Abstract
Chondroadherin (the 36-kD protein) is a leucine-rich, cartilage matrix protein known to mediate adhesion of isolated chondrocytes. In the present study we investigated cell surface proteins involved in the interaction of cells with chondroadherin in cell adhesion and by affinity purification. Adhesion of bovine articular chondrocytes to chondroadherin-coated dishes was dependent on Mg2+ or Mn2+ but not Ca2+. Adhesion was partially inhibited by an antibody recognizing β1 integrin subunit. Chondroadherin-binding proteins from chondrocyte lysates were affinity purified on chondroadherin-Sepharose. The β1 integrin antibody immunoprecipitated two proteins with molecular mass ∼110 and 140 kD (nonreduced) from the EDTA-eluted material. These results indicate that a β1 integrin on chondrocytes interacts with chondroadherin. To identify the α integrin subunit(s) involved in interaction of cells with the protein, we affinity purified chondroadherin-binding membrane proteins from human fibroblasts. Immunoprecipitation of the EDTA-eluted material from the affinity column identified α2β1 as a chondroadherin-binding integrin. These results are in agreement with cell adhesion experiments where antibodies against the integrin subunit α2 partially inhibited adhesion of human fibroblast and human chondrocytes to chondroadherin. Since α2β1 also is a receptor for collagen type II, we tested the ability of different antibodies against the α2 subunit to inhibit adhesion of T47D cells to collagen type II and chondroadherin. The results suggested that adhesion to collagen type II and chondroadherin involves similar or nearby sites on the α2β1 integrin. Although α2β1 is a receptor for both collagen type II and chondroadherin, only adhesion of cells to collagen type II was found to mediate spreading.
The cartilage extracellular matrix is highly specialized in its composition and organization to adapt to and withstand mechanical forces. A number of the matrix molecules are found predominantly or exclusively in cartilage (20). The major matrix components are collagens and proteoglycans (19), with collagen type II representing ∼95% of the collagens (11) and aggrecan ∼95% of the proteoglycans (16). Collagen type II fibers provide tensile strength to the tissue, whereas aggrecan, bound to hyaluronan, provides resilience. The interplay between these molecules is essential for cartilage function (33). Several other matrix components are involved in maintaining the specific cartilage properties, where some have primarily structural roles and others are associated with the chondrocytes and are likely to be involved in monitoring matrix properties and mediating signals to the cells (20). The chondrocytes, being the only type of cell in cartilage, have a key function in cartilage homeostasis. Their roles include controlling normal turnover of matrix molecules, depositing molecules into a functioning matrix, and responding to alterations in load with appropriate remodeling.
Chondroadherin (CHAD)1, originally described as a 36-kD protein, is a prominent noncollagenous extracellular protein in cartilage (31). Although the protein has been detected in extracts from cartilage and bone (31), recent data show very low expression of CHAD mRNA in bone while it is prominently expressed in certain zones of cartilage in young rats (Shen, Z., D. Heinegård, and Y. Sommarin, unpublished results). CHAD contains only a short oligosaccaride lacking sialic acid and hexosamines on serine 122 (31, 35). More recently its sequence was determined, both at the protein and cDNA level, showing that CHAD is a unique member of the leucine-rich repeat (LRR) protein family (35). Other members of this diverse family include the small cartilage proteoglycans biglycan (12), decorin (28), fibromodulin (36), lumican (2), and keratocan (6), as well as PRELP (1).
It has been shown earlier that isolated chondrocytes adhere to chondroadherin immobilized on plastic culture dishes (44) indicating that one function of this protein is to mediate interactions between the chondrocytes and the extracellular matrix. Fibroblasts and osteoblasts also adhered to CHAD (44), suggesting that a cell surface protein common to several cell types may be the receptor for the protein.
Integrins, a family of membrane glycoproteins, are of prime importance for adhesion of most cells to extracellular matrix proteins (22, 25, 37). They consist of two subunits, α and β, where the extracellular domain of the α subunits has several divalent, cation-binding sites. The integrins α1β1, α2β1, α3β1, α5β1, and α6β1 αvβ3 and αvβ5 have been found on chondrocytes (8, 50; Holmvall, K., L. Camper, and E. Lundgren-Åkerlund, unpublished results), but their ligands in cartilage have not been fully defined. Integrins α1β1 and α2β1 have been found to mediate binding to collagen type II (8, 24) and α5β1 mediates binding to fibronectin (38). In the present study we investigated the interaction of cells with the cartilage matrix protein CHAD to identify the cellular receptor that is involved.
Materials and Methods
Antibodies
Monoclonal antibodies against the human integrin subunits β1 (P4C10), α2 (P1E6), α3 (P1B5), α5 (P1D6), and αv (VNR147) (unpurified ascites fluid) were from Life Technologies Inc. (Grand Island, NY). Monoclonal antibody against the human integrin β3 (RUU-PLF12, purified IgG) were purchased from Becton Dickinson (Bedford, MA). Monoclonal antibodies against the human integrins αvβ5 (P1F6) and αvβ3 (LM609) (purified IgG) were from Chemicon International, Inc. (Temecula, CA). The monoclonal antibodies against the human integrin subunits α1 (TS2/7; hybridoma supernatant) and α2 (P1H5; hybridoma supernatant) and rabbit polyclonal antibodies against rat β1 integrin were kind gifts from Drs. William Carter, (Fred Hutchinson Cancer Research Center, Seattle, WA; 3), Timothy Springer (Boston Blood Center, Boston, MA; 23), and Kristofer Rubin (Uppsala University, Uppsala, Sweden; 15), respectively. The monoclonal antibodies Gi9, Gi14, Gi19, and Gi26 (hybridoma supernatant), recognizing human α2 integrin subunit, were kind gifts from Dr. Sentot Santoso (Justus-Liebig University, Giessen, Germany; 39).
Cell Isolation and Culture
Bovine chondrocytes were isolated by collagenase (CLS1; Worthington Biochemical Corp., Lakewood, NJ) digestion of articular cartilage from 4–6-month old calves as described elsewhere (43). Briefly, cartilage slices were digested by collagenase in EBSS (Earle's balanced salt solution; GIBCO BRL, Gaithersburg, MD) for 15–16 h at 37°C. The cells were filtered through a 100 μm nylon filter, washed three times in Dulbecco's modified PBS (GIBCO BRL), and used immediately after isolation. Human chondrocytes from knee joint cartilage were isolated by pronase (Calbiochem, La Jolla, CA) digestion for 1 h followed by collagenase (Boehringer Mannheim, Indianapolis, IN) digestion for 15–18 h as described by Häuselmann et al. (17). The cells were filtered and washed as described above. Human lung carcinoma fibroblasts (A549) and human mammary tumor cells (T47D) obtained from the American Type Culture Collection (Rockville, MD) were cultured in Dulbecco's minimal essential medium (DMEM) supplemented with 10% fetal calf serum, 50 UI penicillin, and 50 μg/ml streptomycin (GIBCO BRL). Human chondrocytes were cultured in DMEM and F12 (1:1) supplemented with 10% fetal calf serum, 25 μg/ml ascorbic acid, 50 UI penicillin, and 50 μg/ml streptomycin (GIBCO BRL). To harvest cells, the culture dish was washed three times with Ca/Mg-free PBS, and the cells were incubated with 0.5% trypsin and 1 mM EDTA (GIBCO BRL) in PBS (−Ca and Mg) for 5 min. Detached cells were suspended in medium containing 10% FCS or in PBS containing 1 mg/ml trypsin inhibitor (Sigma Chemical Co., St. Louis, MO) and then washed in PBS.
Cell Adhesion
Tissue culture-treated, 48-well dishes (Nunclon, Nunc, Denmark) were coated overnight at room temperature with 5 or 10 μg/ml CHAD in 4M guanidine-HCl, 50 mM Tris-HCl, pH 7.6, or 10 μg/ml collagen type II (CII) in PBS and blocked with 0.25% BSA (Serva Feinbiochemica, Wichita Falls, TX; Sigma Chemical Co.) in PBS. Collagen type II was isolated from nasal cartilage by pepsin digestion (34). The dishes were rinsed four times with PBS before the experiment. When the effects of divalent cations were studied, the cells were washed three times in Ca/Mg-free PBS and resuspended in the same buffer supplemented either with 1 mM CaCl2, 1 mM MgCl2, 50 μM MnCl2, 1 mM of both CaCl2 and MgCl2 or supplemented with all the divalent cations. The cells suspended in PBS containing 0.1% BSA were added to the wells at a concentration of 100,000/well of bovine chondrocytes and 50,000/well of human chondrocytes, A549, or T47D-cells. Cells were allowed to adhere for 1 h at 37°C. When the effect of antibodies on adhesion was investigated, the cells were suspended in PBS (+Ca and Mg) and incubated with antibodies for 20 min at room temperature before plating of the cells. The monoclonal antibodies β1, α2 (P1E6), α3, α5, αv, and αvβ5 (unpurified ascites fluid) were diluted 1:100, P1H5 (hybridoma supernatant) was diluted 1:25, and Gi9, Gi14, Gi19, and Gi26 (hybridoma supernatant) were diluted 1:10. The monoclonal antibodies β3 and αvβ3 were used at a concentration of 10 μg/ml. After 1 h of incubation the wells were gently rinsed with PBS to remove nonadherent cells. Adhesion was determined by measuring lysosomal hexosaminidase as described by Landegren (29).
Cell Spreading
Chamber slides (8 chamber; Lab-Tek®, Nunc Inc., Naperville, IL) were coated with 5 μg/ml of CHAD in 4M guanidine-HCl, 50 mM Tris-HCl, pH 7.5, or 5 μg/ml of collagen type II in PBS and blocked with 0.25% BSA in PBS. T47D-cells (20,000/well) were added to the chambers, allowed to adhere, and spread for 3 h at 37°C in the absence or in the presence of 10−8 M phorbol 12-myristate 13-acetate (PMA; Sigma Chemical Co.). Nonadherent cells were removed by washing, and the adherent cells were fixed with 2% paraformaldehyde in PBS and stained with Mayers's hematoxylin and 0.3% erythrosin. Spreading was visualized by light microscopy, and mean cell area was calculated by image analysis using the Zeiss Software KS400/V2.00 (Zeiss, Inc., Oberkochen, Germany).
Surface Labeling with 125I
Bovine chondrocytes or human lung carcinoma fibroblasts A549 were suspended in 1 ml of PBS containing 1 mg/ml glucose. 125I (1 mCi; Nordion Inc., Kanata, ON, Canada) was added to the cells together with 4 U of lactoperoxidase (Sigma Chemical Co.; 120 U/mg) and 0.05 U of glucose oxidase (Sigma Chemical Co.; 1010 U/ml) prepared fresh in PBS-glucose. The cells were kept on ice for 15 min, whereafter the reaction was stopped by adding 10 ml of Dulbecco's culture medium. The cells were then washed three times with PBS and lysed for 1h on ice in 2 ml of 1% Triton X-100, 100 μg/ml aprotinin, 2 μg/ml leupeptin, 2 μg/ml pepstatin A, 1 mM PMSF (Sigma Chemical Co.), 1 mM MnCl2, 1 mM MgCl2 in 10 mM Tris-HCl, pH 7.4. Cell lysates were centrifuged at 10,000 rpm for 30 min at 4°C, and the pellets were discarded.
Isolation and Coupling of CHAD to Agarose
CHAD was purified from bovine tracheal cartilage essentially according to the published procedure (31). For coupling, CHAD (2.5 mg) was solubilized in 0.5% SDS and coupled to 2 ml of Mini-Leak agarose (Biocarb Chemicals, Lund, Sweden) according to the manufacturer's instructions. The control agarose was treated in a similar manner but in the absence of protein.
Affinity Purification of CHAD-binding Protein
The CHAD agarose (0.5 ml) and the control agarose (0.5 ml) were packed in mini-columns (Bio Rad, Hercules, CA) and equilibrated with at least 20 vol of 0.1% Triton X-100, 10 mM Tris-HCl, pH 7.4, 1 mM MnCl2, and 1 mM MgCl2. The lysates from the 125I-labeled cells were passed over the control agarose two times and then incubated with the CHAD agarose in the mini-columns for 2–3 h with continuous end-over-end mixing. The CHAD agarose was washed with 15 vol of the equilibration buffer containing 75 mM NaCl, and the column was then eluted with 20 mM EDTA, 1 mM PMSF, 10 mM Tris-HCl, pH 7.4. The eluted protein peak was passed over a desalting column (PD-10; Pharmacia Fine Chemicals, Uppsala, Sweden) equilibrated with 50 mM Tris, pH 7.4, 0.3 M NaCl, 1% Triton X-100, 0.1% BSA, 1 mM CaCl2, 1 mM MgCl2, and 1 mM PMSF. Samples of the affinity-purified proteins were then either precipitated by methanol/chloroform (48) or immunoprecipitated by antibodies followed by separation on 4–12% SDS-PAGE and visualized by autoradiography or image analysis using the BioImaging Analyzer Bas2000 (Fuji Photo Film Co., Tokyo, Japan).
Immunoprecipitation
Radiolabeled proteins were immunoprecipitated from cell lysate and from affinity-purified material. In experiments where lysates were immunoprecipitated they were passed over a desalting column (PD-10; Pharmacia Fine Chemicals) equilibrated and eluted with 50 mM Tris, pH 7.4, 0.3 M NaCl, 1% Triton X-100, 0.1% BSA, 1 mM CaCl2, 1 mM MgCl2, 1 mM PMSF. The cell lysate or the affinity-purified samples were incubated with continuous end-over-end mixing overnight with 5 μl/ml of monoclonal antibodies (β1, β3, α1, α2 (P1E6), α3, α5, αv, and αvβ5) or 50 μg/ml of the polyclonal antibody (β1) followed by addition of 75 μl anti–mouse IgG-agarose (Sigma Chemical Co.) or 100 μl protein A-Sepharose (Pharmacia Fine Chemicals) and incubation for 2 h. The beads were centrifuged for 4 min at 4,000 rpm, washed three times with 1% Triton X-100, 0.5 M NaCl, and 10 mM Tris-HCl, pH 7.4. All steps were performed at 4°C. SDS-PAGE sample buffer (100 μl) was added to the washed immunoprecipitates, and the samples were boiled for 5 min with or without 2-mercaptoethanol (5%). The immunoprecipitated proteins were separated by SDS-PAGE (4–12%) and visualized by autoradiography or by using the phosphoimager.
Statistics
Results are presented as means ± SD. Student's t test was used to determine statistical significance.
Results
Adhesion of Cells to Chondroadherin
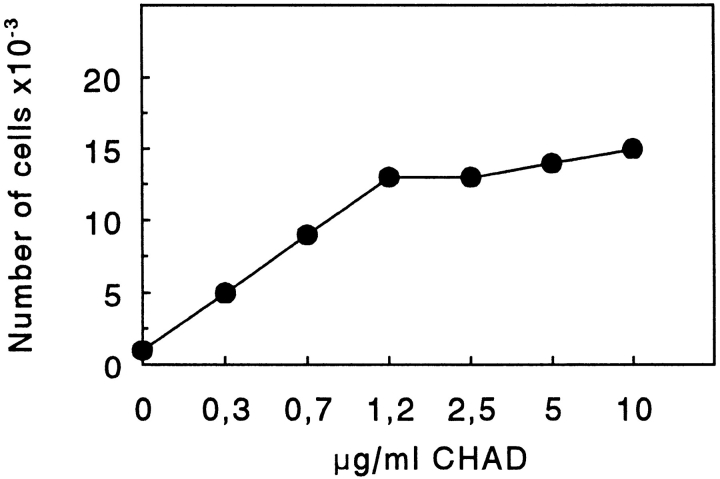
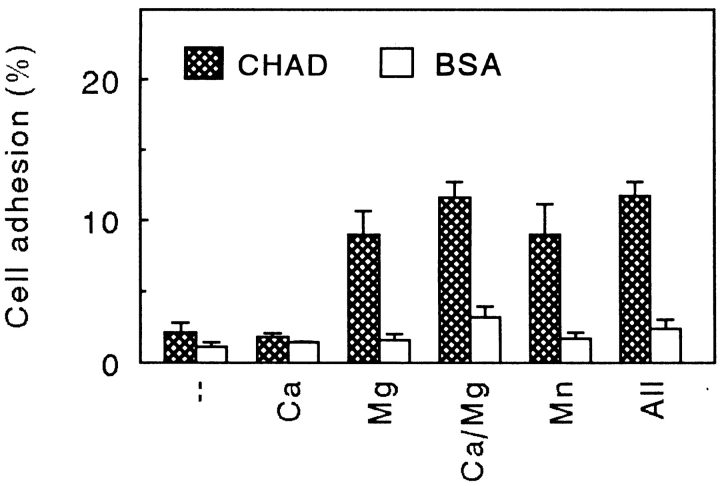
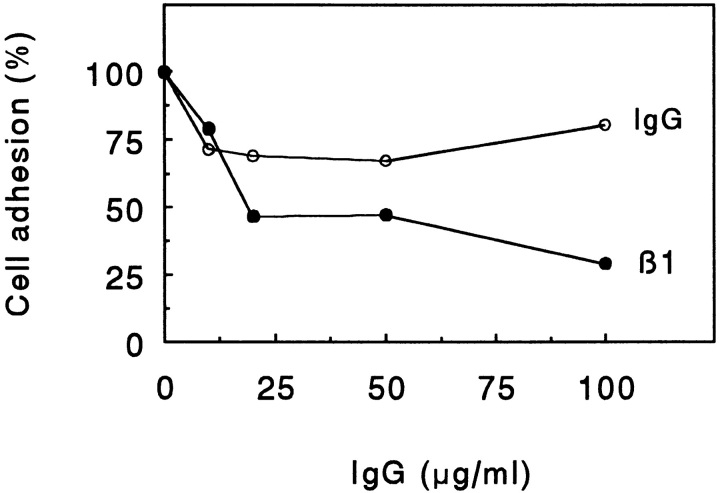
CHAD, immobilized on culture dishes, mediated adhesion of cells in a dose-dependent manner (Fig. 1). Maximal adhesion was seen at a coating concentration of 1.2 μg/ml. Adhesion of bovine chondrocytes to CHAD was dependent on divalent cations such that Mg2+ or Mn2+ but not Ca2+ was required (Fig. 2). Only a low number of cells adhered to the control BSA (Fig. 2). The adhesion to CHAD decreased by 2/3 in the presence of 100 μg/ml of a polyclonal rat β1 integrin antibody compared to adhesion in the absence of antibody (Fig. 3). This indicated that β1 integrins are involved in the adhesion of chondrocytes to CHAD. The control antibody had only a minor effect on the adhesion.
Figure 1.
Adhesion of T47D cells to dishes coated with CHAD. Culture dishes (48 well) were coated with various concentrations of CHAD and blocked for nonspecific binding with BSA (0.25%). T47D cells (50,000/well) were allowed to adhere for 1 h at 37°C. Nonadherent cells were removed by washing, and adhesion was determined by analyzing lysosomal hexosaminidase. The results presented are the mean adhesion in duplicates from one of two experiments.
Figure 2.
Divalent cation-dependent adhesion of chondrocytes to CHAD. Culture dishes (48 well) were coated with CHAD (5 μg/ ml) and blocked for nonspecific binding with BSA (0.25%). Bovine chondrocytes were allowed to adhere to the dishes for 1 h at 37°C in the absence of divalent cations or in the presence of Ca2+ (1 mM), Mg2+ (1 mM), or Mn2+ (50 μM). Nonadherent cells were removed by washing, and adhesion was determined by analyzing lysosomal hexosaminidase. Adhesion is expressed as a percentage of the total number of cells added to the dish. The numbers represent the mean adhesion from three wells ±SD from one of three experiments.
Figure 3.
β1 integrin-dependent adhesion of chondrocytes to CHAD. Culture dishes (48 well) were coated overnight with chondroadherin (5 μg/ml) and blocked for nonspecific binding with BSA (0.25%). Bovine chondrocytes were allowed to adhere to the dishes for 1 h at 37°C in the presence of various concentrations of a polyclonal antibody against the rat β1 integrin subunit or control IgG. Nonadherent cells were removed by washing, and adhesion was determined by analyzing lysosomal hexosaminidase. The adhesion is expressed as a percentage of the control, and the numbers represent mean of duplicate adhesion from one of three experiments.
Affinity Chromatography of CHAD-binding Cell Surface Proteins
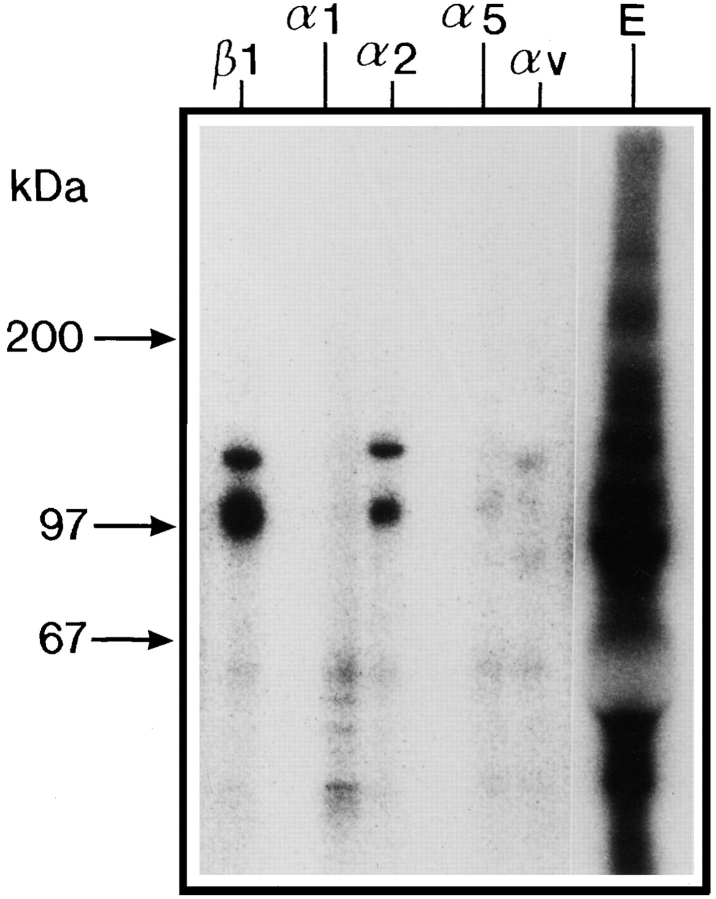
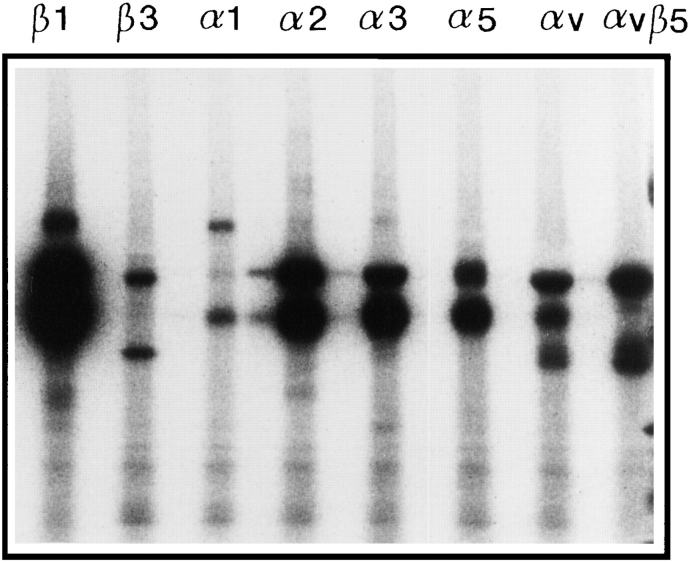
To identify integrins with affinity for CHAD, Triton X-100– solubilized, 125I-labeled cell surface proteins from bovine chondrocytes were affinity purified on CHAD coupled to agarose. As shown in Fig. 4, two proteins with molecular weight ∼110 and 140 kD (nonreduced) were eluted from the CHAD column with EDTA. These proteins were immunoprecipitated with a polyclonal antibody against β1 integrin. As shown in the figure, this β1 integrin showed two bands migrating corresponding to 120 (β1 chain) and 150 kD (α chain) upon SDS-PAGE under reducing conditions. In addition, a protein band with mobility corresponding to 100 kD was found, which may represent a degradation product of the β1 integrin. We were not able to further identify the α chain from the chondrocyte integrin, since available antibodies against human integrins showed too low cross-reactivity to bovine integrins. To identify the β1-associated α chain with affinity for CHAD, Triton X-100–solubilized, 125I-labeled cell surface proteins from human fibroblasts were affinity purified on the CHAD column. Proteins eluted from the CHAD affinity purification experiments were immunoprecipitated with monoclonal antibodies against the human integrin subunits β1, α1, α2, α5, and αv. Fig. 5 shows that the antibodies against the integrin subunits β1 and α2 immunoprecipitated an integrin dimer of similar appearance, while antibodies against α1, α5, and αv did not specifically immunoprecipitate integrins from the EDTA eluate. In a control experiment (Fig. 6) it was shown that these cells express a number of different integrins. Taken together, these results strongly indicate that the integrin α2β1 is a receptor for CHAD.
Figure 4.
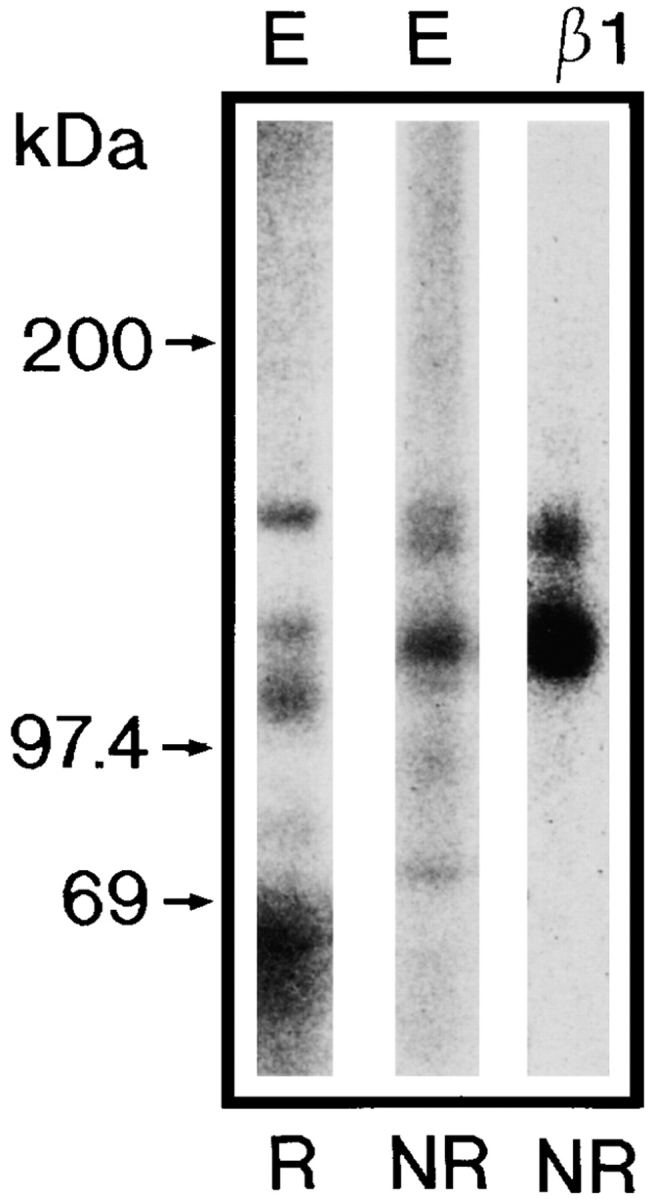
Affinity purification of CHAD-binding cell surface proteins. Bovine chondrocytes were 125I-labeled and lysed with 1% Triton X-100, 100 μg/ml aprotinin, 2 μg/ml leupeptin, 2 μg/ml pepstatin A, 1 mM PMSF, 1 mM MnCl2, and 1 mM MgCl2 in 10 mM Tris-HCl, pH 7.4. The lysate was passed over control agarose followed by CHAD agarose. Proteins with affinity for CHAD were eluted by EDTA (20 mM), passed over a desalting column (PD-10) equilibrated, and eluted with 0.3 M NaCl, 1% Triton X-100, 0.1% BSA 1 mM CaCl2, 1 mM MgCl2, 1 mM PMSF, in 50 mM Tris-HCl, pH 7.4. An aliquot of the protein peak was immunoprecipitated with the polyclonal rat β1 integrin antibody. Proteins in the eluate (E) and in the immunoprecipitate (β1) were separated by 4–12% SDS-PAGE under reducing (R) or nonreducing (NR) conditions.
Figure 5.
Immunoprecipitation of CHAD-binding integrins from human fibroblasts. 125I-labeled A549 fibroblasts were lysed with 1% Triton X-100, 100 μg/ml aprotinin, 2 μg/ml leupeptin, 2 μg/ml pepstatin A, 1 mM PMSF, 1 mM MnCl2, and 1 mM MgCl2 in 10 mM Tris-HCl, pH 7.4. The lysate was passed over control agarose followed by CHAD agarose. Proteins with affinity for CHAD were eluted by EDTA (20 mM), passed over a desalting column (PD-10) equilibrated, and eluted with 0.3 M NaCl, 1% Triton X-100, 0.1% BSA, 1 mM CaCl2, 1 mM MgCl2, 1 mM PMSF in 50 mM Tris-HCl, pH 7.4. Aliquots of the protein peak were immunoprecipitated with monoclonal antibodies against the integrin subunits β1 (P4C10), α1 (TS2/7), α2 (P1E6), α5 (P1D6), and αv (VNR147). The immunoprecipitated proteins were separated by SDS-PAGE (4–12%) under nonreducing conditions and visualized by autoradiography.
Figure 6.
Immunoprecipitation of integrins from human fibroblasts. 125I-labeled A549 fibroblasts were lysed with 1% Triton X-100, 100 μg/ml aprotinin, 2 μg/ml leupeptin, 2 μg/ml pepstatin A, 1 mM PMSF, 1 mM MnCl2, and 1 mM MgCl2 in 10 mM Tris-HCl, pH 7.4. Aliquots of the lysate were immunoprecipitated with monoclonal antibodies against the integrin subunits β1 (P4C10), β3 (RUU-PLF12), α1 (TS2/7), α2 (P1E6), α3 (P1B5), α5 (P1D6), αv (VNR147), and αvβ5 (P1F6). The immunoprecipitated proteins were separated by SDS-PAGE (4–12%) under nonreducing conditions and visualized by autoradiography.
Inhibition of Cell Adhesion to CHAD by Integrin Antibodies
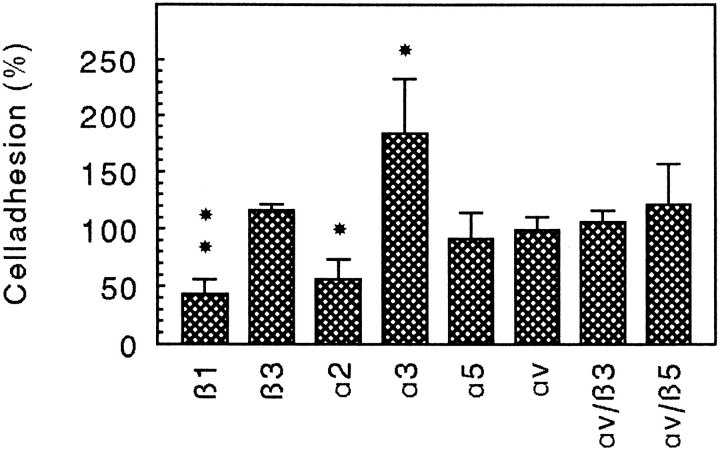
Fibroblasts were adhered to CHAD in cell adhesion experiments in the presence of antibodies against various integrin subunits. As shown in Fig. 7 antibodies against the α2 or β1 integrin subunits inhibited the cell adhesion to >50% while antibodies against β3, α5, αv, αvβ3, or αvβ5 had no or only a minor effect on the adhesion. In contrast to the other antibodies, the α3 antibody stimulated the adhesion of fibroblasts to CHAD. In agreement with the affinity purification experiments, these results show that α2β1 is a CHAD-binding integrin.
Figure 7.
Inhibition of fibroblast adhesion to CHAD by integrin antibodies. Culture dishes (48 well) were coated with CHAD (5 μg/ml) and blocked for nonspecific binding with BSA (0.25%). Human A549 fibroblasts were allowed to adhere to the dishes for 1 h at 37°C in the presence of monoclonal antibodies against the human integrin subunits β1 (P4C10), β3 (RUU-PLF12), α2 (Gi9), α3 (P1B5), α5 (P1D6), αv (VNR147), αvβ3 (LM609), and αvβ5 (P1F6). Nonadherent cells were removed by washing, and adhesion was determined by analyzing lysosomal hexosaminidase. The adhesion is expressed as a percentage of the control, and the numbers represent the mean of duplicate adhesion from three individual experiments ±SD. *P < 0.05; **P < 0.01; P β1 = 0.002; P α2 = 0.011; P α3 = 0.021.
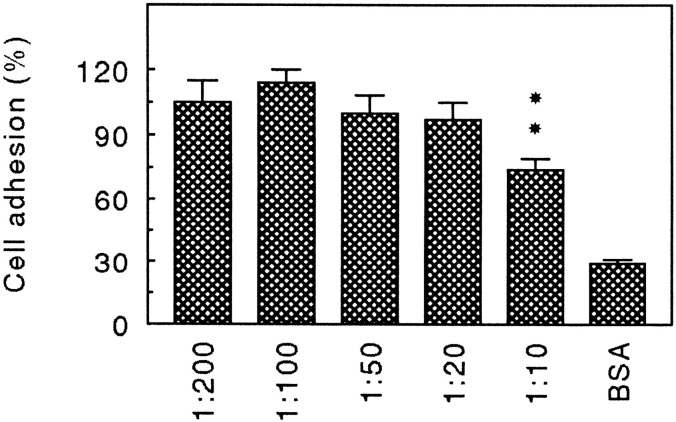
To study whether α2β1 is involved in the adhesion of chondrocytes to CHAD, human chondrocytes were adhered to immobilized CHAD in the absence or in the presence of an antibody against the integrin subunit α2. The antibody partially inhibited the adhesion of chondrocytes in a dose-dependent manner (Fig. 8). Around 30% of the adhesion was inhibited at the highest antibody concentration. This result confirmed that α2β1 is a CHAD-binding integrin on chondrocytes.
Figure 8.
Inhibition of human chondrocyte adhesion to CHAD by α2 integrin antibodies. Culture dishes (48 well) were coated with CHAD (5 μg/ml) and blocked for nonspecific binding with BSA (0.25%). Human chondrocytes were allowed to adhere to the dishes for 1 h at 37°C in the presence of various concentrations of the monoclonal antibody against the human integrin subunit α2 (Gi9). Nonadherent cells were removed by washing, and adhesion was determined by analyzing lysosomal hexosaminidase. The adhesion is expressed as a percentage of the control, and the numbers represent the mean adhesion ±SD from three wells in one of two experiments.
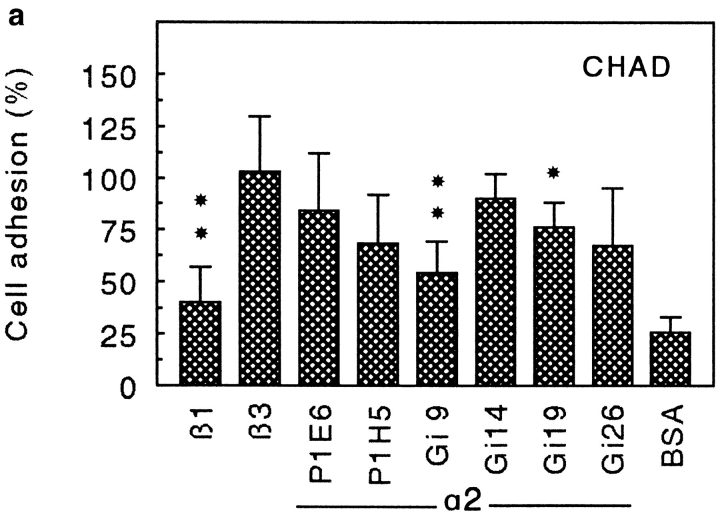
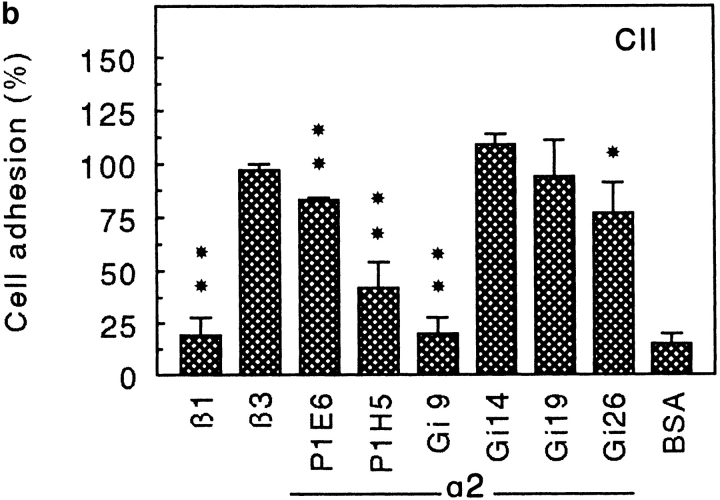
Since α2β1 is a receptor for both collagen type II (24) and CHAD, we investigated the interaction of T47D-cells (cells that express the α2 but not the α1 subunit) with these two substrates, using various antibodies to the α2 integrin subunit (Fig. 9). The α2 antibodies inhibited cell adhesion to collagen type II and CHAD in a similar manner, although they were somewhat less effective in the CHAD experiment. Higher concentrations of the antibodies did not change the inhibition pattern (data not shown). This indicates that similar or nearby sites on the α2β1 integrin are binding to the two substrates.
Figure 9.
Inhibition of adhesion of T47D cells to CHAD (a) and to collagen type II (CII; b) by various α2 antibodies. Culture dishes (48 wells) were coated with 5 μg/ml of CHAD or CII and blocked for nonspecific binding with BSA (0.25%). T47D cells were allowed to adhere to the dishes for 1 h at 37°C in the absence or in the presence of monoclonal antibodies against the integrin subunits β1 (P4C10), β3 (RUU-PLF12), or various α2 antibodies. Nonadherent cells were removed by washing, and adhesion was determined by analyzing lysosomal hexosaminidase. The adhesion is expressed as a percentage of the control, and the numbers represent the mean of duplicate adhesion from three individual experiments ±SD. *P < 0.05; **P < 0.01; (a) Pβ1 = 0.004; PGi9 = 0.006; PGi19 = 0.026. (b) Pβ1 = 0.000; PP1E6 = 0.001; PP1H5 = 0.001; PGi9 = 0.000; PGi26 = 0.047.
Spreading of Cells on Collagen Type II or CHAD
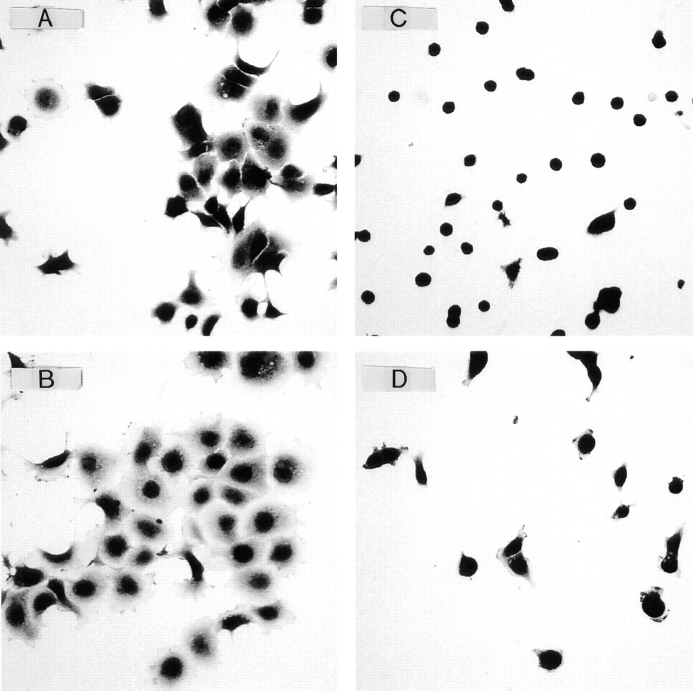
It has earlier been shown (44) that chondrocytes adhered to CHAD appeared to stay round, while chondrocytes immobilized on collagen type II spread on the substratum. We found, similarly, that T47D cells (Fig. 10 and Table I) and fibroblasts (data not shown) spread when they were adhered to collagen type II but not to CHAD. The average cell area of T47D cells that were adhered to CHAD for 3 h was ∼2/3 of those adhered to collagen type II (Table I). Addition of PMA (10−8 M) to the adhered cells stimulated spreading and increased the cell area with ∼40% on both CHAD and collagen type II (Fig. 10 and Table I).
Figure 10.
Spreading of T47D cells on collagen type II (CII) or CHAD. Chamber slides (eight chambers) were coated with 5 μg/ml of CII (A and B) or CHAD (C and D) and blocked for nonspecific binding with BSA (0.25%). Human T47D cells (20,000/ well) were plated onto the chambers and allowed to adhere and spread for 3 h at 37°C in the absence (A and C) or in the presence (B and D) of 10−8 M PMA. Nonadherent cells were removed by washing, and the adherent cells were fixed with 2% paraformaldehyde in PBS and stained with Meyer's hematoxilin and erythrosin. Spreading was visualized by light microscopy, and mean cell area (Table I) was calculated by image analyses using the Zeiss software KS400/ V2.00.
Table I.
Spreading of T47D Cells on CII or CHAD in the Absence or in the Presence of PMA
| CII | CHAD | |||
|---|---|---|---|---|
| − PMA | 123 ± 20 | 83 ± 8 | ||
| + PMA | 208 ± 38 | 140 ± 10 |
The numbers represent mean cell area (μm2) of cells ± SD (n = 4).
Discussion
In the present investigation we show that CHAD, a relatively abundant noncollagenous protein in cartilage extracellular matrix, interacts with β1 integrins on bovine chondrocytes. This interesting finding identifies CHAD as a candidate for mediating signals between the chondrocytes and the cartilage matrix.
CHAD is a member of the LRR protein family (35). Among other members in this family are the small cartilage proteoglycans biglycan, decorin, fibromodulin, and lumican. These proteoglycans are all known to interact with collagen (18, 41, 47), but it is not known if CHAD interacts with collagen or other matrix molecules.
Chondrocyte adhesion to CHAD was partially inhibited by a rat polyclonal antibody against β1 integrin. Species differences between cells and antibodies may explain why the inhibition was not total. Alternatively, other receptors than β1 integrins may also be involved in the adhesion to CHAD. We found that adhesion of chondrocytes to CHAD was dependent on Mg2+ or Mn2+ but not on Ca2+. This is consistent with results from extensive studies of regulation of integrin activity by divalent cations. The activity of several integrins, including α2β1, is stimulated by Mg2+ or Mn2+ and inhibited by Ca2+ (14).
Available antibodies did not immunoprecipitate the β1-associated α integrin subunit from bovine chondrocytes that mediated the adhesion to CHAD. The most likely explanation for this is that antibodies raised against human integrin subunits show weak or no cross-reactivity to many of the bovine chondrocyte integrins. From the molecular weight of the CHAD-binding α integrin subunit (140 kD nonreduced and 150 kD reduced), the fact that the apparent size increased upon reduction and that the adhesion was Mg2+ dependent, it is likely that the α subunit involved is α2. Since we know from FACS® analysis that isolated human primary chondrocytes from articular cartilage have relatively small amounts of the α2β1 integrin (Holmvall, K., L. Camper, and E. Lundgren-Åkerlund, unpublished results), we chose to investigate CHAD-binding integrins on human fibroblasts. These cells express α2β1 as well as other integrins (Fig. 6). In affinity purification experiments we were able to show that the integrin α2β1 indeed is a CHAD-binding integrin. Antibodies against α5 and αv immunoprecipitated orders of magnitude–lower amounts of their respective integrins from the CHAD-agarose eluate. We further found that monoclonal antibodies against the subunits β1 or α2 inhibited the adhesion of cells to culture dishes coated with CHAD, while antibodies against the other integrin subunits had minor or no effect on the adhesion. In contrast to the lack of effects of the other integrin antibodies, the α3-integrin antibody appeared to stimulate the adhesion to CHAD. The findings corroborated further that the integrin α2β1 mediates the interaction between cells and CHAD. To confirm a participation of α2β1 integrins also in chondrocyte adhesion, we studied the adhesion of human chondrocytes to CHAD in the presence of the α2 antibody Gi9. We found that the antibody partially inhibited adhesion of cultured chondrocytes to CHAD (Fig. 8), which confirms that the integrin α2β1 indeed is involved in adhesion of chondrocytes to this substrate. In agreement with the fibroblast experiment the Gi9 antibody only partially inhibited the adhesion of human chondrocytes. This may indicate that another receptor in addition to α2β1 is involved in the adhesion of cells to CHAD. It is also possible that the immobilized CHAD mediate a high degree of nonspecific binding.
In previous experiments, it has been shown that adhesion of chondrocytes and chondrosarcoma cells to collagen type II was mediated by the integrins α1β1 and α2β1 (24). Since α1β1 is present on both chondrocytes (24) and fibroblasts (Fig. 5) and since this integrin appeared not to interact with CHAD in the affinity chromatography experiments (Figs. 4 and 5), it is unlikely that collagen contaminants in the CHAD preparation were mediating the cell binding.
Since integrin α2β1 is also a receptor for collagen type II (24) we asked whether adhesion to collagen type II and to CHAD were mediated by similar mechanisms. One observation indicating that there is a difference in the α2β1 integrin binding to these ligands is that chondrocytes (37), T47D-cells (Fig. 10), and fibroblasts (data not shown) all spread on immobilized collagen type II, while adhesion to CHAD did not promote spreading. One explanation may be that different sites on the α2 chain are involved in adhesion to collagen type II and CHAD. To study this, we adhered T47D cells to the two substrates in the presence of various α2 antibodies. The monoclonal antibodies P1E6, P1H5, and Gi9 are known to block adhesion of cells to collagen. The monoclonal antibodies Gi19 and Gi29 have some inhibitory effect on adhesion of platelets to collagen, while Gi14 does not inhibit adhesion. (Santoso, S., personal communication) The T47D cells do not express collagen type II binding integrins other than α2β1 and were therefore particularly informative in these studies (45; Camper, L., and E. Lundgren-Akerlund, unpublished results). We found that the different α2 antibodies inhibited adhesion to collagen type II and CHAD in a similar manner, indicating that these ligands bind to similar or nearby sites on the α2β1 integrin (Fig. 9). Further experiments using α2 integrin antibodies recognizing other epitopes on the α2 subunit will be needed to elucidate the binding sites. Another explanation is that the binding of collagen type II and CHAD is regulated differently. It has previously been shown that α2β1 integrins from different cell types show different ligand specificity. α2β1 on platelets and melanoma cells bind collagen (27, 46), while α2β1 on other cell types binds both collagen and laminin (9, 30). Several factors including divalent cations, proteoglycans, and phospholipids have been suggested to modulate integrin activity, and it has been suggested that the degree of activation may regulate their ligand specificity (4). Since Mn2+ has been shown to increase the affinity between integrins and their ligands in affinity chromatography (13) and cell adhesion (10, 32), we tested the possibility that Mn2+ could stimulate cell spreading. However, Mn2+ appeared not to stimulate spreading of T47D cells on immobilized chondroadherin (data not shown). Phorbol esters such as PMA are known to mimic the effect of several different integrin-activating stimuli and to induce clustering of integrins (7, 42, 49). Protein kinase C may therefore be an important regulator of the integrin affinity and ligand specificity. Our finding that cells showed some spreading on CHAD in the presence but not in the absence of PMA (Fig. 10 and Table I) indicates that spreading of cells may require activation and altered affinity of the integrins. It also lends strong support to the involvement of integrins in the cell attachment.
It is likely that integrin α2β1, being a receptor for two different proteins in cartilage, has an important function in mediating signals between the chondrocytes and the cartilage matrix. We and others (8, 24, 50) have found that isolated chondrocytes express relatively small amounts of α2β1 integrin. The collagen type II binding integrin α1β1, on the other hand, is one of the major integrins on isolated chondrocytes. It is possible that α2β1 is a more dynamic integrin that is upregulated during changes in cell–matrix interactions such as matrix turnover, remodelling, or mechanical stress. We have found that the integrin subunit α2 was upregulated in chondrosarcoma cells during mechanical stress while the expression of α1 was not changed (24). It has also been shown that the integrin subunit α2, but not α1, is upregulated in fibroblasts in contracting collagen gels (26, 40) during reorganization of the collagen matrix. This supports the idea that the integrin α2β1 can respond to changes in the extracellular matrix. Furthermore, it has also been shown that growth factors such as TGFβ (21) and EGF (5) stimulate expression of the integrin α2β1, indicating that growth factors can regulate the α2β1-mediated interactions with the extracellular matrix. However, further investigations are needed to understand the functional role of the specific interaction of α2β1 with CHAD in cartilage and to elucidate the differences between interaction of α2β1 with CHAD and collagen type II.
Acknowledgments
We are grateful to Dr. Arnoud Sonnenberg for valuable advice and for the generous gift of T47D cells.
Footnotes
Please address all correspondence to Dr. Evy Lundgren-Åkerlund, Department of Cell and Molecular Biology, Section for Connective Tissue Biology, Lund University, P.O. Box 94, S-221 00 Lund, Sweden. Tel.: (46) 46-222-3311; FAX: (46) 46-211-3417.
1. Abbreviation used in this paper: CHAD, chondroadherin.
Grants were obtained from the Swedish Medical Research Council, the Medical Faculty, Lund University, Anna-Greta Crafoord's stiftelse, Crafoord's stiftelser, Gustav V's 80-års fond, Axel och Margaret Ax:son Johnson's stiftelse, Greta och Johan Kock's stiftelse, Kungliga fysiografiska sällskapets stiftelse, Riksföreningen mot reumatism, Åke Wiberg's stiftelse, Thelma Zoe′ga's ‘fond, and Alfred Österlund's stiftelse.
References
- 1.Bengtsson E, Neames PJ, Heinegard D, Sommarin Y. The primary structure of a basic leucine-rich repeat protein, PRELP, found in connective tissue. J Biol Chem. 1995;270:25639–25644. doi: 10.1074/jbc.270.43.25639. [DOI] [PubMed] [Google Scholar]
- 2.Blochberger TC, Vergnes J-P, Hempel J, Hassell JR. cDNA to chick lumican (corneal keratan sulfate proteoglycan) reveals homology to the small interstitial proteoglycan gene family and expression in muscle and intestine. J Biol Chem. 1992;267:347–352. [PubMed] [Google Scholar]
- 3.Carter W, Wayner E, Bouchard T, Kaur P. The role of integrins α2β1 in cell-cell and cell-substrate adhesion of human epidermal cells. J Cell Biol. 1990;110:1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan BMC, Hemler ME. Multiple functional forms of integrin VLA-2 can be derived from a single α2 cDNA clone: interconversion of forms induced by an anti-β1 antibody. J Cell Biol. 1993;120:537–543. doi: 10.1083/jcb.120.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen JD, Kim JP, Zhang K, Sarret Y, Wynn KC, Kramer RH, Woodley DT. Epidermal growth factor (EGF) promotes human keratinocyte locomotion on collagen by increasing the α2 integrin subunit. Exp Cell Res. 1993;209:216–223. doi: 10.1006/excr.1993.1304. [DOI] [PubMed] [Google Scholar]
- 6.Corpuz LM, Funderburgh JL, Funderburgh ML, Bottomley GS, Prakash S, Conrad GW. Molecular cloning and tissue distribution of keratocan. J Biol Chem. 1996;271:9759–9763. doi: 10.1074/jbc.271.16.9759. [DOI] [PubMed] [Google Scholar]
- 7.Danilov YN, Juliano RL. Phorbol ester modulation of integrin-mediated cell adhesion: a postreceptor event. J Cell Biol. 1989;108:1925–1933. doi: 10.1083/jcb.108.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dürr J, Goodman S, Potocnik A, von der Mark H, von der Mark K. Localization of β1-integrins in human cartilage and their role in chondrocyte adhesion to collagen and fibronectin. Exp Cell Res. 1993;207:235–244. doi: 10.1006/excr.1993.1189. [DOI] [PubMed] [Google Scholar]
- 9.Elices MJ, Hemler ME. The human integrin VLA-2 is a collagen receptor on some cells and a collagen/laminin receptor on others. Proc Natl Acad Sci USA. 1989;86:9906–9910. doi: 10.1073/pnas.86.24.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elices MJ, Urry LA, Hemler ME. Receptor functions for the integrin VLA-3: fibronectin, collagen, and laminin binding are differentially influenced by ARG-GLY-ASP peptide and by divalent cations. J Cell Biol. 1991;112:169–181. doi: 10.1083/jcb.112.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eyre, D.R., J.-J. Wu, and P. Woods. 1992. Cartilage-specific collagens. Structural studies. In Articular Cartilage and Osteoarthritis. K.E. Kuettner, R. Schleyerbach, J.G. Peyron, and V.C. Hascall, editors. Raven Press, Ltd., New York. 119–131.
- 12.Fisher LW, Termine JD, Young MF. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homolgy with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J Biol Chem. 1989;264:4571–4576. [PubMed] [Google Scholar]
- 13.Gailit J, Ruoslahti E. Regulation of the fibronectin receptor affinity by divalent cations. J Biol Chem. 1988;263:12927–12932. [PubMed] [Google Scholar]
- 14.Garratt AN, Humphries MJ. Recent insights into ligand binding, activation and signalling by integrin adhesion receptors. Acta Anat. 1995;154:34–45. doi: 10.1159/000147750. [DOI] [PubMed] [Google Scholar]
- 15.Gullberg D, Terracio L, Borg TK, Rubin K. Identification of integrin-like matrix receptors with affinity for interstitial collagens. J Biol Chem. 1989;264:12686–12694. [PubMed] [Google Scholar]
- 16.Hardingham, T.E., A.J. Fosang, and J. Dudhia. 1992. Aggrecan, the chondroitin sulfate/keratan sulfate proteoglycan from cartilage. In Articular Cartilage and Osteoarthritis. K.E. Kuettner, R. Schleyerbach, J.G. Peyron, and V.C. Hascall, editors. Raven Press, Ltd., New York. 5–20.
- 17.Häuselmann HJ, Aydelotte MB, Schumacher BL, Kuettner KE, Gitelis SH, Thonar EJ-MA. Synthesis and turnover of proteoglycans by human and bovine adult articular chondrocytes cultured in alginate beads. Matrix. 1992;12:116–129. doi: 10.1016/s0934-8832(11)80053-3. [DOI] [PubMed] [Google Scholar]
- 18.Hedbom E, Heinegård D. Binding of fibromodulin and decorin to separate sites on fibrillar collagens. J Biol Chem. 1993;268:27307–27312. [PubMed] [Google Scholar]
- 19.Heinegård, D., and M. Paulsson. 1987. Cartilage. In Methods in Enzymology. Structural and Contractile Proteins. Part E. Extracellular Matrix. L.W. Cunningham, editor. Academic Press Inc., Orlando, FL. 336–363.
- 20.Heinegård D, Oldberg Å. Structure and biology of cartilage and bone matrix noncollagenous macromolecules. FASEB (Fed Am Soc Exp Biol) J. 1989;3:2042–2051. doi: 10.1096/fasebj.3.9.2663581. [DOI] [PubMed] [Google Scholar]
- 21.Heino J, Massagué J. Transforming growth factor-β switches the pattern of integrins expressed in MG-63 human osteosarcoma cells and causes a selective loss of cell adhesion to laminin. J Biol Chem. 1989;264:21806–21811. [PubMed] [Google Scholar]
- 22.Hemler ME. VLA-proteins in the integrin family: structure, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- 23.Hemler ME, Sanchez-Madrid F, Flotte TJ, Krensky AM, Burakoff SJ, Bhan AK, Springer TA, Strominger JL. Glycoproteins of 210,000 and 130,000 m.w. on activated T cells: cell distribution and antigenic relation to components on resting cells and T cell lines. J Immunol. 1984;132:3011–3018. [PubMed] [Google Scholar]
- 24.Holmvall K, Camper L, Johansson S, Rubin K, Kimura JH, Lundgren-Åkerlund E. Chondrocyte and chondrosarcoma cell integrins with affinity for collagen type II and their response to mechanical stress. Exp Cell Res. 1995;221:496–503. doi: 10.1006/excr.1995.1401. [DOI] [PubMed] [Google Scholar]
- 25.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 26.Klein CE, Dressel D, Steinmayer T, Mauch C, Eckes B, Krieg T, Bankert RB, Weber L. Integrin α2β1 is upregulated in fibroblasts and highly aggressive melanoma cells in three-dimensional collagen lattices and mediates the reorganization of collagen I fibrils. J Cell Biol. 1991;115:1427–1436. doi: 10.1083/jcb.115.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kramer RH, Marks N. Identification of integrin collagen receptors on human melanoma cells. J Biol Chem. 1989;264:4684–4688. [PubMed] [Google Scholar]
- 28.Krusius T, Ruoslahti E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci USA. 1986;83:7683–7687. doi: 10.1073/pnas.83.20.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landegren U. Measurement of cell numbers by means of the endogenous enzyme hexosaminidase. Application to detection of lymphokines and cell surface antigens. J Immunol Methods. 1984;67:379–388. doi: 10.1016/0022-1759(84)90477-0. [DOI] [PubMed] [Google Scholar]
- 30.Languino LR, Gehlsen KR, Wayner E, Carter WG, Engvall E. Endothelial cells use α2β1 integrin as a laminin receptor. J Cell Biol. 1989;109:2455–2462. doi: 10.1083/jcb.109.5.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larsson T, Sommarin Y, Paulsson M, Antonsson P, Hedbom E, Wendel M, Heinegård D. Cartilage matrix proteins. A basic 36-kDa protein with a restricted distribution to cartilage and bone. J Biol Chem. 1991;266:20428–20433. [PubMed] [Google Scholar]
- 32.Lundgren E, Berger E, Arfors K-E. Effect of divalent cations on adhesion of polymorphonuclear leukocytes to matrix molecules in vitro. J Leukocyte Biol. 1992;51:603–608. doi: 10.1002/jlb.51.6.603. [DOI] [PubMed] [Google Scholar]
- 33.Maroudas, A. 1979. Physicochemical properties of articular cartilage. In Adult Articular Cartilage. M.A.R. Freeman, editor. Pittman, London. 215–290.
- 34.Miller EJ. Structural studies on cartilage collagen employing limited cleavage and solubilization with pepsin. Biochemistry. 1972;11:4903–4909. doi: 10.1021/bi00776a005. [DOI] [PubMed] [Google Scholar]
- 35.Neame PJ, Sommarin Y, Boynton RE, Heinegård D. The structure of a 38-kDa leucine-rich protein (chondroadherin) isolated from bovine cartilage. J Biol Chem. 1994;269:21547–21554. [PubMed] [Google Scholar]
- 36.Oldberg Å, Antonsson P, Lindblom K, Heinegård D. A collagen-binding 59-kd protein (fibromodulin) is structurally related to the small interstitial proteoglycans PG-S1 and PG-S2 (decorin) EMBO (Eur Mol Biol Organ) J. 1989;8:2601–2604. doi: 10.1002/j.1460-2075.1989.tb08399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science (Wash DC) 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 38.Salter DM, Hughes DE, Simpson R, Gardner DL. Integrin expression by human articular chondrocytes. Br J Rheumatol. 1992;31:231–234. doi: 10.1093/rheumatology/31.4.231. [DOI] [PubMed] [Google Scholar]
- 39.Santoso S, Kalb R, Walka M, Kiefel V, Mueller-Eckhardt C, Newman PJ. The human platelet alloantigens Bra and Brbare associated with a single amino acid polymorphism on glycoprotein Ia (integrin subunit α2) J Clin Invest. 1993;92:2427–2432. doi: 10.1172/JCI116849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schiro JA, Chan BMC, Roswit WT, Kassner PD, Pentland AP, Hemler ME, Eisen AZ, Kupper TS. Integrin α2β1 (VLA-2) mediates reorganization and contraction of collagen matrices by human cells. Cell. 1991;67:403–410. doi: 10.1016/0092-8674(91)90191-z. [DOI] [PubMed] [Google Scholar]
- 41.Schonherr E, Witsch-Prehm P, Harrach B, Robenek H, Rauterberg J, Kresse H. Interaction of biglycan with type I collagen. J Biol Chem. 1995;270:2776–2783. doi: 10.1074/jbc.270.6.2776. [DOI] [PubMed] [Google Scholar]
- 42.Shimitzu Y, Van Seventer GA, Horgan KJ, Shaw S. Regulated expression and binding of three VLA (β1) integrin receptors on T cells. Nature (Lond) 1990;345:250–253. doi: 10.1038/345250a0. [DOI] [PubMed] [Google Scholar]
- 43.Sommarin Y, Heinegård D. Specific interaction between cartilage proteoglycans and hyaluronic acid at the chondrocyte cell surface. Biochem J. 1983;214:777–784. doi: 10.1042/bj2140777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sommarin Y, Larsson T, Heinegård D. Chondrocyte-matrix interactions. Attachment to proteins isolated from cartilage. Exp Cell Res. 1989;184:181–192. doi: 10.1016/0014-4827(89)90376-5. [DOI] [PubMed] [Google Scholar]
- 45.Sonnenberg A, Linders CJT, Modderman PW, Damsky CH, Aumailley M, Timpl R. Integrin recognition of different cell-binding fragments of Laminin (P1, E3, E8) and evidence that α6β1 but not α6β4 functions as a major receptor for fragment E8. J Cell Biol. 1990;110:2145–2155. doi: 10.1083/jcb.110.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Staaz WD, Rajpara SM, Wayner EA, Carter WG, Santoro SA. The membrane glycoprotein Ia-IIIa (VLA-2) complex mediates the Mg2+-dependent adhesion of platelets to collagen. J Cell Biol. 1989;108:1917–1924. doi: 10.1083/jcb.108.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vogel K, Paulsson M, Heinegard D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J. 1984;223:587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wessel D, Flügge U I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- 49.Wilkins JA, Stupack D, Stewart S, Caixia S. β1 integrin-mediated lymphocyte adherence to extracellular matrix is enhanced by phorbol ester treatment. Eur J Immunol. 1991;21:517–522. doi: 10.1002/eji.1830210239. [DOI] [PubMed] [Google Scholar]
- 50.Woods VL, Jr, Schreck PJ, Gesink DS, Pacheco HO, Amiel D, Akeson WH, Lotz M. Integrin expression by human articular chondrocytes. Arthritis Rheum. 1994;37:537–544. doi: 10.1002/art.1780370414. [DOI] [PubMed] [Google Scholar]