | ||
|---|---|---|---|---|
| β1 v787a | .A........... | − | ||
| β1 n792a | ......A...... | − | ||
| β1 n792d | ......D...... | − | ||
| β1 y792a | .........A... | − | ||
| β1 y792f | .........F... | + | ||
| β1 nΔ-8 | AVTTVVNPKYEGK | + | ||
| | |||||||• | ||||
| β2 nΔ-8 | ATTTVVNPKFAES | − | ||
| β2 aes/egk | ..........EGK | − | ||
| β2 f766y | .........Y... | − | ||
| β2 t758v | .V........... | + |
The amino acid replaced in each β1 integrin mutant is indicated. Dots denote the sequences that are identical to the β1 nΔ-8 or β2 nΔ-8 sequences.
Positive interaction denotes growth of blue colonies on X-gal indicator plates lacking leucine.
ICAP-1α Is a Phosphoprotein
In addition to the 20- and 16-kD polypeptides corresponding to ICAP-1α and ICAP-1β, heterogeneous bands migrating slower than the 20-kD band were detected in Western analysis of 293T cell lysates using polyclonal anti-ICAP1 antisera (data not shown). Because the amino acid composition of ICAP-1 is rich in Ser and Thr, the possibility that this mobility difference is due to protein phosphorylation was raised. When total detergent lysates of several different cell lines were surveyed by Western analysis, there was a significant variation in the relative amount of slow migrating species (Fig. 3 A). In particular, two osteosarcoma cell lines, SaOS (Fig. 3 A, lane 2) and UTA-6 (Fig. 3 A, lane 3), displayed high abundance of the slow migrating species. To directly confirm that the slow migrating species represented phosphorylated forms of ICAP-1α, UTA-6 cell lysates incubated at 30°C to activate endogenous phosphatases (Fig. 3 B). The slow migrating species disappeared with a concomitant increase in the 20 kD ICAP-1α upon 30°C incubation (Fig. 3 B, lane 3). The addition of phosphatase inhibitors, sodium vanadate, and calyculin A, completely prevented the conversion of the slow migrating species to the 20 kD ICAP-1α (Fig. 3 B, lanes 2 and 4), confirming the mobility difference is due to protein phosphorylation. Interestingly, during in vitro phosphatase experiment, we failed to detect any mobility shift in ICAP-1β, suggesting that either ICAP-1β is not phosphorylated or the mobility of ICAP-1β does not change significantly by protein phosphorylation.
Figure 3.
ICAP-1α is a phosphoprotein. (A) Western blot analysis showing ICAP-1 expression. Total cell lysates (15 μg) from various cell lines were analyzed on a Western blot using anti-ICAP1 antibody. The positions of ICAP-1α and ICAP-1β as well as the slow migrating species corresponding to the phosphorylated ICAP-1α (p-ICAP1α) are indicated. (B) In vitro phosphatase treatment experiment. 15 μg of UTA-6 cell lysates were incubated at 30°C for 30 min. Lane 1, input; lane 2, incubation in the presence of sodium vanadate and calyculin A; lane 3, incubation at 30°C.
Regulation of ICAP-1α Phosphorylation by Integrin-dependent Cell–Matrix Interaction
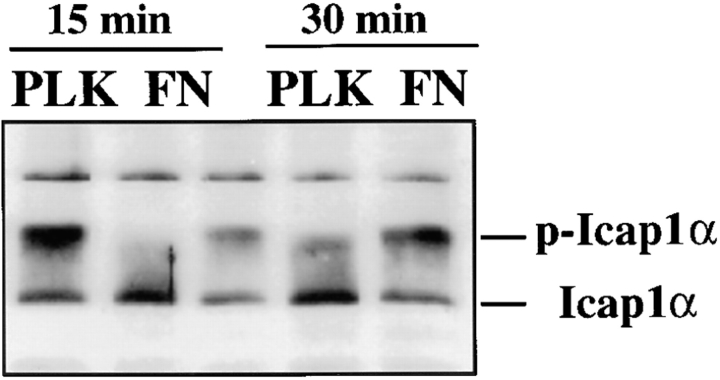
Many focal contact proteins, FAK, paxillin, and tensin being a few representative ones, have been shown to undergo protein phosphorylation during cell attachment (Clark and Brugge, 1995). The possibility that the phosphorylation of ICAP-1α may be regulated in a similar manner during cell attachment was directly addressed by binding UTA-6 cells to either PLK or fibronectin (FN)- coated surface (Fig. 4). Western analyses of cell lysates revealed an enhanced phosphorylation of ICAP-1α when cells adhere to the FN-coated surface. The maximum enhancement occurred within the minimum 30 min required for sufficient number of cells to adhere and undergo cell spreading (Fig. 4, lanes 2 and 4). A longer incubation did not further enhance ICAP-1α phosphorylation (data not shown). This enhancement was specific for cell attachment to FN-coated surface, which requires β1 integrins. On PLK-coated surface, cells adhere very efficiently, but fail to initiate subsequent cell spreading. Under these conditions, the phosphorylation of ICAP-1α remained at a reduced level (Fig. 4, lanes 1 and 3).
Figure 4.
Regulation of ICAP-1α phosphorylation during cell adhesion. UTA-6 cells were trypsinized and replated on plates coated with poly-l-lysine (PLK) (lanes 1 and 3) or fibronectin (FN) (lanes 2 and 4). Adherent cells were lysed in 0.5% NP-40 at t = 15 min (lanes 1 and 2), and t = 30 min (lanes 3 and 4) and the extent of ICAP-1α phosphorylation was determined on a Western blot using anti-ICAP-1 antibody.
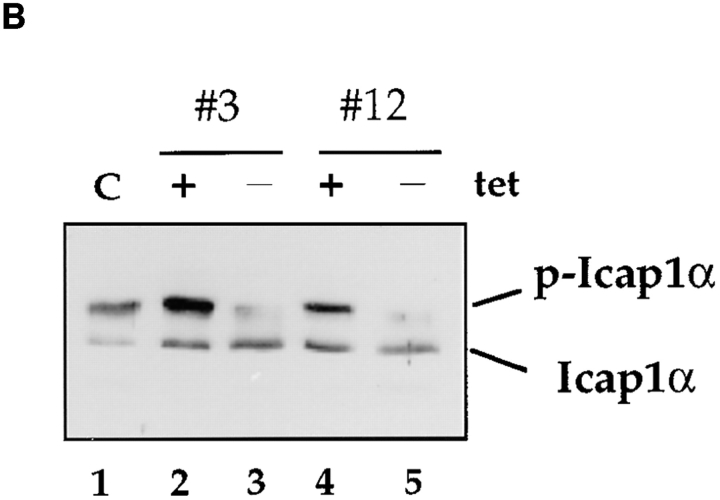
During integrin-dependent cell adhesion, the rearrangement of cytoskeletal actin stress fibers and assembly of focal adhesion plaques are regulated by Rho-family GTPases (Hall, 1994; Burridge and Chrzanowska-Wodnicka, 1996). In UTA-6 derivative cell lines expressing constitutively activated RhoA(Q63L) under the control of tetracycline- inducible system, the induction of RhoA(Q63L) expression results in the formation of dense stress fibers and increase in the number of focal adhesion plaques (Wong, C., and D. Chang, manuscript in preparation). These cells display a significantly delayed and incomplete cell spreading when plated on FN-coated surface (Fig. 5 A). To test whether the alteration in cell–matrix interaction induced by the expression of RhoA(Q63L) had any effect on the phosphorylation status of ICAP-1α, a Western analysis was carried out using the lysates from RhoA(Q63L) expressing cells. The lysates prepared from cells grown in the presence of tetracycline, which represses the expression of RhoA(Q63L), were used as controls. Fig. 5 B shows that the expression of constitutively activated RhoA(Q63L) reduces the extent of ICAP-1α phosphorylation in two independently derived cell lines. Thus, taken together, these findings suggest that phosphorylation of ICAP-1α is regulated during integrin-dependent cell adhesion and spreading.
Figure 5.
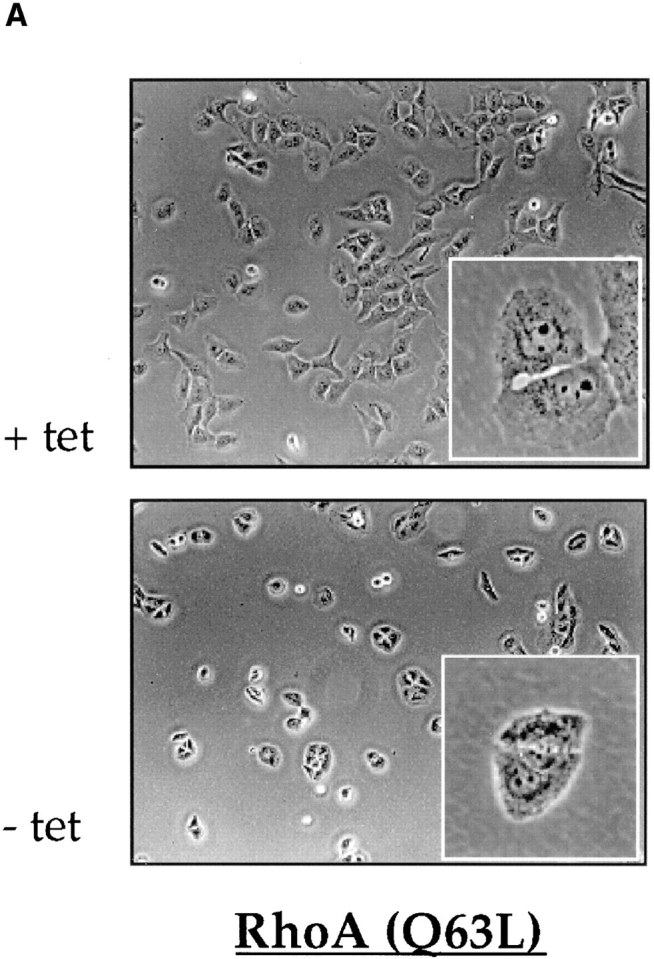
Control of ICAP-1α phosphorylation by RhoA protein. (A) Expression of activated RhoA interferes with cell spreading. The morphology of UTA-6 cells expressing RhoA(Q63L) under the control of tetracycline-inducible promoter is shown. In the absence of tetracycline, which induces the expression of RhoA (Q63L), cells remain rounded and fail to spread. (B) Expression of activated RhoA results in dephosphorylation of ICAP-1α. The extent of ICAP-1α phosphorylation in two independently derived UTA-6 cell lines expressing RhoA (Q63L) was assessed in Western blot analysis using anti-ICAP1 antibody. In parental cells (lane 1) or in the presence of tetracycline (lanes 2 and 4), a significant portion of ICAP-1α is phosphorylated. Induction of RhoA(Q63L) is associated with the loss of ICAP-1α phosphorylation is both cell lines (lanes 3 and 5).
Discussion
Integrins, through binding to both extracellular matrix proteins and cytoskeletal structures, provide a direct linkage between the extracellular environment and the cell interior. The cytoplasmic domains of integrins have been implicated in the integrin affinity regulation and localization of integrins to the focal contacts. Understanding how the short cytoplasmic tails of integrins affect the functions of integrins requires characterization of cellular proteins that bind, either directly or indirectly, to the integrin cytoplasmic domains. To this end, we have used a yeast two-hybrid screen to identify proteins that directly bind to the β1 integrin cytoplasmic domain. In particular, we have focused on the COOH-terminal 21-aa region of the β1 integrin, which is required for the localization of integrins to the focal contacts. In this study, we report identification and characterization of ICAP-1α, a novel 200-aa protein that specifically interacts with the β1 integrin cytoplasmic domain through a conserved and functionally important NPXY sequence motif. In addition, ICAP-1α undergoes protein phosphorylation that is subject to regulation, suggesting that ICAP-1α plays an important role during integrin-dependent cell adhesion.
The broad tissue distribution of ICAP-1α mRNA and the detection of ICAP-1α protein in cell lines of various tissue origins indicate ICAP-1α, like β1 integrins, is ubiquitously expressed. In contrast, the expression of ICAP-1β, an alternatively spliced variant of ICAP-1α that does not bind to the β1 integrin cytoplasmic domain, was more variable in cell lines we have tested. In particular, the ICAP-1β expression was low or absent in three cell lines, SaOS (human osteosarcoma line), TPH-1 (human monocytic line), and Cos-7 (monkey kidney line). The differences in the ability to interact with the β1 cytoplasmic domain and in the expression level provide a mechanism for regulation of ICAP-1α function by ICAP-1β. It is noteworthy that β3-endonexin likewise has an alternatively spliced variant that does not interact with the β3 integrin cytoplasmic domain (Shattil et al., 1995).
The amino acid sequence of ICAP-1α is unique and displays no similarities to any known proteins. Several proteins, including known focal contact proteins, α-actinin, paxillin, talin, and FAK, as well as recently identified potential regulatory proteins such as β3-endonexin, ILK-1, and cytohesin-1 have been shown to bind integrins through the β subunit cytoplasmic domain (for review see Sastry and Horwitz, 1993; Shattil et al., 1995; Hannigan et al., 1996; Kolanus et al., 1996). In contrary to α-actinin, FAK, and ILK-1, which interact with more than one type of β subunit, the interaction of ICAP-1α is restricted to the β1 subunit only. This property of ICAP-1α is similar to two recently identified proteins, β3-endonexin and cytohesin-1, which bind specifically to integrins β3 and β2, respectively (Shattil et al., 1995; Kolanus et al., 1996). As these proteins are not related in sequence to each other or to ICAP-1α, it remains to be seen how these unrelated proteins are coupled to the β integrin cytoplasmic domains, which are related and functionally interchangeable in some cases.
The results from our mutagenesis studies indicate that the COOH-terminal 13-aa region of the β1 subunit, which includes a conserved NPXY motif, is sufficient to bind ICAP-1α. Several studies have shown that this region, especially the NPXY motif, is important for the recruitment of β1 integrins to the focal contacts (Marcantonio et al., 1990; Reszka et al., 1992; Peter and O'Toole, 1995; Ylanne et al., 1995) and the colocalization of talin, FAK, and actin with β1 integrins (Lewis and Schwartz, 1995). Our finding that Asn to Ala (or Glu), or Tyr to Ala substitution within the NPXY motif completely abolished the interaction between ICAP-1α and integrins, while a more conservative replacement of Tyr with Phe had no effect, demonstrates a remarkable similarity between the sequence requirement for the binding of ICAP-1α to integrins and localization of β1 integrins to the focal contacts and suggests that ICAP-1α may play a role in the recruitment of β1 integrins to the focal contacts. Alternatively, the COOH-terminal 13-aa region may be involved in initiating signal transduction events required to trigger cell spreading and ICAP-1α may participate in the initiation of this signaling event. As β2, β3, and β5 integrin cytoplasmic domains (which do not bind ICAP-1α) can also direct integrins to the focal contacts (LaFlamme et al., 1994; Peter and O'Toole, 1995), there must be parallel mechanisms for recruiting various integrins to the focal contacts.
The COOH-terminal regions of different β integrins are somewhat diverse in amino acid sequence, which may explain in part the observed specificity of ICAP-1α interaction with the β1 integrin cytoplasmic domain. The basis of this restricted specificity, however, was attributed to a single Val residue NH2-terminal to the conserved NPXY motif. In β2 or β3 subunits, the corresponding position is occupied by Thr (Sastry and Horwitz, 1993). Confirming the importance of Val at this position, the replacement of the Thr with a Val in the β2 subunit cytoplasmic tail allowed it to bind ICAP-1α. The β3 integrin cytoplasmic domain, in addition, has a less conserved NITY in place of the NPXY motif, which may also account for the lack of interaction with ICAP-1α. A recent study on the amino acid sequence requirement for the interaction between β3-endonexin and β3 integrin cytoplasmic domain indicated that the NITY motif is critical for the binding specificity (Eigenthaler et al., 1997). Altogether these findings suggest that the COOH-terminal regions of different β integrins may constitute specific binding sites for different cytoplasmic proteins. In addition, the existence of integrin β subunit specific binding proteins indicates that the functions of individual subunits can be differentially regulated.
The presence of ICAP-1α immunoreacting species, which migrated slower than the expected molecular weight of 20 kD predicted from the ICAP-1α reading frame suggested that ICAP-1α undergoes posttranslational modification. The conversion of slower migrating species to the expected 20-kD species during 30°C incubation, which activates the endogenous phosphatases present in nonionic detergent lysates, and the observation that this conversion can be effectively blocked by the addition of known phosphatase inhibitors demonstrate that ICAP-1α is a phosphoprotein.
The most intriguing property of ICAP-1α is that its phosphorylation is regulated during cell adhesion and by RhoA protein. We suspect that the effect on ICAP-1α phosphorylation seen during cell adhesion on FN-coated surface and in cells expressing constitutively activated RhoA(Q63L) are related events, reflective of the cytoskeletal rearrangement that occurs during cell adhesion. It is well known that cells, soon after making an initial contact on FN-coated surface, undergo cell spreading that involves the formation of focal adhesion plaques and actin stress fibers (for review see Hall, 1994). Both these events are known to be regulated by RhoA (Ridley and Hall, 1992; Nobes and Hall, 1995). Furthermore, both matrix assembly and cell spreading are integrin-dependent processes and require integrin cytoplasmic domain (for review see Burridge and Chrzanowska-Wodnicka, 1996; LaFlamme et al., 1994). On a nonspecific PLK-coated surface, cells make initial contact but fail to promote matrix assembly and initiate spreading. Cells expressing RhoA(Q61L) also display a delayed and incomplete spreading, presumably due to the interference from dense stress fibers. According to this scenario, the diminished ICAP-1α phosphorylation observed during cell adhesion on PLK-coated surface and in RhoA(Q63L) expressing cells is a result of inefficient cell spreading. Our findings, however, do not rule out the possibility that phosphorylation of ICAP-1α may be a direct consequence of the interaction between integrins and fibronectin. Regardless of the exact mechanism underlying the control of ICAP-1α phosphorylation, our data clearly demonstrate that the phosphorylation of ICAP-1α is regulated during the cell–matrix interaction.
It remains to be seen whether the enhancement in ICAP-1α phosphorylation during cell attachment involves Ser/Thr phosphorylation or Tyr phosphorylation. Although Ser/Thr phosphorylation is suspected based on the amino acid composition of ICAP-1α (21% Ser/Thr) and the presence of potential protein kinase C and protein kinase A phosphorylation sites, the exact amino acid residues that are phosphorylated are not known. We have not been able to demonstrate the immunoreactivity of ICAP-1α with PY20 anti-phosphotyrosine antibody (data not shown).
In summary, we present initial characterization of ICAP-1α, a novel β1 integrin cytoplasmic domain binding protein. Two observations, (a) the binding of ICAP-1α to a conserved region of β1 integrin cytoplasmic domain that previously has been shown to be important for the adhesive function and focal contact localization of integrins, and (b) the extent of ICAP-1α phosphorylation is regulated during cell–matrix interaction, suggest that ICAP-1α plays a role during integrin-dependent cell adhesion. β Integrin cytoplasmic domains likely contain overlapping binding sites for both structural and regulatory proteins that coordinate cell adhesion and subsequent cytoskeletal rearrangement. The identification of ICAP-1α together with the characterization of the sequences on β1 integrin cytoplasmic domain required for the ICAP-1α binding should facilitate future studies on how specific integrin- dependent cellular events are regulated.
Acknowledgments
We are grateful to R. Brent (Massachusetts General Hospital, Boston, MA) for providing the yeast strains and plasmids for the yeast genetic screening, to J. Gutkind (National Institutes of Health, Bethesda, MD), for providing mAbs, cell lines, and DNA constructs. We thank T. Kim for technical assistance and K. Shuai for helpful comments on the manuscript.
This work was supported by the grants from Searle Scholars Program/ The Chicago Community Trust, James S. McDonnell Foundation, and Jonnson Comprehensive Cancer Center of UCLA.
Abbreviations used in this paper
- aa
amino acid
- FAK
focal adhesion molecule
- FN
fibronectin
- GST
glutathione-S-transferase
- ICAP-1
integrin cytoplasmic domain–associated protein-1
- PLK
poly-l-lysine
Footnotes
Please address all correspondence to David D. Chang, UCLA School of Medicine, Division of Heme-Onc, Factor 11-934, 10833 Le Conte Avenue, Los Angeles, CA 90095. Tel.: (310) 825-9759. Fax: (310) 825-6192. e-mail: dchang@medicine.medsch.ucla.edu
References
- Altieri DC, Edgington TS. The saturable high affinity association of factor X to ADP-stimulated monocytes defines a novel function of the Mac-1 receptor. J Biol Chem. 1988;263:7007–7015. [PubMed] [Google Scholar]
- Ausubel, F.M., R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl. 1994. Current Protocols in Molecular Biology. John Wiley & Sons, Inc., New York.
- Balzac F, Belkin AM, Koteliansky VE, Balabanov YV, Altruda F, Silengo L, Tarone G. Expression and functional analysis of a cytoplasmic domain variant of the β1integrin subunit. J Cell Biol. 1993;121:171–178. doi: 10.1083/jcb.121.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal A, Geirasch LM. The NPXY internalization signal of the LDL receptor adopts a reverse-turn conformation. Cell. 1991;67:1195–1201. doi: 10.1016/0092-8674(91)90295-a. [DOI] [PubMed] [Google Scholar]
- Bennett JS, Vilaire G. Exposure of platelet fibrinogen receptors by ADP and epinephrine. J Clin Invest. 1979;64:1393–1401. doi: 10.1172/JCI109597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–519. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- Chen W-J, Goldstein JL, Brown MS. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990;265:3116–3123. [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science (Wash DC) 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Coso OA, Chiariello M, Yu JC, Teramoto H, Crespo P, Xu N, Miki T, Gutkind JS. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- Eigenthaler M, Hofferer L, Shattil S J, Ginsberg M H. A conserved sequence motif in the integrin β3 cytoplasmic domain is required for its specific interaction with β3-endonexin. J Biol Chem. 1997;272:7693–7698. doi: 10.1074/jbc.272.12.7693. [DOI] [PubMed] [Google Scholar]
- Englert C, Hou X, Maheswaran S, Bennett P, Ngwu C, Re GG, Garvin AJ, Rosner MR, Haber DA. WT1 suppresses synthesis of the epidermal growth factor receptor and induces apoptosis. EMBO (Eur Mol Biol Organ) J. 1995;14:4662–4675. doi: 10.1002/j.1460-2075.1995.tb00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faull RJ, Kovach NL, Harlan JM, Ginsberg MH. Affinity modulation of integrin α5β1: regulation of the functional response by soluble fibronectin. J Cell Biol. 1993;121:155–162. doi: 10.1083/jcb.121.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman AS, Rhynhart K, Nojima Y, Svahn J, Eliseo L, Benjamin CD, Morimoto C, Vivier E. Stimulation of protein tyrosine phosphorylation in human B cells after ligation of the β1integrin VLA-4. J Immunol. 1993;150:1645–1652. [PubMed] [Google Scholar]
- Glass DB, el-Maghrabi MR, Pilkis SJ. Synthetic peptides corresponding to the site phosphorylated in 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase as substrates of cyclic nucleotide-dependent protein kinases. J Biol Chem. 1986;261:2987–2993. [PubMed] [Google Scholar]
- Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyuris J, Golemis E, Chertkov H, Brent R. Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell. 1993;75:791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC, Dedhar S. Regulation of cell adhesion and anchorage-dependent growth by a new β1-integrin-linked protein kinase. Nature (Lond) 1996;379:91–96. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- Haverstick DM, Sakai H, Gray LS. Lymphocyte adhesion can be regulated by cytoskeleton-associated, PMA-induced capping of surface receptors. Am J Physiol. 1992;262:916–926. doi: 10.1152/ajpcell.1992.262.4.C916. [DOI] [PubMed] [Google Scholar]
- Hermanowski-Vosatka A, Detmers PA, Gotze O, Silverstein SC, Wright SD. Clustering of ligand on the surface of a particle enhances adhesion to receptor-bearing cells. J Biol Chem. 1988;263:17822–17827. [PubMed] [Google Scholar]
- Hibbs ML, Jakes S, Stacker SA, Wallace RW, Springer TA. The cytoplasmic domain of the integrin lymphocyte function-associated antigen 1β subunit: sites required for binding to intercellular molecule 1 and the phorbol ester-stimulated phosphorylation site. J Exp Med. 1991;174:1227–1238. doi: 10.1084/jem.174.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz A, Duggan K, Buck C, Beckerle M C, Burridge K. Interaction of plasma membrane fibronectin receptor with talin—a transmembrane linkage. Nature (Lond) 1986;320:531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Kolanus W, Nagel W, Schiller B, Zeitlmann L, Godar S, Stockinger H, Seed B. Alpha L beta 2 integrin/LFA-1 binding to ICAM-1 induced by cytohesin-1, a cytoplasmic regulatory molecule. Cell. 1996;86:233–242. doi: 10.1016/s0092-8674(00)80095-1. [DOI] [PubMed] [Google Scholar]
- Kozak M. Regulation of translation in eukaryotic systems. Annu Rev Cell Biol. 1992;8:197–225. doi: 10.1146/annurev.cb.08.110192.001213. [DOI] [PubMed] [Google Scholar]
- LaFlamme SE, Thomas LA, Yamada SS, Yamada KM. Single subunit chimeric integrins as mimics and inhibitors of endogenous integrin functions in receptor localization, cell spreading and migration, and matrix assembly. J Cell Biol. 1994;126:1287–1298. doi: 10.1083/jcb.126.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Languino LR, Ruoslahti E. An alternative form of the integrin beta 1 subunit with a variant cytoplasmic domain. J Biol Chem. 1992;267:7116–7120. [PubMed] [Google Scholar]
- Lewis JM, Schwartz MA. Mapping in vivo associations of cytoplasmic proteins with integrin beta 1 cytoplasmic domain mutants. Mol Biol Cell. 1995;6:151–160. doi: 10.1091/mbc.6.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcantonio EE, Guan J-L, Trevithick JE, Hynes RO. Mapping of the functional determinants of the integrin β1 cytoplasmic domain by site-directed mutagenesis. Cell Regul. 1990;1:597–604. doi: 10.1091/mbc.1.8.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith J, Jr, Takada Y, Fornaro M, Languino LR, Schwartz MA. Inhibition of cell cycle progression by the alternatively spliced integrin β1C. Science (Wash DC) 1995;269:1570–1572. doi: 10.1126/science.7545312. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Otey CA, Pavalko FM, Burridge K. An interaction between α-actinin and the β1integrin subunit in vitro. J Cell Biol. 1990;111:721–729. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole T E, Ylanne J, Culley B M. Regulation of integrin affinity states through an NPXY motif in the beta subunit cytoplasmic domain. J Biol Chem. 1995;270:8553–8558. doi: 10.1074/jbc.270.15.8553. [DOI] [PubMed] [Google Scholar]
- Pasqualini R, Hemler M E. Contrasting roles for integrin β1 and β5cytoplasmic domains in subcellular localization, cell proliferation, and cell migration. J Cell Biol. 1994;125:447–460. doi: 10.1083/jcb.125.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter K, O'Toole TE. Modulation of cell adhesion by changes in αL β2(LFA-1, CD11a/CD18) cytoplasmic domain/cytoskeleton interaction. J Exp Med. 1995;181:315–326. doi: 10.1084/jem.181.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reszka A A, Hayashi Y, Horwitz A F. Identification of amino acid sequences in the integrin β1cytoplasmic domain implicated in cytoskeletal association. J Cell Biol. 1992;117:1321–1330. doi: 10.1083/jcb.117.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Sastry SK, Horwitz AF. Integrin cytoplasmic domains: mediators of cytoskeletal linkages and extra- and intracellular initiated transmembrane signaling. Curr Opin Cell Biol. 1993;5:819–831. doi: 10.1016/0955-0674(93)90031-k. [DOI] [PubMed] [Google Scholar]
- Schaller MD, Otey CA, Hildebrand JD, Parsons JT. Focal adhesion kinase and paxillin bind to peptides mimicking β integrin cytoplasmic domains. J Cell Biol. 1995;130:1181–1187. doi: 10.1083/jcb.130.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Shattil SJ, O'Toole T, Eigenthaler M, Thon V, Williams M, Babior BM, Ginsberg MH. β3-endonexin, a novel polypeptide that interacts specifically with the cytoplasmic tail of the integrin β3subunit. J Cell Biol. 1995;131:807–816. doi: 10.1083/jcb.131.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart MP, Cabanas C, Hogg N. T cell adhesion to intercellular adhesion molecule-1 (ICAM-1) is controlled by cell spreading and the activation of integrin LFA-1. J Immunol. 1996;156:1810–1817. [PubMed] [Google Scholar]
- Studier FW, Moffatt BA. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- van Kooyk Y, Weder P, Heije K, Figdor CG. Extracellular Ca2+modulates leukocyte function-associated antigen-1 cell surface distribution on T lymphocytes and consequently affects cell adhesion. J Cell Biol. 1994;124:1061–1070. doi: 10.1083/jcb.124.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nhieu GT, Krukonis ES, Reszka AA, Horwitz AF, Isberg RR. Mutations in the cytoplasmic domain of the integrin β1chain indicate a role for endocytosis factors in bacterial internalization. J Biol Chem. 1996;271:7665–7672. doi: 10.1074/jbc.271.13.7665. [DOI] [PubMed] [Google Scholar]
- Wayner EA, Orlando RA, Cheresh DA. Integrin, αvβ3 and αvβ5contribute to cell attachment to vitronectin but differentially distribute on the cell surface. J Cell Biol. 1991;113:919–929. doi: 10.1083/jcb.113.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgett JR, Gould KL, Hunter T. Substrate specificity of protein kinase C. Use of synthetic peptides corresponding to physiological sites as probes for substrate recognition requirements. Eur J Biochem. 1986;161:177–184. doi: 10.1111/j.1432-1033.1986.tb10139.x. [DOI] [PubMed] [Google Scholar]
- Ylanne J, Huuskonen J, O'Toole TE, Ginsberg MH, Virtanen I, Gahmberg CG. Mutation of the cytoplasmic domain of the integrin beta 3 subunit. Differential effects on cell spreading, recruitment to adhesion plaques, endocytosis, and phagocytosis. J Biol Chem. 1995;270:9550–9557. doi: 10.1074/jbc.270.16.9550. [DOI] [PubMed] [Google Scholar]
- Yurochko AD, Liu DY, Eierman D, Haskill S. Integrins as a primary signal transduction molecule regulating monocyte immediate-early gene induction. Proc Natl Acad Sci USA. 1992;89:9034–9038. doi: 10.1073/pnas.89.19.9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhidkova NI, Belkin AM, Mayne R. Novel isoform of β1integrin expressed in skeletal and cardiac muscle. Biochem Biophys Res Commun. 1995;214:279–285. doi: 10.1006/bbrc.1995.2285. [DOI] [PubMed] [Google Scholar]