Abstract
Two mechanisms have been proposed for regulating rolling velocities on selectins. These are (a) the intrinsic kinetics of bond dissociation, and (b) the reactive compliance, i.e., the susceptibility of the bond dissociation reaction to applied force. To determine which of these mechanisms explains the 7.5–11.5-fold faster rolling of leukocytes on L-selectin than on E- and P-selectins, we have compared the three selectins by examining the dissociation of transient tethers. We find that the intrinsic kinetics for tether bond dissociation are 7–10-fold more rapid for L-selectin than for E- and P-selectins, and are proportional to the rolling velocities through these selectins. The durations of pauses during rolling correspond to the duration of transient tethers on low density substrates. Moreover, applied force increases dissociation kinetics less for L-selectin than for E- and P-selectins, demonstrating that reactive compliance is not responsible for the faster rolling through L-selectin. Further measurements provide a biochemical and biophysical framework for understanding the molecular basis of rolling. Displacements of tethered cells during flow reversal, and measurements of the distance between successive pauses during rolling provide estimates of the length of a tether and the length of the adhesive contact zone, and suggest that rolling occurs with as few as two tethers per contact zone. Tether bond lifetime is an exponential function of the force on the bond, and the upper limit for the tether bond spring constant is of the same order of magnitude as the estimated elastic spring constant of the lectin–EGF unit. Shear uniquely enhances the rate of L-selectin transient tether formation, and conversion of tethers to rolling adhesions, providing further understanding of the shear threshold requirement for rolling through L-selectin.
Binding of selectins to cell-surface carbohydrate ligands enables leukocytes that are free in vascular shear flow to tether to vessel walls and to roll in response to hydrodynamic drag. Rolling is a remarkable class of adhesive interaction because the zone of adhesive contact is rapidly translated along the vessel wall. Only certain adhesion molecules are specialized to support rolling (46). L-selectin, expressed on leukocytes, binds to carbohydrate ligands that are expressed on high endothelial venules (HEVs) of secondary lymphoid tissues (5) as well as on certain types of leukocytes (2, 15). The ligand for L-selectin is a sulfated sLex-related carbohydrate (21, 22). This carbohydrate ligand on HEV is expressed on a mixture of mucinlike sialoglycoproteins termed peripheral node addressin (PNAd)1. PNAd and various components of PNAd including CD34 have been shown to support rolling of L-selectin–expressing leukocytes in hydrodynamic flow (6, 28, 39). The vascular selectins, P-selectin and E-selectin, can be induced on endothelium by inflammatory mediators, and both bind to carbohydrate ligands on myeloid cells and subsets of lymphocytes (31, 42). The major P-selectin counterreceptor on human leukocytes, P-selectin glycoprotein ligand–1 (PSGL-1), is a mucin decorated with sLex (33, 43) and with sulfated tyrosines (37, 44, 55). Both L-selectin (36) and PSGL-1 (33) on neutrophils are concentrated on the tips of microvilli, where they are likely to support the initial contact with the vessel wall under physiological flow.
Although bonds between selectins and their counterreceptors must be rapidly formed and broken to support translation of the adhesive contact zone during rolling, there are two markedly different mechanisms by which rolling velocity can be regulated. These are (a) intrinsic bond kinetics, i.e., kinetics in the absence of applied force; and (b) the susceptibility of bond kinetics to applied force, which is termed reactive compliance. Tensile force is generally expected to increase the rate of bond dissociation, but the amount of increase, i.e., reactive compliance, may differ markedly for different types of adhesion molecules. According to one view, rapid bond dissociation kinetics are required for rolling (1, 25), and kinetic differences may underlie the 7.5–11.5-fold faster rolling through L-selectin than through E-selectin or P-selectin when ligand densities are adjusted to give rolling adhesions of similar strength (40). According to another view, the susceptibility of bond kinetics to force may be the dominant factor in regulating rolling velocity and the intrinsic bond dissociation rate constant, i.e., k off in the absence of force, or k off o, may be so slow that bond dissociation requires 0.5–5 h (9, 19, 49). However, reactive compliance is predicted to be variable only within a certain range; if bonds were highly compliant, force would shorten bond lifetime so much that tethering and rolling could not be supported (9, 19).
In this paper, we have investigated the basis for the markedly faster rolling through L-selectin than E- or P-selectins (40), and tested the relative contributions to rolling velocities on selectins of intrinsic bond dissociation kinetics and the compliance of the bond dissociation reaction. We have examined transient tethers on PNAd and E-selectin. Transient tethers have previously been studied on P-selectin (1), and occur when the densities of selectins or their ligands on the wall of a flow chamber are too low to support rolling. Transient tethers are the smallest unit of adhesive interaction that is observable in shear flow. Transient tethers have properties that suggest but do not prove that they represent single selectin–ligand bonds. We find that the intrinsic kinetics of dissociation of transient tethers correlates with rolling velocities for L-, E-, and P-selectins. Furthermore, we find that the L-selectin tether bonds are less sensitive to applied force than E-selectin or P-selectin tether bonds, which is opposite to what would be expected if bond compliance dominated the differences between the selectins in rolling velocity. The exponential constants that quantitate the effect of force on bond dissociation are interesting quantities in themselves. These constants are compared to estimates of the elastic spring constant for selectins.
Rolling cells do not move smoothly, but advance in a series of jerky movements that may reflect bond dissociation events (8, 25). New bonds to the substrate are thought to be formed after each step forward, but forward motion is more likely to be restrained by previously formed bonds, which after cell movement find themselves at the rear of the contact zone, than by newly formed bonds. We have examined a number of parameters of cell rolling through L-selectin that are essential to understanding its molecular and cellular basis, and to relating transient tether dissociation to rolling velocity. Transient tethers are a uniform population with regard to dissociation kinetics, and therefore may be considered quantal units (1). To determine whether the same quantal tether dissociation units underlie the jerky rolling behavior of leukocytes in shear flow, pauses in rolling were measured and were found to have the same time scale as transient tethers. To measure biophysical parameters including the relationship between force on a cell and force on a tether bond, the length of the tether, and the dimension of the adhesive contact zone between the cell and the substrate, we measured the distance that transiently tethered cells pivot when the flow direction is reversed. Furthermore, we relate this distance to the distance moved between pauses during rolling. We show that as few as two tether bonds to the substrate are sufficient to support rolling.
Adhesive interactions through L-selectin have the remarkable property that they require shear above a threshold value for their promotion and maintenance (13, 29). Rolling leukocytes accumulate on PNAd above but not below this shear threshold of ∼0.4 dyn/cm2. Furthermore, when leukocytes rolling on PNAd or on monolayers of adherent leukocytes above the shear threshold are subjected to a rapid decrease in shear to below the threshold, in less than 1 s they cease to interact with the substrate and move at the hydrodynamic velocity. When shear is raised above the threshold, leukocytes rapidly resume rolling interactions. The shear threshold phenomenon is also observed in vivo (13), and is hypothesized to help prevent inappropriate accumulation of leukocytes in vessels with inherently low wall shear rates and in hypoperfusion. Investigation of the kinetics of L-selectin tethers, and of rolling on L-selectin, particularly around the shear threshold, shed further light on this mysterious phenomenon.
Materials and Methods
Monoclonal Antibodies
mAbs used in this study as purified Igs were the anti–L-selectin mAb DREG 56 (24), mAb MECA-79 (47), and mAb 581 to CD34 (39). Control IgG were X63 myeloma IgG1 and the anti-CD44 mAb, A3D8 (20).
Preparation of Substrates
Human PNAd was purified from tonsil lysates by MECA-79 mAb affinity chromatography as previously described (5, 39). PNAd aliquots (0.001–1 μg/ml in PBS/0.5% octyl glycoside) were diluted 1:10 in PBS/10 mM sodium bicarbonate, pH 8.0, and 25 μl was immediately spotted onto polystyrene dishes for 3 h at 24°C. Substrates were washed and quenched with 2% human serum albumin (HSA) (fraction V; Calbiochem-Novabiochem Corp., La Jolla, CA). The site density of CD34 in the PNAd-coated substrates was determined by saturation binding of radiolabeled anti-CD34 mAb (39). CD34 contributes ∼50% of the total L-selectin tethering and rolling activity in human PNAd (39); therefore the densities given in the text for PNAd are twofold that determined for CD34. For example, a final concentration of 0.45 μg/ml PNAd yielded 90 sites per μm2 of CD34 and therefore the estimated density of PNAd was 180 sites per μm2. Site densities at low input concentrations (⩽0.15 μg/ml) were below the detection limit and were extrapolated from higher site density determinations; since the concentration of detergent in the adsorption media was kept constant, the site density of adsorbed PNAd was assumed proportional to PNAd concentration. Proportionality has previously been demonstrated in the range of 90–290 sites per μm2 (39). Purified E-selectin (26) was reconstituted in glass-supported lipid bilayers as described for P-selectin (1).
Isolation of Leukocytes
Human neutrophils were isolated from citrate anticoagulated whole blood by dextran sedimentation and density separation over Ficoll-Hypaque (32). Leukocytes were stored in Mg2+- and Ca2+-free HBSS, containing 10 mM Hepes, pH 7.4, for up to 2 h at 24°C.
Shear Flow Assays
PNAd substrates were assembled as the lower wall in the flow chamber and mounted on an inverted phase-contrast microscope (25, 26). Leukocytes were resuspended in binding medium (HBSS/Hepes containing 2 mM Ca2+ and 2 mg/ml HSA) and perfused through the chamber at different flow rates to obtain the indicated shear stresses at the chamber wall (25, 29).
For inhibition studies, cells were preincubated for 5 min in binding medium with 0.3–3 μg/ml of L-selectin blocking mAb or control mAbs. The cell suspension was perfused into the flow chamber without washing out the antibodies. Blockade of L-selectin function was also carried out by perfusing cells in the presence of 5 mM EDTA or 50 μg/ml fucoidan. For comparisons of interactions in the presence or absence of inhibition, at different shear stresses, or with different cell types, identical fields of view were used to ensure that the results reflected uniform site density and distribution of the immobilized adhesive proteins on the substrate. Between each comparison, any adherent cells were removed by perfusion with binding medium supplemented with 5 mM EDTA.
Determination of Cell Displacements
Images from Nikon plan ×20 or ×40 objectives and a TEC-470 CCD video camera (Optronics, Goleta, CA) were recorded and played back on a Mitsubishi S-VHS (U-67) recorder. Motions of neutrophils were analyzed by determination of cell center positions in successive video frames. Coordinates of cell centers were determined to an accuracy of ±0.68 μm and 0.34 μm with ×20 and ×40 objectives, respectively, and the velocity between each frame of individual cells was calculated.
Determination of Duration of Transient Tethering Events
Transient tethers were defined as cell attachment events separated by at least 50 μm of motion at the hydrodynamic velocity, and when no cell motion (<2 μm displacement) occurred while the cell was tethered to the immobilized ligand (1). The duration of transient tethers on PNAd was calculated by counting both the frames during which cells were motionless and the fraction of the immediately preceding and succeeding frames for which cells were tethered. The distance moved in these frames divided by the distance moved between frames by a freely moving cell yielded the fraction of time the cell was not tethered in the preceding and succeeding frames. Using the latter technique, tethering events lasting even less than one frame could be reliably detected, with proportionately higher accuracy at higher shear because of the greater difference between cell position in succeeding frames. The detection limit was ∼0.01 s at 1.5 dyn/cm2. The durations of transient tethers on P-selectin were measured in an integral number of frames (1). Sufficient videotape was analyzed (0.5–2 min) to obtain 30–40 tethering events, and the natural log of the number of cells that remained bound as a function of time after initiation of tethering was plotted. The slope = −k off.
Measurement of the Lever Arm Acting on a Tether
Neutrophils were allowed to interact in flow with P-selectin reconstituted in a lipid bilayer on a glass slide (1, 25). Flow was controlled by switching a pressurized air reservoir between the two ends of the flow chamber with an air valve. Shear stress was calibrated from the hydrodynamic velocity of nonadherent cells perfused at different flow rates in the same chamber using a syringe pump. The lever arm is defined as half the distance moved during flow reversal while cells were tethered to P-selectin.
Results
The Micromotion of Neutrophils During Rolling on PNAd
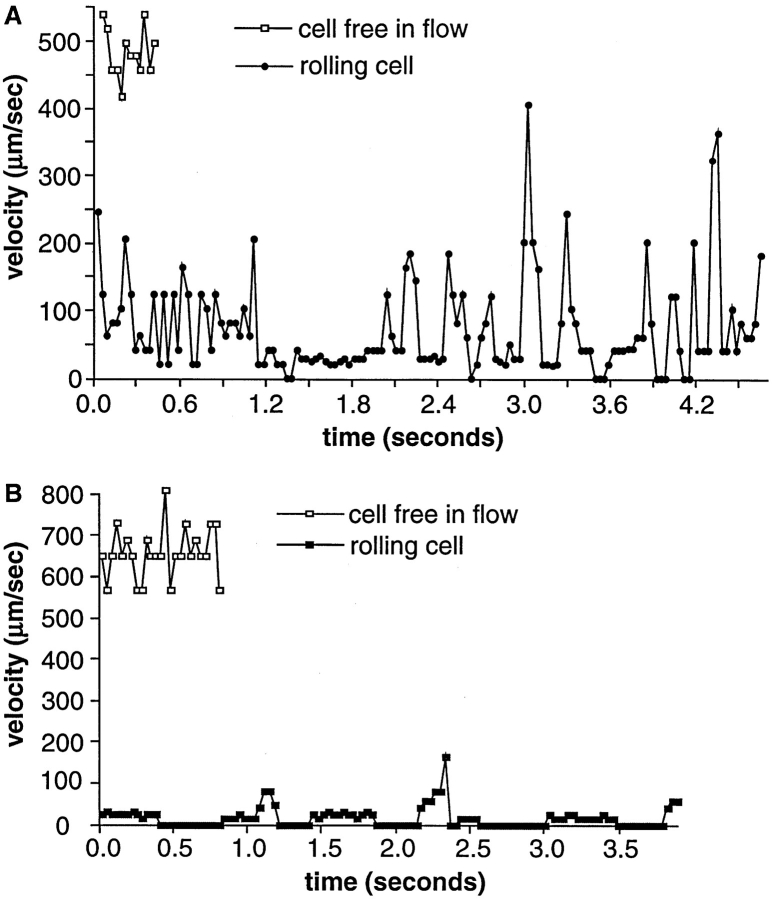
To examine the jerky movements during rolling in hydrodynamic flow that are thought to occur when selectin– ligand bonds dissociate, we studied motion on a time scale smaller than selectin bond lifetimes, i.e., between each 0.033-s video frame (Fig. 1, A and B). Densities of PNAd (60 sites per μm2; Fig. 1 A) and P-selectin (30 sites per μm2; Fig. 1 B) were just above the threshold required to support rolling, in order to minimize the number of selectin–ligand bonds between the rolling leukocyte and the substrate, and thereby maximize the effect of individual bonds on behavior. The average rolling velocity was much faster through L-selectin on PNAd (Fig. 1 A) than through PSGL-1 on P-selectin (Fig. 1 B). Neutrophils paused during rolling on both substrates, but the pauses were of markedly longer duration on P-selectin than PNAd, correlating with the slower rolling velocity on P-selectin. The five pauses on PNAd (Fig. 1 A) were 0.073 ± 0.026 s (mean ± SD) and the six pauses on P-selectin (Fig. 1 B) were 0.30 ± 0.14 s.
Figure 1.
The microkinetics of rolling of individual neutrophils interacting with the lowest densities of purified PNAd or P-selectin that can support rolling. (A) Neutrophils were perfused in a parallel wall flow chamber on PNAd at 60 sites per μm2 at a wall shear stress of 1.5 dyn/cm2. (B) Neutrophils were perfused on P-selectin at 30 sites per μm2 at 1.8 dyn/cm2. Velocities of cells free in flow adjacent to the wall are shown for comparison. Coordinates of cell centers were determined within ±0.68 μm and the velocity between each frame was calculated for individual cells.
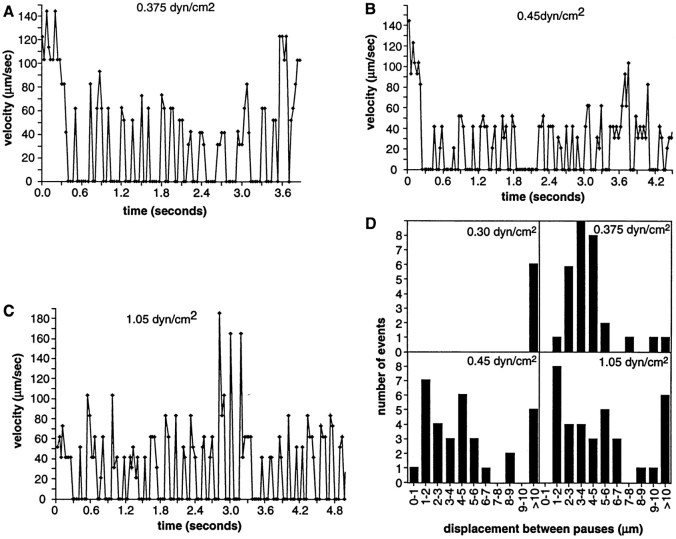
Previous studies have shown that rolling through L-selectin on PNAd requires a wall shear stress above a threshold value of ∼0.4 dyn/cm2 (13); therefore, we examined micromotion around the shear threshold. At 0.3 dyn/cm2, there was no rolling, and only transient tethers were seen. At 0.375 dyn/cm2, a transitional type of rolling behavior was seen. Rolling occurred in relatively short runs, a longer representative of which is shown in Fig. 2 A. Neutrophils rolled with pauses of 0.11 ± 0.051 s (n = 19). A remarkable feature of these pauses was their separation by leukocyte displacements that were regular in length. The modal displacement between pauses at 0.375 dyn/cm2 was 3–4 μm (Fig. 2 D). This displacement represents the distance between successive tethers to the substrate. At 0.45 and 1.05 dyn/cm2, neutrophils rolled for substantially longer times per run, and there was a broader distribution of displacement between pauses, including many movements of 1–2 μm (Fig. 2, B–D). The duration of pauses during rolling at 0.45 dyn/cm2 was 0.113 ± 0.077 s, and at 1.05 dyn/cm2 was 0.094 ± 0.026 s (Fig. 2, B and C). At 0.3 dyn/cm2, tethers were separated by >10 μm, consistent with the lack of rolling (Fig. 2 D).
Figure 2.
The microkinetics of neutrophil rolling near the shear threshold. (A) The position of a neutrophil selected for continued interaction with a 100 sites per μm2 PNAd substrate at 0.375 dyn/ cm2 was determined to ±0.34 μm accuracy in each video frame and interframe velocities were calculated. (B) As in A, at 0.45 dyn/cm2. (C) As in A, at 1.05 dyn/cm2. Cells rolled for shorter distances at 0.375 dyn/cm2 than at 0.45 and 1.05 dyn/cm2; the cell in A rolled for 3.2 s. (D) The distance between pauses during rolling is modal near the shear threshold. The distance traveled between each pause (velocity = 0) was determined at each shear stress for two representative neutrophils that showed substantial interaction with the substrate after tethering within the field of view, in the same experiment as in A–C. Note that fewer events were seen at 0.3 dyn/cm2 because only transient tethers occurred.
Transient Tethers on PNAd
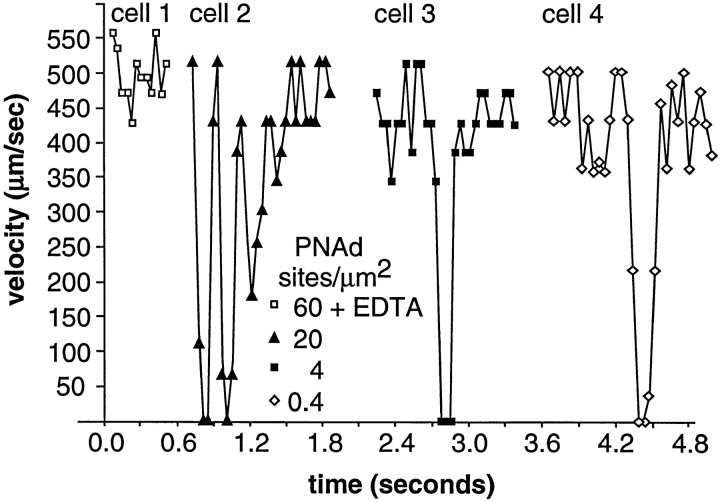
At PNAd densities lower than 60 sites per μm2, rolling became faster and jerkier. At 20 sites per μm2, tethered neutrophils failed to continuously roll on PNAd (Fig. 3, cell 2), and below this density, tethering became transient and was separated by distances over which cells traveled at the hydrodynamic velocity (Fig. 3, cells 3 and 4). Neutrophil tethering to PNAd was highly specific, since it was completely inhibited by pretreatment of cells with L-selectin mAb, EDTA (Fig. 3, cell 1), soluble fucoidan, or pretreatment of the substrate with O-sialoglycoprotease to degrade mucinlike constituents of PNAd (data not shown) (39).
Figure 3.
The microkinetics of transient tethers on PNAd. Neutrophils were perfused at 1.5 dyn/cm2 on substrates with PNAd at the indicated density. Velocities of representative cells on each substrate are shown. Cell 1 in EDTA is shown to demonstrate the hydrodynamic velocity. Coordinates of cell centers were determined within one pixel accuracy (±0.9 μm) and the velocity between each frame was calculated for individual cells.
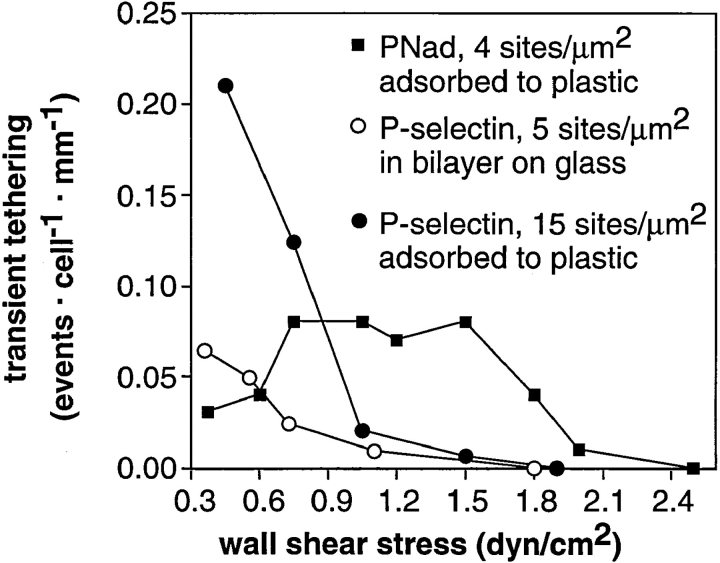
The efficiency of transient tethers was studied on substrate densities below the density required to support rolling (Fig. 4 A). Tethering efficiency decreased at higher shear stresses on both P-selectin and PNAd substrates and declined to zero at 1.8 dyn/cm2 on P-selectin and at 2.5 dyn/cm2 on PNAd. Extraction from the substrate did not appear to occur, because the upper shear limit for tethering was identical whether P-selectin was adsorbed to plastic or incorporated in lipid bilayers supported on glass (Fig. 4 A). Below, we describe the kinetics of transient tethers. At the highest shear stresses at which transient tethers occurred, their lifetimes were well within the measurable range of >0.01 s, and were 0.2 s for P-selectin and 0.04 s for L-selectin. The finding that the lifetime of transient tethers through L-selectin is much shorter than through PSGL-1 (see below), despite the observation that L-selectin tethers withstand higher shear stresses, argues that the kinetics are unrelated to extraction and are due to receptor–ligand dissociation.
Figure 4.
L-selectin forms tethers at higher shear forces than PSGL-1. The number of transient tethers was counted and divided by the distance cells were transported by flow across the field of view and by the number of cells that flowed across the field that were within the focal plane of the substrate. This yields the density of tethers. L-selectin blocking mAb DREG-56 added to neutrophils in the binding medium at 3 μg/ml, shortly before perfusion on the substrate, inhibited 95% of tethering events on PNAd. The density of leukocyte tethering to control substrates coated with HSA was <0.005 events cell−1 · mm−1. No tethering events were observed in the presence of 5 mM EDTA.
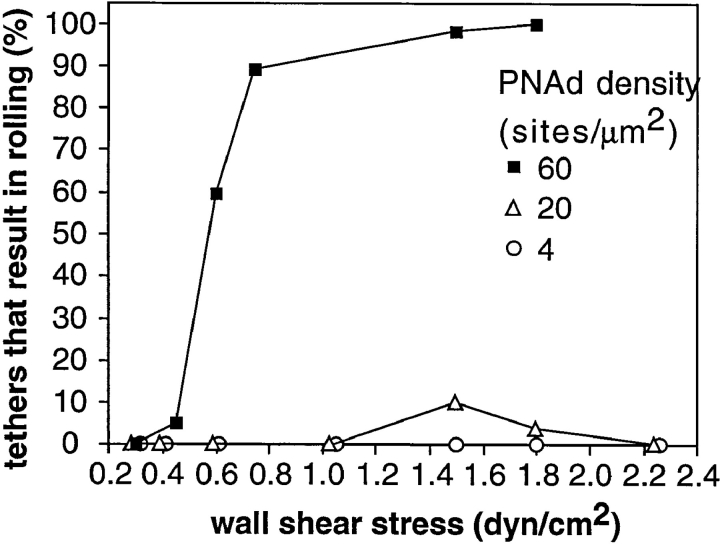
We examined transient tether efficiency around the shear threshold for rolling on L-selectin. Transient tethers occurred at 0.30 dyn/cm2 (Fig. 2 D) and 0.35 dyn/cm2 (Fig. 4). Thus, transient tethers occur at wall shear stresses below the threshold, where rolling does not occur. However, transient tether efficiency on PNAd at wall shear stresses of 0.35 and 0.6 dyn/cm2 was lower than that at 0.75–1.5 dyn/cm2 (Fig. 4). This drop occurred despite correction for the lower cell flux at lower wall shear stresses, i.e., with tethering efficiency expressed as a function of the cell transport distance across the substrate. By contrast, tethering efficiency on P-selectin substrates was higher at 0.3 dyn/cm2 than at 0.75 dyn/cm2 (Fig. 4). On PNAd, the percentage of tethers that resulted in rolling adhesions, as opposed to transient tethers, increased dramatically between 0.45 and 0.75 dyn/cm2 at 60 sites per μm2 (Fig. 5). At 20 and 4 PNAd sites per μm2, little or no rolling adhesions occurred at any shear (Fig. 5). Thus, a density of PNAd above 20 sites per μm2 and shear stress above about 0.45 dyn/cm2 are required for tethers to progress to rolling adhesions. In conclusion, shear enhances both the density of transient tethers and the conversion of transient tethers events on the substrate to multivalent tethers that can support rolling.
Figure 5.
Conversion of tethering events to rolling is a function of wall shear stress and site density of PNAd. Tethers were considered to result in rolling when neutrophils moved for at least 2 s at a mean velocity of at least fivefold lower than the mean hydrodynamic velocity of untethered cells at a given shear stress.
First Order Kinetics of Dissociation of Transient Tethers on PNAd
The duration of transient tethers on PNAd was measured for multiple cells. Synchronizing the initiation of tethering to t = 0, transient tethers were seen to dissociate with first order kinetics (Fig. 6 A). The data fit a single straight line with a high correlation coefficient, and k off was independent of PNAd density (Figs. 6, A–C). L-selectin tethers dissociated from PNAd markedly faster than P-selectin tethers at 0.75 dyn/cm2 (Fig. 6 A). The k off on PNAd at 0.75 dyn/cm2 corresponded to a tether lifetime (the reciprocal of k off) of 0.09 s. This lifetime is comparable to the duration of pauses seen above in rolling on PNAd. The homogeneity in the dissociation rate constant, as shown by fit to a straight line and lack of dependence on PNAd density at 12 sites per μm2 and below, suggested that we were measuring the smallest detectable unit of binding between the cell and the substrate, and that this binding unit was homogeneous or quantal. In evidence against a contribution of multiple independent receptor–ligand interactions to transient tethers, partial saturation with an inhibitory mAb to L-selectin, which reduced the frequency of tethers by 70%, or inclusion of the L-selectin antagonists fucoidan and mannose-6-phosphate, had no effect on k off (Fig. 6 B; and data not shown). Moreover, partial desialylation of the PNAd immobilized in the flow chamber with neuraminidase, which reduced the frequency of transient tethers by 77%, had no significant effect on k off (not shown).
Figure 6.
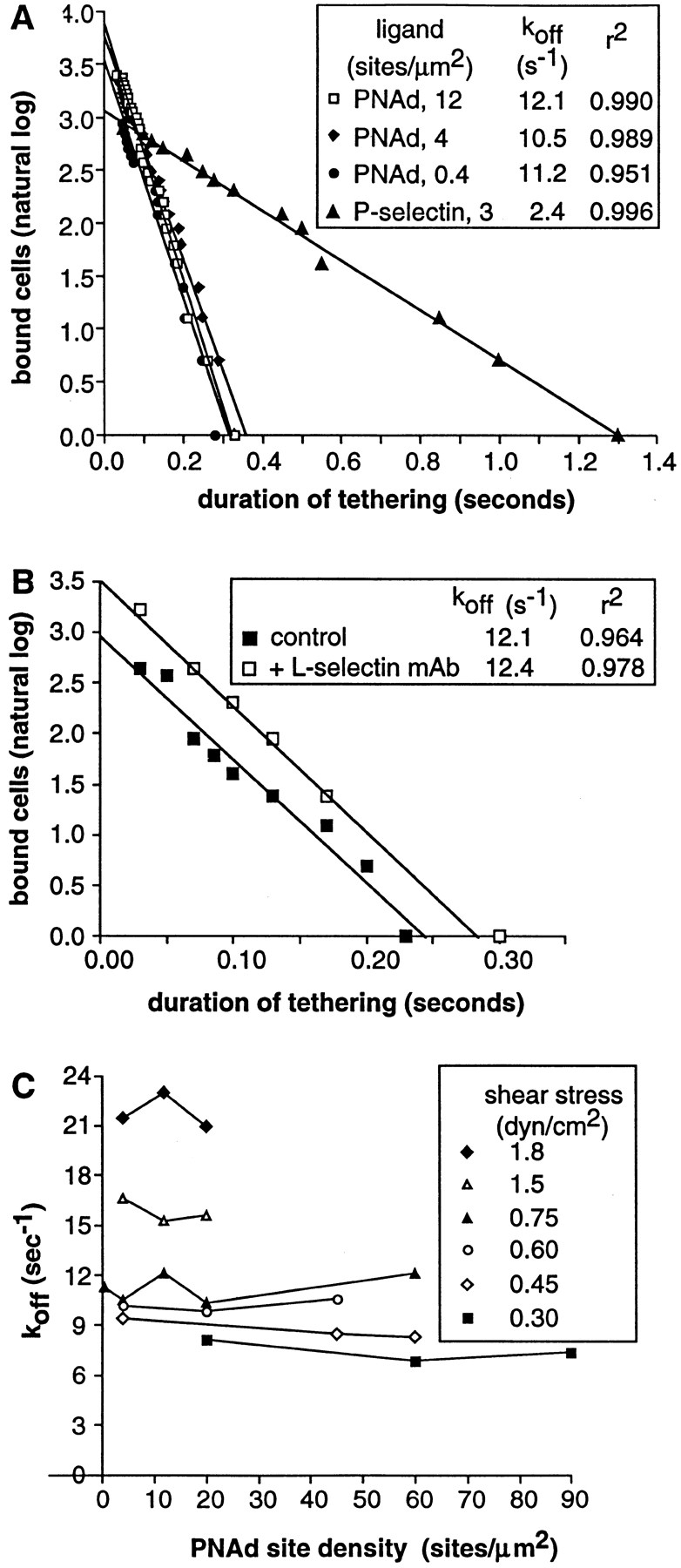
The kinetics of dissociation of transiently tethered neutrophils from PNAd and its independence from PNAd or L-selectin densities. The k off values equal the negative slope of the lines through the dissociation data. (A) Kinetics at a wall shear stress of 0.75 dyn/cm2 on different low densities of PNAd. Data at the same wall shear stress on P-selectin (1) are shown for comparison. (B) Kinetics at a wall shear stress of 0.75 dyn/cm2 on higher density PNAd (60 sites per μm2) in the presence of subsaturating concentrations of control mAb AD38 to CD44 or mAb DREG-56 to L-selectin at 0.3 μg/ml. The L-selectin mAb reduced tethering frequency by 70% and abolished all rolling adhesions. (C) Dissociation rate constants at different wall shear stresses, as a function of PNAd site density. At low shear stresses, when both transiently tethered and continuously rolling cells could be observed, the few transient events that occurred had the same dissociation kinetics as those derived at low densities.
The dissociation rate constant for the L-selectin–PNAd tether increased as shear was increased (Figs. 6 C and 7 A).
Figure 7.
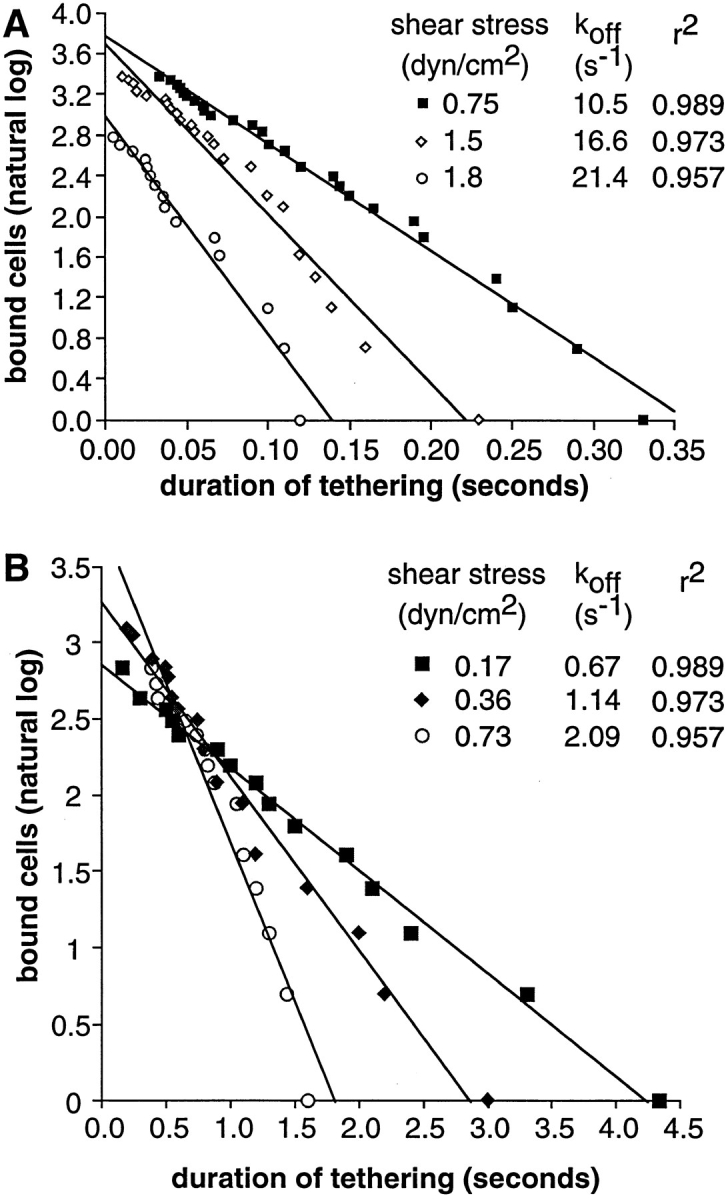
Effect of wall shear stress on the kinetics of neutrophil dissociation from PNAd and E-selectin. (A) PNAd at four sites per μm2. (B) E-selectin at seven sites per μm2.
For comparison, we examined transient tethers of neutrophils on E-selectin at seven sites per μm2. Transient tethers required Ca2+ and were sensitive to neuraminidase treatment of neutrophils. Like L-selectin tethers, dissociation of E-selectin tethers showed first order kinetics with a single rate constant, which was independent of density when E-selectin was present at 10 sites per μm2 or below. As with other selectins, the k off of transient E-selectin tethers increased as shear was increased (Fig. 7 B). E-selectin tethers had a longer lifetime than L-selectin tethers.
Parameters of the Adhesive Contact Zone
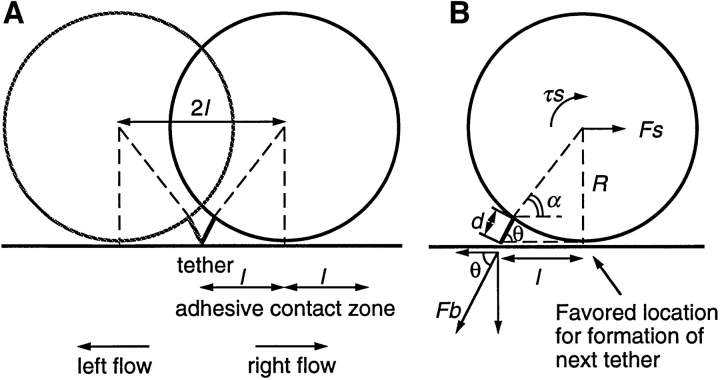
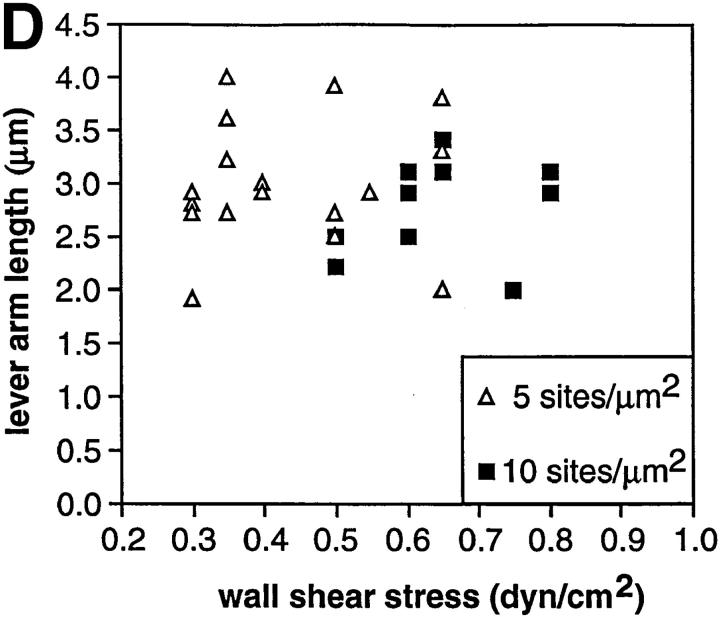
An understanding of the mechanics of transient tethers and rolling requires knowledge of where the cell is tethered to the substrate (Fig. 8, A and B). Therefore, the direction of flow was reversed every few seconds during transient tethering experiments. Measurements were made on P-selectin substrates, since the short lifetime made measurements on PNAd impractical; however, tether parameters are expected to be similar or identical for L-selectin because of its colocalization with the P-selectin ligand PSGL-1 on microvilli tips (33, 36). Thus, tethers to both PNAd and P-selectin are predicted to be through microvilli tips. When, by chance, flow reversal occurred while a cell was transiently tethered, the distance moved by the cell was measured (Fig. 8 C). Cells that had transiently tethered as shown by lack of movement between one or more successive video frames (Fig. 8 C, 0.333–0.733 s) were observed to move during flow reversal, to then again remain stationary for one or more successive video frames (Fig. 8 C, 0.933–1.633 s), and to subsequently dissociate and move at the hydrodynamic velocity (Fig. 8 C, 1.833– 2.000 s). The lever arm is the distance between (a) the point on the substrate where the adhesion molecule is attached to which the cell is tethered, and (b) the projection of the center of the cell on the substrate, and is equal to half the distance the cell moves during flow reversal (Fig. 8 A). Measurements on 27 tether events that coincided with flow reversal (Fig. 8 D) showed a mean ± SD of 3.06 ± 0.53 μm for the length of the lever arm. This length was independent of shear from 0.3 to 0.8 dyn/cm2 (Fig. 8 D). No change in cell dimension in any direction within the plane of focus was visible microscopically during tethering or flow reversal. The distance moved during flow reversal defines the maximal length of the adhesive contact zone, because a cell would be predicted to be capable of interacting with the substrate between the tether point and the projection of the center of the cell on the substrate, and at most for an equal distance on the other side of the projection of the center of the cell (Fig. 8 A). Using the lever arm length and the model of a spherical nondeformable cell (8.5 μm in diam) with a tether that is flexible where it joins the cell body, and balancing the forces and torques on a tethered cell in shear flow, the length of the tether (d) can be estimated to be 1.00 ± 0.32 μm (Fig. 8 B). This length may include microvillus and adhesion molecule lengths, as well as deviations of the cell from its idealization as a nondeformable sphere.
Figure 8.

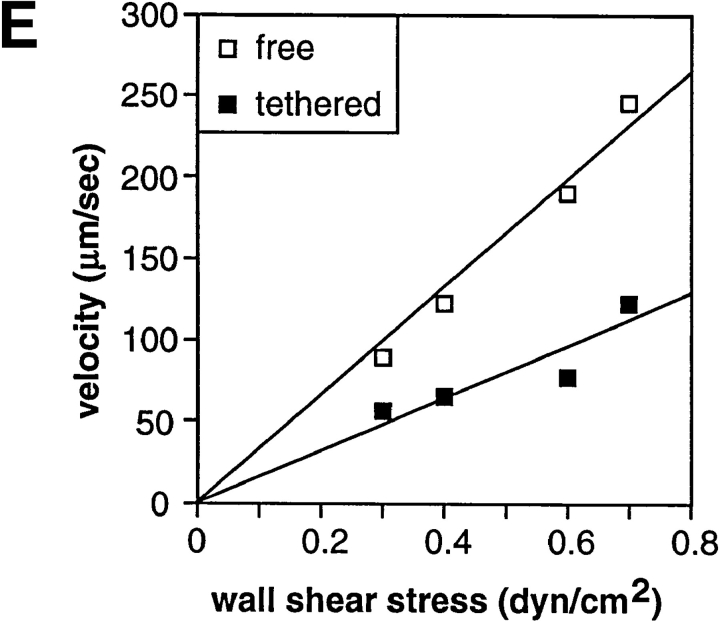
The lever arm acting on a transient tether, the dimension of the adhesive contact zone, and the force on the tether bond. (A) Scheme for measurement of the lever arm by reversing the direction of flow. The lever arm, l, is the distance between the tether point and the projection of the center of the neutrophil on the substrate. (B) Estimation of forces on a neutrophil tethered in shear flow (drawn to scale). The force balance equations are F b cosθ = F s and F b lsinθ = τs + RF s, where F b is the force on the tether bond, F s is the force on the cell, and τs is the torque on the cell. The exact solution for F s for a motionless hard sphere in shear flow near a wall is F s = 6π·viscosity·R·h·shear·C where R is sphere radius, h is the distance from the center of the cell to the wall, and C is a numerical factor determined by integration that depends on h/R and ranges from 1 to 1.7 (17). With R = 4.25 μm for a neutrophil (48), variation of h–R from 0 to 0.5 μm has little effect on F s (±3%), and we have assumed h = R for a tethered cell. The assumption of hardness is good because no neutrophil deformation is visible microscopically within the range of shear used here. Roughness such as from microvilli is thought to increase F s only modestly; for a rough object, F s is intermediate between F s on a sphere contained in the object and F s on a sphere containing the object. We estimate uncertainty in R to measurement variation (48) and its effective increase by microvilli as ±0.25 μm. The calculated value of F s and its uncertainty are 59.8 ± 6.9 pN per dyn/cm2 wall shear stress. Both the uncertainty in R and in the lever arm measurement l = 3.06 ± 0.53 μm introduce uncertainties into the other calculated values. The uncertainties stemming from R and l were estimated separately for these values and their variances were added. We calculate θ = 62.3 ± 4.2°, d = 1.0 ± 0.32 μm, F b/F s = 2.15 ± 0.32, and F b = 124.4 ± 26.1 pN per dyn/cm2 wall shear stress. (C) Movement of a representative transiently tethered neutrophil on P-selectin at five sites per μm2 during reversal of flow at 0.3 dyn/cm2. The vertical dashed line marks the left boundary of the tethered cell before flow reversal. (D) Measurement of the lever arm. Each point is a measurement on an individual cell; the lever arm is defined as half the distance moved during flow reversal while cells were tethered to the indicated density of P-selectin. Shear stress was calibrated from the hydrodynamic velocity of cells in the same chamber using a syringe pump. (E) Velocities after flow reversal of untethered neutrophils and of neutrophils transiently tethered to P-selectin (five sites per μm2). The velocity of tethered cells was derived from a frame by frame analysis of the number of video frames required for a transiently tethered cell to move the distance of 2 l after flow reversal. Values are the average of two velocity determinations at each wall shear stress. Velocities of cells free in flow (i.e., untethered, traveling at the hydrodynamic velocity) were determined in the same video segments. Velocities of cells free in flow are the mean of four determinations. Similar velocity determination of cells free in flow was performed on an identical field using a syringe pump generating known wall shear stresses, and the shear stress was calibrated from these velocities. The movement of indicator nontethered cells in the same field of view was observed to mark flow reversal, and time was not counted until indicator cells reached full velocity (dead time was about one frame at the lowest shears).
Both tether k off and the time required for a cell to move between successive tethers, i.e., to pivot about a tether, will affect rolling velocity. Therefore, we determined the time required for transiently tethered cells to move the distance of 2 l (l is the distance between the tether point and the projection of the center of the neutrophil on the substrate) after flow reversal (Fig. 8 E). This took approximately twice as long as for untethered cells in the same field of view and focal plane, perhaps because slippage and rotation of tethered cells relative to the substrate in shear (17) is retarded by the tether.
The Intrinsic koff and Effect of Force on koff for Selectins
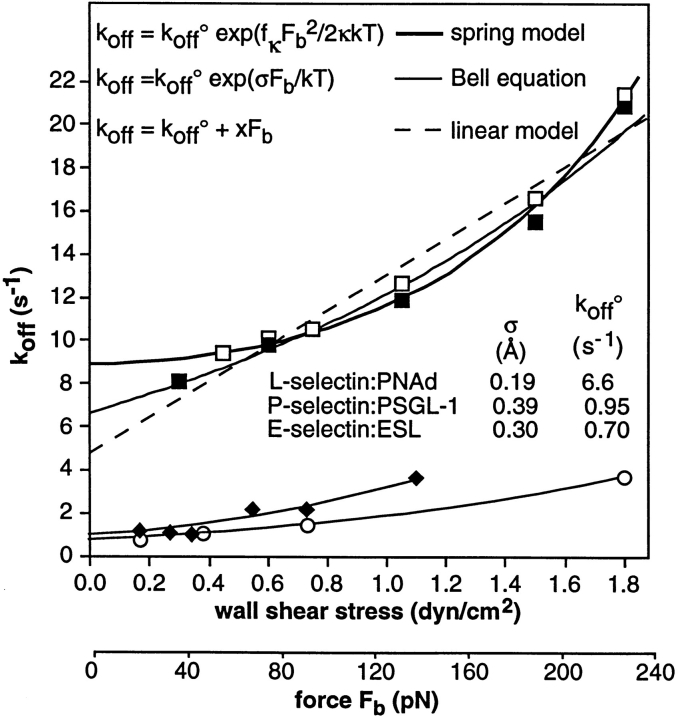
The lever arm measurement allows the relationship between wall shear stress and force on the tether bond to be estimated. Given the lever arm length, the force on the tether bond (F b), can be calculated to be 2.15 ± 0.47 times the hydrodynamic drag force on the cell (F s) (Fig. 8 B). Using the solution for F s for a sphere near a wall in shear flow (17), the relationship between wall shear stress and F b can be estimated (Fig. 8, legend). Thus, data on k off for L-selectin and E-selectin determined here and for P-selectin (1) can be plotted as a function of wall shear stress and the estimated force on the tether bond (Fig. 9). There are several important conclusions: first, the k off for L-selectin is markedly faster than that for the vascular E- and P-selectins at all forces examined, and when extrapolated to zero shear stress to yield k off° (unstressed k off; Fig. 9). Therefore, the faster k off of L-selectin will make an important contribution to its faster rolling velocity. Second, the increase in k off with increased force, i.e., the mechanical property of reactive compliance, is not related to differences in rolling velocity between selectins. To quantitate reactive compliance, all data were fit to Bell's equation, in which the potential energy stored in the bond increases linearly with the applied force and is proportional to σ, the “bond interaction distance” (Fig. 9). The value of σ can quantitatively represent reactive compliance. There is less variation in σ than in k off° for selectins; furthermore, L-selectin has the lowest reactive compliance. Therefore, differences in mechanical properties cannot explain faster rolling through L-selectin than through E- and P-selectins because greater, not lesser, compliance would lead to faster rolling. Third, the most robust data set, that on L-selectin off rates, supports an exponential relationship between F b and k off. In addition to Bell's equation, these data were fit to a Hookean spring model in which k off increases exponentially with the second power of F b, and a straight line model in which k off increases linearly with F b. The experimental data on L-selectin show a good fit to the Bell expression (χ2 = 0.305 with 9 degrees of freedom), and a somewhat better fit to the Hookean spring model (χ2 = 0.213, with 9 degrees of freedom). Both exponential equations appear to be adequate approximations to the data; neither fit the data significantly better than the other. By contrast, the straight line model yields χ2 = 0.848, with 9 degrees of freedom. The Hookean spring and Bell expressions both fit the data significantly better than the linear relationship as shown with an F-test of the χ2 values (10) (P = 0.014 and 0.03, respectively), providing support for an exponential dependence of k off on F b.
Figure 9.
Fit to theoretical predictions of the effect of F b on k off. k off was measured on PNAd at 4 and 20 sites per μm2 (▪ and □, respectively) or E-selectin (○); data on P-selectin (⋄) (1) are plotted against F b using the lever arm measured in the current paper. F b was calculated as described in the legend to Fig. 7 B. The thin lines are fit of all three selectin interactions to Bell's expression (4) k off = k off° exp (σF b/kT) where k off° is the unstressed k off, σ is the separation between receptor and ligand that weakens the bond, k is Boltzmann's constant, and T is the absolute temperature. The fits yield for PNAd: L-selectin k off° = 6.8 ± 0.2 s−1, σ = 0.20 ± 0.01 Å; for P-selectin k off° = 0.93 ± 0.15 s−1, σ = 0.40 ± 0.08 Å; for E-selectin k off° = 0.7 ± 0.05 s−1, σ = 0.31 ± 0.02 Å. The thick line is fit of the Hookean spring model, k off = k off° exp (f κ F b 2/2κkT), where κ is the spring constant for the bond; in the formalism of Dembo et al. (9) and Hammer and Apte (19), f κ is the fraction of the bond spring constant that is applied to bond dissociation; the remainder is applied to bond association. In other words, f κ is the fraction of bond strain that is devoted to bond dissociation, and is also known as the fractional spring slippage (9, 19). The fit yields for the PNAd: L-selectin k off° = 8.8 ± 0.2 s−1 and κ/f κ = 7.1 ± 0.4 N/m. Data was fit using the program Igor (WaveMetrics, Inc., Lake Oswego, OR). Values for σ and k off° shown in the figure are from the fit to Bell's equation.
Discussion
To understand the molecular basis of selectin-mediated rolling, we have examined the kinetic and mechanical properties of the transient tether, and its relationship to properties of rolling adhesions including velocity, pause duration, and step length. We have focused on L-selectin because rolling through L-selectin is faster in velocity than through the vascular selectins and exhibits a shear threshold dependence (13, 29). We have compared the intrinsic k off and mechanical properties of L-, P-, and E-selectins by studying the dissociation of transient tethers. In all cases, dissociation of transient tethers followed first order kinetics and was independent of ligand density over a wide range of densities below the threshold required to support rolling. These properties suggest but do not prove that transient tethers represent the formation and dissociation of single receptor–ligand bonds. Elsewhere, we have directly compared rolling velocities on PNAd, P-selectin, and E-selectin at densities on the substrate that were similar, and that gave rolling adhesions of equal strength, as shown by resistance to detachment by increased shear. Over a wide range of wall shear stresses, rolling through L-selectin was 7.5–9-fold faster than on P-selectin, and 8–11.5-fold faster than on E-selectin (40). An important question addressed in the current study was the relative importance of the intrinsic bond dissociation rate constant, k off°, and the susceptibility of k off to applied force, i.e., reactive compliance, in determining the more rapid rolling velocity through the leukocyte L-selectin molecule than through the vascular E- and P-selectin molecules.
Biologically, L-selectin appears specialized to mediate fast interactions that generally represent the initial event in adhesion cascades. L-selectin nucleates leukocyte–leukocyte interactions (2) that subsequently lead to slower rolling on E- and P-selectins (1, 53) and firm adhesion through leukocyte integrins (41). Similarly, rolling interactions through L-selectin appear to precede slower rolling through mucosal cell adhesion molecule-1 (MAdCAM-1) in Peyer's patches (3). Fast rolling through L-selectin occurs across species including rabbit (50), mouse (6), cow (2), and human (28); thus it appears from its evolutionary conservation to be biologically important. We have not examined the association rate constant, k on, because this requires knowledge of the concentration and diffusion of the selectin and the ligand in the adhesive contact zone, and may also be affected by transport of the cell by shear flow and the shear threshold phenomenon. Maintenance of stable rolling requires that the average number of bonds that are formed and broken be equal. The faster rate of tether dissociation through L-selectin than through E- and P-selectins requires an equally faster overall rate of L-selectin tether bond formation, and if the effective concentrations of reactants were identical, this would require a faster k on. Thus, while our investigations have focused on k off , they have implications for the overall rate of bond formation, and for understanding the basis for the fast interactions through L-selectin that are important in initiating adhesion cascades in the vasculature.
Both k off° and mechanical properties must be important for selectin-mediated rolling. Reactive compliance has been predicted to be low to obtain conditions that are permissive for rolling (9, 19). Reactive compliance in the Hookean spring model is inversely related to the exponential constant κ/f κ, i.e., the tether bond spring constant divided by the fraction of the bond spring constant that is applied to bond dissociation; the range of permissive values has been predicted to correspond to values of κ/f κ in Newtons (N)/m that are between 0.1 and infinity (19). Variation within this wide range would have a dramatic effect on k off in the presence of force, and hence on rolling velocity. Therefore, we have sought to determine whether k off° or reactive compliance is varied biologically to obtain the considerably faster rolling through L-selectin than through the vascular selectins. The comparisons between selectins on k off° and the reactive compliance for bond dissociation were clear cut. The intrinsic L-selectin k off in the absence of force, k off°, is sevenfold faster than for P-selectin and 9.4-fold faster than for E-selectin. This is in excellent agreement with the 7.5–9-fold faster rolling through L-selectin than through P-selectin and 8–11.5-fold faster rolling than through E-selectin, on substrates of equal adhesive strength (40). The k off° are 6.8, 0.93, and 0.7 s−1 for L-, P-, and E-selectins, respectively, including our previous estimate for P-selectin (1); a previous estimate of a stressed k off for E-selectin obtained by measuring pauses during rolling on endothelium is 0.5 s−1 (23). By contrast, current biophysical models assume k off° of 5 × 10−4–5 × 10−5 s−1, which corresponds to unstressed bond lifetimes of 0.5–5 h (19, 49). Reactive compliance must increase the unstressed k off° by >1,000-fold to obtain a stressed k off that can support rolling in these models. Thus bond compliance has a major role in determining rolling velocity in current biophysical models, whereas the effect of short intrinsic bond lifetimes has not been modeled. The difference between the k off°'s measured here and k off°'s assumed in these models range between 2 × 103 and 106-fold, and could impact model predictions.
By contrast with k off°, we found that mechanical compliance, i.e., σ, varied only twofold among selectins, and did not correlate with rolling velocity. Greater compliance means a greater increase in k off with applied force, thus giving rise to faster rolling velocity. However, L-selectin has lower reactive compliance than the vascular selectins, and therefore this does not contribute to its faster rolling velocity. To the contrary, the lower reactive compliance of L-selectin may help compensate for its faster k off, so that its already short bond lifetime is not excessively diminished by the hydrodynamic drag forces experienced by adherent leukocytes in postcapillary venules.
We have recently measured the L-selectin–carbohydrate interaction in the other direction, i.e., with L-selectin adsorbed to the substrate interacting with a carbohydrate ligand for L-selectin that is expressed on the surface of neutrophils (Alon, R., S. Chen, R. Fuhlbrigge, K.D. Puri, and T.A. Springer, manuscript in preparation). Despite the difference in direction and nature of the carbohydrate ligand, the kinetics (k off° = 7.0 ± 0.5 s−1) and reactive compliance (σ = 0.24 ± 0.02 Å) are quite similar to those reported here for the L-selectin–PNAd interaction (k off° = 6.8 ± 0.2 s−1 and σ = 0.20 ± 0.01 Å). We have also found that rolling on L-selectin is faster than on E- and P-selectins when substrate densities are adjusted to give rolling adhesions of similar strength, further supporting the generality of the findings reported here. It has been suggested that proteolytic shedding of L-selectin may contribute to the rapidity with which it supports rolling (52). The equivalent kinetics of rolling on PNAd and L-selectin argue against this, because the protease is cell associated and cannot function in trans as would be required to shed L-selectin from a substrate (12, 38).
To demonstrate a relationship between tether k off and rolling velocity, it was important to examine the kinetics of rolling on a time scale shorter than the tether lifetime. The duration of pauses during rolling was measured on low densities of PNAd and P-selectin, and near the shear threshold on PNAd, to minimize the number of receptor–ligand bonds and hence to accentuate the importance of dissociation of individual tethers. At a wall shear stress of 1.5–1.8 dyn/cm2, the duration of pauses on P-selectin of 0.30 ± 0.14 s was longer than on PNAd of 0.073 ± 0.026 s, correlating with the longer lifetime of P-selectin than PNAd tethers. On PNAd at 1.5 dyn/cm2, pause duration during rolling was 0.073 ± 0.026 s compared to a transient tether lifetime of 0.061 s. On PNAd at 0.375 and 0.45 dyn/cm2, pause durations were 0.11 ± 0.051 and 0.113 ± 0.077 s compared to tether lifetimes of 0.121 and 0.116 s at these shear stresses, respectively. This close agreement between transient tether lifetime and rolling pause duration provides strong evidence that pauses during rolling through L-selectin on PNAd at the tested shear stresses correspond to the time required for the dissociation of tether bonds, and that movements during rolling reflect dissociation of tether bonds. Further support for this comes from a comparison of the estimate for the k off of E-selectin of 0.5 s−1 derived from analysis of neutrophil pauses during rolling on stimulated endothelium (23) and our measurement of k off° of 0.7 s−1 from transient tether kinetics. Thus, the agreement between tether lifetime and pause duration during rolling provides an explicit link between k off and rolling velocity.
Measurement of the distance that transiently tethered cells moved during flow reversal yielded information on the geometry of the contact region between the cell and the substrate. The lever arm distance of 3.06 ± 0.53 μm yields an estimate of 1.0 ± 0.32 μm for the length of the tether between the cell and the substrate, including both adhesion molecule and microvillus lengths and the effects of cell roughness and deformation. This is in reasonable agreement with recent estimates by a different measurement technique (45). Furthermore, the dimension of the adhesive contact zone in the direction parallel to flow can be estimated to be twice the lever arm distance, 6.12 ± 1.06 μm. On PNAd at 0.375 dyn/cm2, near the shear threshold where the number of tethers formed to the substrate is predicted to be the minimum required to support rolling, the step distance was found to be unimodal, with a peak at 3–4 μm. The close correspondence between the step distance of 3–4 μm and the lever arm distance suggests that when a cell is restrained by a tether, the most favored location for the formation of the next tether is under the center of the cell. Dividing the dimension of the contact zone of 6 μm by the step distance of 3 μm during rolling on low densities of PNAd near the shear threshold yields an estimate of two tethers per contact zone. Two tethers is the minimum required to support rolling; one tether would give a transient tether. The minimum number of tethers was expected under these conditions, near the transition between rolling and transient tethering. The results support the idea that rolling can be supported by a small number of bonds between the cell and the substrate (25). Several models assume a large number of receptor–ligand bonds (9, 49), whereas one finds that the number of bonds never exceeds 100 (19).
Our data show a significantly better fit to two different models of exponential relationships between F b and k off than to a linear relationship. Thus, it is valid to consider the meaning of the exponential constants, σ and κ/f κ. There are two caveats to the measurements of these constants. First, neither exponential model fits significantly better than the other, and thus each should be viewed as no more than an approximation to the data. Second, although measurements of selectin bond mechanical properties with leukocytes is clearly of the highest biological significance, it is not clear whether leukocytes are accurate biophysical measuring devices; we cannot rule out some systematic error in the estimate of F b because leukocytes are not rigid as modeled, but are viscoelastic. Nonetheless, we have estimated the error in the calculation of F b as ±21% (Fig. 8, legend) and the estimates of the constants may be viewed at least as useful first approximations. The absolute values of these constants might differ between adhesion molecules and molecules that do not have to function while withstanding force. σ was proposed as the increase in distance between the receptor and ligand that leads to an increased rate of bond dissociation (4); note that σ is not only distinct from, but also less than the unbinding distance and that separation by larger distances than σ would be required to abolish interaction. σ was found to be 0.20 ± 0.01 Å. The constant κ/f κ is the tether bond spring constant divided by the fraction of tether bond stress devoted to bond dissociation (9), and it was found to be 7.1 ± 0.4 N/m. Note that membrane and cytoskeletal elements of the microvillus that bear L-selectin (36) may also contribute to elasticity; we acknowledge this with the designation “tether bond”. Tether bond stress is modeled as decreasing K eq; i.e., a portion increases k off and the remainder decreases k on. Since f κ ⩽ 1, the value for κ/f κ derived here places an upper limit on the tether bond spring constant, κ, of 7.1 N/m. This may be compared to estimates of the elastic spring constant for the EGF–lectin domain unit, since the elastic spring constant for the binding domain will be linked by the binding site dislocation that accompanies domain elongation to the spring constant for the bond dissociation reaction. Domains that fold up independently of one another act independently in elasticity (11). The elastic unit thus is the lectin and EGF domain pair, because they can be expressed independently of the short consensus repeat domains (7, 54). This unit as shown for E-selectin by x-ray crystallography is a cylinder ∼3 nm in diam and 5.2 nm long (18). Because of the high homology to P-and L-selectins, essentially identical dimensions can be safely predicted. Ligand binding to the lectin domain and connection of the EGF domain to the more membrane-proximal short consensus repeats occur on opposite ends of the cylinder, and stretching is therefore along its axis. Using this model, the elastic spring constant can be calculated from the Young's moduli for globular and structural proteins, which are in the range of 1.4–4 × 109 N/m2 (16, 34, 51), to be 2–6 N/m. This estimated elastic spring constant is thus of the same order of magnitude as the upper limit for the tether bond spring constant for L-selectin of 7.1 N/m.
Using the highest F b at which we consistently saw transient tethers, 230 pN, and the range of estimates for the elastic spring constant, the lectin–EGF unit is predicted to be stretched by 0.38–1.15 Å; i.e., the strain (percentage increase in the rest length) would be 0.76–2.3%. This is less than the critical strain of 3–5% observed for globular proteins, i.e., the strain at which denaturation occurs (35). The EGF–lectin domain pair would also be assumed to denature at these strains, and this could contribute to the upper shear threshold for transient tethers.
L-selectin is remarkable for its requirement for shear above a threshold value for adhesive interactions (13). Tensile force could theoretically increase bond lifetime as in a Chinese finger prison (9). Such “catch-bonds” might be theorized to account for the shear threshold phenomenon. However, our data show instead a decrease in bond lifetime at higher shear forces, and therefore rule out this explanation. Several other observations are relevant to the shear threshold phenomenon. We found that transient tethers occur below the shear threshold, and that conversion of transient tethers to rolling adhesions is sharply dependent on shear, with a 10-fold increase in the probability of converting a transient tether to a rolling adhesion between 0.45 and 0.6 dyn/cm2. The frequency of transient tethers also increased from 0.4–0.7 dyn/cm2, by contrast to results on P-selectin, where tethering frequency decreased with increasing shear. Thus, shear appears to promote formation of tether bonds with the substrate, both in the case of the initial transient tether, and more so in the formation of subsequent tethers so that rolling can be supported. Several factors might contribute to the shear threshold. The force on the tether bond can be resolved into two components, one equal to F s, and another at a right angle to this, which will push the cell onto the substrate at the point of contact below the cell's center of mass (Fig. 8 B). This may flatten the cell at the area of contact, and hence enlarge the adhesive zone and promote bond formation (14). Furthermore, shear flow can elongate mucin molecules (30), and this might better expose the carbohydrates that they bear for recognition by selectins.
In conclusion, we have found that L-selectin has a fast k off, and that this is related to its ability to support fast rolling. Rolling through L-selectin is faster than through any other known adhesion molecule. The fast rate of bond dissociation must be compensated by a correspondingly fast overall rate of bond formation. These rapid kinetics may be biologically important for L-selectin's function in the earlier steps of adhesion cascades. We have examined two distinct mechanisms that could account for differences in rolling velocity, and have found that differences between selectins are explained by intrinsic bond kinetics, and not by the mechanical property of reactive compliance. Indeed, k off increases less for L-selectin than for other selectins with increasing shear; this may be important for L-selectin's function in initiating adhesion cascades, and may help compensate for its fast intrinsic k off°. Our measurements on cell–substrate contact parameters have elucidated a number of remarkable features of selectin-mediated rolling, including its ability to be supported by as few as two tether bonds in the zone of adhesive contact.
Acknowledgments
We would like to thank U. von Andrian for providing purified PNAd used in preliminary investigations, R. McEver for purified P-selectin and mAb to PSGL-1, and D. Hammer, M. Dembo, H. Brenner, and A. Nadim for helpful discussion.
This work was supported by a National Institutes of Health grant (HL 48675).
Abbreviations used in this paper
- HSA
human serum albumin
- PNAd
peripheral node addressin
- PSGL-1
P-selectin glycoprotein ligand–1
Footnotes
Please address all correspondence to Timothy A. Springer, The Center for Blood Research and Department of Pathology, Harvard Medical School, 200 Longwood Avenue, Room 251, Boston, MA 02115. Tel.: (617) 278-3200. Fax: (617) 278-3232.
R. Alon's present address is Weizmann Institute of Science, Department of Immunology, 76100 Rehovot, Israel.
References
- 1.Alon R, Hammer DA, Springer TA. Lifetime of the P-selectin; carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature (Lond) 1995;374:539. doi: 10.1038/374539a0. [DOI] [PubMed] [Google Scholar]
- 2.Bargatze RF, Kurk S, Butcher EC, Jutila MA. Neutrophils roll on adherent neutrophils bound to cytokine-induced endothelial cells via L-selectin on the rolling cells. J Exp Med. 1994;180:1785–1792. doi: 10.1084/jem.180.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bargatze RF, Jutila MA, Butcher EC. Distinct roles of L-selectin and integrins α4β7 and LFA-1 in lymphocyte homing to Peyer's patch-HEV in situ: the multistep model confirmed and refined. Immunity. 1995;3:99–108. doi: 10.1016/1074-7613(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 4.Bell GI. Models for the specific adhesion of cells to cells: a theoretical framework for adhesion mediated by reversible bonds between cell surface molecules. Science (Wash DC) 1978;200:618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- 5.Berg EL, Robinson MK, Warnock RA, Butcher EC. The human peripheral lymph node vascular addressin is a ligand for LECAM-1, the peripheral lymph node homing receptor. J Cell Biol. 1991;114:343–349. doi: 10.1083/jcb.114.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg EL, McEvoy LM, Berlin C, Bargatze RF, Butcher EC. L-selectin-mediated lymphocyte rolling on MAdCAM-1. Nature (Lond) 1993;366:695–698. doi: 10.1038/366695a0. [DOI] [PubMed] [Google Scholar]
- 7.Bowen BR, Fennie C, Lasky LA. The Mel 14 antibody binds to the lectin domain of the murine peripheral lymph node homing receptor. J Cell Biol. 1990;110:147–153. doi: 10.1083/jcb.110.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunk DK, Goetz DJ, Hammer DA. Sialyl Lewisx/E-selectin-mediated rolling in a cell-free system. Biophys J. 1996;71:2902–2907. doi: 10.1016/S0006-3495(96)79487-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dembo M, Torney DC, Saxman K, Hammer D. The reaction-limited kinetics of membrane-to-surface adhesion and detachment. Proc R Soc Lond B Biol Sci. 1988;234:55–83. doi: 10.1098/rspb.1988.0038. [DOI] [PubMed] [Google Scholar]
- 10.Dowdy, S., and S. Wearden. 1985. Statistics for Research. John Wiley & Sons, New York. 629 pp.
- 11.Erickson HP. Reversible unfolding of fibronectin type III and immunoglobulin domains provides the structural basis for stretch and elasticity of titin and fibronectin. Proc Natl Acad Sci USA. 1994;91:10114–10118. doi: 10.1073/pnas.91.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feehan C, Darlak K, Kahn J, Walcheck B, Spatola AF, Kishimoto TK. Shedding of the lymphocyte L-selectin adhesion molecule is inhibited by a hydroxamic acid-based protease inhibitor: identification with an L-selectin-alkaline phosphatase reporter. J Biol Chem. 1996;271:7019–7024. doi: 10.1074/jbc.271.12.7019. [DOI] [PubMed] [Google Scholar]
- 13.Finger EB, Puri KD, Alon R, Lawrence MB, von Andrian UH, Springer TA. Adhesion through L-selectin requires a threshold hydrodynamic shear. Nature (Lond) 1996;379:266–269. doi: 10.1038/379266a0. [DOI] [PubMed] [Google Scholar]
- 14.Firrell JC, Lipowsky HH. Leukocyte margination and deformation in mesenteric venules of rat. Am J Physiol. 1989;256:1667–1674. doi: 10.1152/ajpheart.1989.256.6.H1667. [DOI] [PubMed] [Google Scholar]
- 15.Fuhlbrigge RC, Alon R, Puri KD, Lowe JB, Springer TA. Sialylated, fucosylated ligands for L-selectin expressed on leukocytes mediate tethering and rolling adhesions in physiologic flow conditions. J Cell Biol. 1996;135:837–848. doi: 10.1083/jcb.135.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gittes F, Mickey B, Nettleton J, Howard J. Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. J Cell Biol. 1993;120:923–934. doi: 10.1083/jcb.120.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldman AJ, Cox RG, Brenner H. Slow viscous motion of a sphere parallel to a plane wall. II. Couette flow. Chem Engineer Sci. 1967;22:653–660. [Google Scholar]
- 18.Graves BJ, Crowther RL, Chandran C, Rumberger JM, Li S, Huang K-S, Presky DH, Familletti PC, Wolitzky BA, Burns DK. Insight into E-selectin/ligand interaction from the crystal structure and mutagenesis of the lec/EGF domains. Nature (Lond) 1994;367:532–538. doi: 10.1038/367532a0. [DOI] [PubMed] [Google Scholar]
- 19.Hammer DA, Apte SM. Simulation of cell rolling and adhesion on surfaces in shear flow: general results and analysis of selectin- mediated neutrophil adhesion. Biophys J. 1992;63:35–57. doi: 10.1016/S0006-3495(92)81577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haynes BF, Harden EA, Telen MJ, Hemler ME, Strominger JL, Palker TJ, Scearce RM, Eisenbarth GS. Differentiation of human T lymphocytes. I. Acquisition of a novel human cell surface protein (p80) during normal intrathymic T cell maturation. J Immunol. 1983;131:1195–1200. [PubMed] [Google Scholar]
- 21.Hemmerich S, Rosen SD. 6′-Sulfated sialyl Lewis x is a major capping group of GlyCAM-1. Biochemistry. 1994;33:4830–4835. doi: 10.1021/bi00182a011. [DOI] [PubMed] [Google Scholar]
- 22.Hemmerich S, Butcher EC, Rosen SD. Sulfation-dependent recognition of high endothelial venules (HEV)-ligands by L-selectin and MECA 79. J Exp Med. 1994;180:2219–2226. doi: 10.1084/jem.180.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplanski G, Farnarier C, Tissot O, Pierres A, Benoliel A-M, Alessi M-C, Kaplanski S, Bongrand P. Granulocyte-endothelium initial adhesion: analysis of transient binding events mediated by E-selectin in a laminar shear flow. Biophys J. 1993;64:1922–1933. doi: 10.1016/S0006-3495(93)81563-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kishimoto TK, Jutila MA, Butcher EC. Identification of a human peripheral lymph node homing receptor: A rapidly down-regulated adhesion molecule. Proc Natl Acad Sci USA. 1990;87:2244–2248. doi: 10.1073/pnas.87.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 26.Lawrence MB, Springer TA. Neutrophils roll on E-selectin. J Immunol. 1993;151:6338–6346. [PubMed] [Google Scholar]
- 27.Lawrence MB, Bainton DF, Springer TA. Neutrophil tethering to and rolling on E-selectin are separable by requirement for L-selectin. Immunity. 1994;1:137–145. doi: 10.1016/1074-7613(94)90107-4. [DOI] [PubMed] [Google Scholar]
- 28.Lawrence MB, Berg EL, Butcher EC, Springer TA. Rolling of lymphocytes and neutrophils on peripheral node addressin and subsequent arrest on ICAM-1 in shear flow. Eur J Immunol. 1995;25:1025–1031. doi: 10.1002/eji.1830250425. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence MB, Kansas GS, Kunkel EJ, Ley K. Threshold levels of fluid shear promote leukocyte adhesion through selectins (CD62L,P,E) J Cell Biol. 1997;136:717–727. doi: 10.1083/jcb.136.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li F, Erickson HP, James JA, Moore KL, Cummings RD, McEver RP. Visualization of P-selectin glycoprotein ligand-1 as a highly extended molecule and mapping of protein epitopes for monoclonal antibodies. J Biol Chem. 1996;271:6342–6348. doi: 10.1074/jbc.271.11.6342. [DOI] [PubMed] [Google Scholar]
- 31.McEver RP, Moore KL, Cummings RD. Leukocyte trafficking mediated by selectin-carbohydrate interactions. J Biol Chem. 1995;270:11025–11028. doi: 10.1074/jbc.270.19.11025. [DOI] [PubMed] [Google Scholar]
- 32.Miller LJ, Bainton DF, Borregaard N, Springer TA. Stimulated mobilization of monocyte Mac-1 and p150,95 adhesion proteins from an intracellular vesicular compartment to the cell surface. J Clin Invest. 1987;80:535–544. doi: 10.1172/JCI113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore KL, Patel KD, Bruehl RE, Fugang L, Johnson DA, Lichenstein HS, Cummings RD, Bainton DF, McEver RP. P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J Cell Biol. 1995;128:661–671. doi: 10.1083/jcb.128.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morozov VN, Morozova TY. What does a protein molecule look like? . Comments Mol Cell Biophys. 1990;6:249–270. [Google Scholar]
- 35.Morozov VN, Morozova TY. Elasticity of globular proteins. The relation between mechanics, thermodynamics and mobility. J Biomol Struct Dyn. 1993;11:459–481. doi: 10.1080/07391102.1993.10508010. [DOI] [PubMed] [Google Scholar]
- 36.Picker LJ, Warnock RA, Burns AR, Doerschuk CM, Berg EL, Butcher EC. The neutrophil selectin LECAM-1 presents carbohydrate ligands to the vascular selectins ELAM-1 and GMP-140. Cell. 1991;66:921–933. doi: 10.1016/0092-8674(91)90438-5. [DOI] [PubMed] [Google Scholar]
- 37.Pouyani T, Seed B. PSGL-1 recognition of P-selectin is controlled by a tyrosine sulfation consensus at the PSGL-1 amino terminus. Cell. 1995;83:333–343. doi: 10.1016/0092-8674(95)90174-4. [DOI] [PubMed] [Google Scholar]
- 38.Preece G, Murphy G, Ager A. Metalloproteinase-mediated regulation of L-selectin levels on leucocytes. J Biol Chem. 1996;271:11634–11640. doi: 10.1074/jbc.271.20.11634. [DOI] [PubMed] [Google Scholar]
- 39.Puri KD, Finger EB, Gaudernack G, Springer TA. Sialomucin CD34 is the major L-selectin ligand in human tonsil high endothelial venules. J Cell Biol. 1995;131:261–270. doi: 10.1083/jcb.131.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puri KD, Finger EB, Springer TA. The faster kinetics of L-selectin than E-selectin and P-selectin rolling at comparable binding strength. J Immunol. 1997;158:405–413. [PubMed] [Google Scholar]
- 41.Rochon YP, Simon SI, Lynam EB, Sklar LA. A role for lectin interactions during human neutrophil aggregation. J Immunol. 1994;152:1385–1393. [PubMed] [Google Scholar]
- 42.Rosen SD, Bertozzi CR. The selectins and their ligands. Curr Opin Cell Biol. 1994;6:663–673. doi: 10.1016/0955-0674(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 43.Sako D, Chang X-J, Barone KM, Vachino G, White HM, Shaw G, Veldman GM, Bean KM, Ahern TJ, Furie B, et al. Expression cloning of a functional glycoprotein ligand for P-selectin. Cell. 1993;75:1179–1186. doi: 10.1016/0092-8674(93)90327-m. [DOI] [PubMed] [Google Scholar]
- 44.Sako D, Comess KM, Barone KM, Camphausen RT, Cumming DA, Shaw GD. A sulfated peptide segment at the amino terminus of PSGL-1 is critical for P-selectin binding. Cell. 1995;83:323–331. doi: 10.1016/0092-8674(95)90173-6. [DOI] [PubMed] [Google Scholar]
- 45.Shao J-Y, Hochmuth RM. Micropipette suction for measuring piconewton forces of adhesion and tether formation from neutrophil membranes. Biophys J. 1996;71:2892–2901. doi: 10.1016/S0006-3495(96)79486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 47.Streeter PR, Rouse BTN, Butcher EC. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol. 1988;107:1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ting-Beall HP, Needham D, Hochmuth RM. Volume and osmotic properties of human neutrophils. Blood. 1993;81:2774–2780. [PubMed] [Google Scholar]
- 49.Tözeren A, Ley K. How do selectins mediate leukocyte rolling in venules? . Biophys J. 1992;63:700–709. doi: 10.1016/S0006-3495(92)81660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Andrian UH, Chambers JD, McEvoy LM, Bargatze RF, Arfors KE, Butcher EC. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte β2 integrins in vivo. . Proc Natl Acad Sci USA. 1991;88:7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wainwright, S.A., W.D. Biggs, J.D. Currey, and J.M. Gosline. 1976. Mechanical Design in Organisms. Edward Arnold, Ltd., London. 423 pp.
- 52.Walcheck B, Kahn J, Fisher JM, Wang BB, Fisk RS, Payan DG, Feehan C, Betageri R, Darlak K, Spatola AF, et al. Neutrophil rolling altered by inhibition of L-selectin shedding in vitro. Nature (Lond) 1996;380:720–723. doi: 10.1038/380720a0. [DOI] [PubMed] [Google Scholar]
- 53.Walcheck B, Moore KL, McEver RP, Kishimoto TK. Neutrophil-neutrophil interactions under hydrodynamic shear stress involve L-selectin and PSGL-1. A mechanism that amplifies initial leukocyte accumulation on P-selectin in vitro. J Clin Invest. 1996;98:1081–1087. doi: 10.1172/JCI118888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walz G, Aruffo A, Kolanus W, Bevilacqua M, Seed B. Recognition by ELAM-1 of the sialyl-Lewisxdeterminant on myeloid and tumor cells. Science (Wash DC) 1990;250:1132–1135. doi: 10.1126/science.1701275. [DOI] [PubMed] [Google Scholar]
- 55.Wilkins PP, Moore KL, McEver RP, Cummings RD. Tyrosine sulfation of P-selectin glycoprotein ligand-1 is required for high affinity binding to P-selectin. J Biol Chem. 1995;270:22677–22680. doi: 10.1074/jbc.270.39.22677. [DOI] [PubMed] [Google Scholar]