Abstract
Integrin-dependent cell adhesion to specific extracellular matrix molecules has been demonstrated to trigger dramatic changes in gene expression that can affect cell fate. However, little is understood about the molecular mechanism by which events at sites of cell– substratum adhesion are communicated to the cell interior to regulate the transcriptional apparatus. By analogy to classical mechanisms of cell surface receptor function, it seems likely that some components of the integrin-activated signal transduction machinery will be colocalized with cell adhesion molecules. Zyxin is a low abundance phosphoprotein that accumulates with integrins at sites of cell–substratum attachment. Here we show that zyxin exhibits a functional nuclear export signal that is required to keep zyxin concentrated in the cytoplasm and is sufficient to direct nuclear proteins to the cytosol. Furthermore, we demonstrate that native zyxin shuttles between the nucleus and sites of cell adhesion in fibroblasts and is thus an excellent candidate for relaying information between these two compartments.
Adhesion of higher eukaryotic cells to their surroundings has been shown to induce profound changes in gene expression that can affect cell fate, progress through the cell cycle, and state of cellular differentiation (Clark and Brugge, 1995; Gumbiner, 1996). One class of receptors that mediates both cell–substratum adhesion and signaling is the integrins. Integrins serve as receptors for a variety of extracellular matrix molecules. In cultured cells, integrins concentrate at focal contacts where they establish a transmembrane linkage between elements of the extracellular matrix and the actin cytoskeleton. In addition to this structural role, integrins have recently been shown to transmit signals from the extracellular environment to the cell interior. Since integrins do not exhibit any catalytic activity, it is thought that signaling occurs via the ability of the receptors to regulate the activities of noncovalently associated signaling partners.
A number of proteins that appear to participate in integrin-dependent signaling have been identified. These include tyrosine kinases such as focal adhesion kinase (Otey, 1996), c-Abl (Lewis et al., 1996), and Src family members (Clark and Brugge, 1993; Cobb et al., 1994); serine/threonine kinases including mitogen-activated protein kinases (Chen et al., 1994; Zhu and Assoian, 1995) and protein kinase C (Chun and Jacobson, 1993; Vuori and Ruoslahti, 1993); the lipid kinase phosphatidylinositol-3 kinase (McNamee et al., 1993; Shimizu et al., 1995); and small GTP-binding proteins including Ras, Rac, Rho, and CDC42 (Lamarche et al., 1996).
Another protein that has been proposed to function in signaling at sites of cell adhesion is zyxin (Sadler et al., 1992; Beckerle, 1997). Zyxin is a low abundance phosphoprotein that colocalizes with integrin receptors at sites of cell substratum attachment in fibroblasts (Crawford and Beckerle, 1991; Crawford et al., 1992; Sadler et al., 1992). Zyxin has two structural features that are compatible with its proposed role in signal transduction. First, zyxin exhibits a region of 146 amino acids that displays a proline content >35%. This proline-rich segment of zyxin contains a number of sequences reminiscent of docking sites for Src homology 3 (SH3) domains, and indeed, the COOH-terminal SH3 domain of the proto-oncogene product, Vav, interacts with zyxin (Hobert et al., 1996). The proline-rich region of zyxin also mediates zyxin's ability to associate with two additional proteins, α-actinin and members of the Ena/VASP family, that are important for the assembly and integrity of the actin cytoskeleton (Pavalko and Burridge, 1991; Crawford et al., 1992; Reinhard et al., 1995; Gertler et al., 1996). Recent work has directly implicated zyxin in the spatial control of actin filament assembly by demonstrating that membrane-targeted zyxin is sufficient to induce the development of actin-rich cell surface protrusions (Golsteyn et al., 1997). A second structural feature in zyxin that is consistent with a function in signal transduction is the presence of three copies of a double zinc finger motif called the LIM motif (Sadler et al., 1992). LIM domains have been shown to participate in specific protein–protein interactions (Schmeichel and Beckerle, 1994; Wu and Gill, 1994) and may also have the capacity to bind nucleic acid (Perez-Alvarado et al., 1994; Schmeichel and Beckerle, 1997). LIM domains are found in a number of proteins that play roles in regulating cell proliferation and differentiation (Sadler et al., 1992; Sanchez-Garcia and Rabbitts, 1994; Gill, 1995). One of zyxin's LIM domains has been shown to bind the product of an early serum response gene called the cysteine-rich protein, CRP (Schmeichel and Beckerle, 1994). CRP family members also exhibit LIM domains and appear to be required for muscle differentiation (Arber et al., 1994, 1997; Crawford et al., 1994; Stronach et al., 1996).
In addition to the proline-rich array and LIM domains, zyxins from human, mouse, rabbit, and chicken, (Macalma et al., 1996; Wang et al., 1994; Sadler et al., 1992) as well as a Drosophila zyxin-related protein (Stronach, B.E., T. Macalma, and M.C. Beckerle, unpublished observations) exhibit a short, highly conserved sequence that is rich in leucines and charged residues. In this paper we demonstrate that this conserved leucine-rich sequence contains a functional nuclear export signal (NES)1 similar to those characterized in PKIα and the HIV protein Rev (Fischer et al., 1995; Wen et al., 1995). Zyxin's NES is required to exclude expressed zyxin from the nucleus, is sufficient to export nuclear proteins to the cytosol, and provides a molecular mechanism for regulating zyxin's in vivo distribution. In addition, we demonstrate that native zyxin shuttles between the cytoplasm and the nucleus and is thus an excellent candidate for relaying information between these two compartments.
Materials and Methods
Unless otherwise stated, reagents were obtained from Sigma Chemical Co. (St. Louis, MO).
Eukaryotic Expression Plasmid Construction
Plasmids used in zyxin expression experiments were created by subcloning PCR-amplified chicken zyxin cDNA into the pcDNA1/Neo eukaryotic expression vector from Invitrogen (San Diego, CA). 5′ HpaI and 3′ XbaI restriction endonuclease sites were designed into the PCR oligonucleotides to facilitate cloning into pcDNA1/Neo's EcoRV and XbaI polylinker sites. The HpaI site immediately precedes zyxin's start (ATG) codon, and the XbaI site immediately follows zyxin's stop (TGA) codon. The PCR template for chicken zyxin constructs was provided by the plasmid cZyx5 (Sadler et al., 1992). Site-directed mutagenesis was performed with Promega's Altered Sites kit (Madison, WI) to generate PCR template for the deletion mutant pZyxΔ322-331.
Construction of zyxin-β-gal expression plasmids entailed cloning PCR-derived β-gal product into pcDNA1/Neo, re-isolation, and subsequent addition of zyxin PCR product. The β-gal DNA insert was amplified using Promega's pSV-β-gal Control Vector as template and engineered with a 5′ XhoI linker sequence and a Kozak consensus ATG start codon in place of the first 21 nucleotides of the wild-type coding sequence. The zyxin PCR product was designed to contain a 5′ HpaI site and a 3′ XhoI site for ready 5′ fusion to β-gal sequences. The zyxin sequences are found at the NH2 termini of the resulting chimeric proteins. All zyxin inserts were sequenced to verify error-free amplification.
Glutathione-S-Transferase Fusion Protein Expression
Glutathione-S-transferase (GST) fusion protein expression constructs were created by cloning PCR-derived chicken zyxin sequences into pGEX2T-128/129 (Schmeichel and Beckerle, 1994). EcoRI sites were engineered into the PCR oligonucleotides to facilitate cloning and allow for in frame 3′ fusion to the GST-FLAG leader sequences. A termination codon, TGA, was introduced into the 3′ end of the zyxin PCR products. All zyxin constructs were sequenced to verify correct amplification.
GST–zyxin fusion proteins were produced by inducing log phase BL21(DE3) bacteria containing the pGEX2T-128/129 construct with IPTG for 2 h, purifying the recombinant protein with glutathione agarose beads (Schmeichel and Beckerle, 1994), and concentrating the eluate with a Centricon-10 concentrator (Amicon, Beverly, MA). The concentrate was equilibrated in PBS.
Microinjection and Immunofluorescence
The rat embryo fibroblast cell line, REF-52, was grown on glass coverslips to ∼80% confluency, and plasmid or protein was injected into cell nuclei using an Eppendorph Micromanipulator/Transjector apparatus (Madison, WI). Cesium chloride-purified plasmids (Sambrook et al., 1989) were injected at a concentration of 0.2–0.25 mg/ml in PBS. Recombinant GST fusion proteins were mixed with FITC-labeled BSA (Molecular Probes, Inc., Eugene, OR) and injected at a final concentration of 5–10 mg/ml in PBS for each protein. The purified m1334 antibody was mixed with FITC-labeled rabbit anti–goat IgG (Cappel, Durham, NC) with final concentrations of m1334 at 4.5 mg/ml and rabbit anti–goat IgG at 3.75 mg/ml in PBS.
Indirect immunofluorescence preparation included washing coverslips four times in PBS; fixing for 10 min in 3.7% formaldehyde in PBS; washing two times 3 min in PBS; permeabilizing for 3 min in 0.2% Triton X-100 in PBS; washing three times for 3 min in PBS; blocking inverted coverslips on Parafilm (Greenwich, CT) with mBB blocking buffer containing 10% normal goat serum (GIBCO BRL, Gaithersburg, MD), 2% BSA, and 0.2% gelatin in PBS for 30 min; incubating blocked coverslips with primary antibody at 37°C for 60 min; washing six times for 3 min in PBS; incubating coverslips with secondary antibody at 37°C for 60 min; washing six times for 3 min in PBS; and mounting in gelvatol containing 2.5% Dabco. Antibodies used in these immunofluorescence preparations included the following: a rabbit polyclonal anti-zyxin antibody, B38 (1:400 in mBB; Macalma et al., 1996); a chicken-specific anti-zyxin mouse monoclonal antibody, m1334 (straight tissue culture supernatant); the anti–β-galactosidase (β-gal) monoclonal antibody GAL-13 (1:4,000 in mBB); the anti-FLAG epitope mouse monoclonal antibody M2 (IBI/Kodak/VWR Scientific, Inc., New Haven, CT; 1:2,000 in mBB); and two fluorochrome-labeled secondary antibodies, Texas red goat anti–rabbit Ig (1:200 in mBB) and FITC goat anti–mouse Ig (1:500 in mBB; Cappel). Cells were photographed on a microscope (Axiophot; Zeiss, Inc., Thornwood, NY); negatives were digitized and adjusted using Adobe Photoshop (Adobe Systems, Inc., Mountain View, CA). Some of the figures shown represent composites generated from two fields; the region where the images are juxtaposed is indicated by a thin white line.
Tissue Culture and Antibody Purification
REF-52 cells were grown as described (McClure et al., 1982). Primary chicken embryo fibroblasts (CEF) were isolated and cultured as described (Beckerle, 1986). For microinjection experiments, cells were trypsinized, plated on glass coverslips, and allowed to spread for 48 h before injection.
The m1334 antibody used in the microinjection experiments was purified from protein- and serum-free tissue culture supernatants. The m1334 mouse monoclonal cell line was initially conditioned to growth in CCM1 (Hyclone, Logan, UT) containing 0.5% serum. Upon growth to near confluency, culture medium was switched to a protein and serum-free media containing RPMI with 10% HyQ PF-mAb growth supplements (Hyclone) and cultured until yellowing of the medium occurred. The m1334 antibody was purified from the culture supernatant by ammonium sulfate precipitation (Harlow and Lane, 1988) and concentrated with a concentrator (Centricon-10; Amicon, Beverly, MA). The antibody concentrate was washed three times with PBS by diluting and concentrating in the microconcentrator.
Results
Zyxin Displays a Conserved Leucine-rich Sequence That Is Important for Regulating its Subcellular Distribution
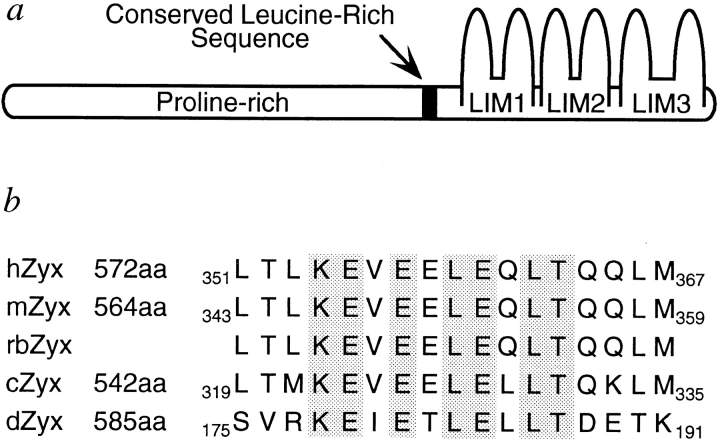
Zyxin has been identified in human, mouse, rabbit, and chicken (Sadler et al., 1992; Wang, et al., 1994; Macalma et al., 1996). A zyxin-related protein has also been described in the fruit fly (Stronach, B.E., T. Macalma, and M.C. Beckerle, unpublished results). These proteins display a conserved molecular architecture consisting of an extensive proline-rich NH2-terminal region and three LIM domains (Fig. 1 a). Comparison of the sequences of zyxins from different species revealed a third conserved feature, a leucine-rich sequence embedded within the proline-rich NH2-terminal domain (Fig. 1 a). In the four vertebrate zyxins characterized to date, this region displays a group of 17 highly conserved amino acids, particularly leucines and charged, mostly acidic, amino acids (Fig. 1 b). This region is characterized by a central core of 10 amino acids that is also highly conserved in the zyxin-related protein from Drosophila (Fig. 1 b). We have performed experiments designed to evaluate the function of this short leucine-rich region of zyxin.
Figure 1.
Schematic representation of zyxin and the conserved leucine-rich region. The zyxin protein (a) is comprised of an extensive proline-rich NH2-terminal region and three COOH-terminal, tandemly arrayed LIM domains that provide binding sites for multiple SH3 and LIM domain proteins, respectively (Crawford et al., 1992; Sadler et al., 1992; Reinhard et al., 1995; Gertler et al., 1996; Hobert et al., 1996). A 17-amino acid, leucine-rich sequence (b) is highly conserved in human, mouse, rabbit, and chicken zyxin (Sadler et al., 1992; Wang, et al., 1994; Macalma et al., 1996) as well as a Drosophila zyxin-related protein (Stronach, B.E., T. Macalma, and M.C. Beckerle, personal communication). The shaded residues are absolutely conserved. The total length of each zyxin species and the location of the conserved leucine-rich region are indicated.
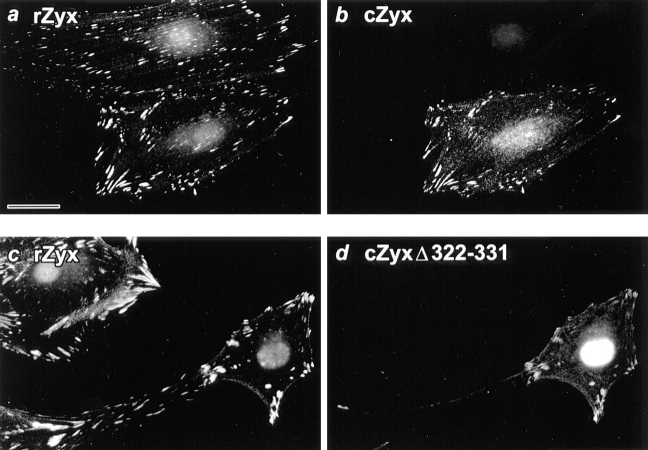
Transient transfection studies illustrate that the conserved, leucine-rich sequence plays an important role in the regulation of zyxin's subcellular distribution. Indirect immunofluorescence staining of REF-52 cells with anti-zyxin antibodies reveals that, at steady state, zyxin is prominently colocalized with integrin receptors at focal contacts (Fig. 2 a). Some faint nuclear staining is also observed, but this is attributable to components present in the pre-immune serum and is not seen when staining with an anti-zyxin monoclonal antibody (data not shown; and Fig. 2 b). If full length chicken zyxin is expressed in REF-52 cells and the distribution of the expressed protein is monitored with a chicken-specific anti-zyxin antibody, we find that the chicken protein incorporates into the rat focal contacts in a manner that is indistinguishable from the endogenous rat protein (Fig. 2, a and b). In contrast, if we express chicken zyxin that lacks 10 amino acids (amino acid 322–331) corresponding to the core conserved, leucine-rich region (Fig. 1 b), the zyxin deletion variant is now prominently concentrated within cell nuclei, in addition to being detected in the focal contacts (Fig. 2 d). Thus, in the absence of amino acids 322–331, the ability of zyxin to be targeted to the nucleus is revealed.
Figure 2.
Deletion of the core conserved leucine-rich sequence from zyxin induces its nuclear accumulation. REF-52 cells were microinjected with a eukaryotic expression construct engineered to express full length chicken zyxin (a and b) or zyxin lacking the central core-conserved, leucine-rich sequence corresponding to amino acid 322–331 (c and d). 24 h after microinjection of the expression plasmid, the cells were prepared for indirect immunofluorescence with two anti-zyxin antibodies: a rabbit polyclonal antibody (B38) that recognizes the endogenous rat zyxin (a and c) and a mouse monoclonal antibody (m1334) that recognizes only the expressed chicken protein (b and d). The slight nuclear staining observed in a and c is also observed with the B38 pre-immune serum and therefore does not reflect recognition of zyxin by the antibody (data not shown). Bar, 33 μM.
Zyxin Sequences Are Sufficient to Redirect the Localization of a Nuclear Protein to the Cytoplasm
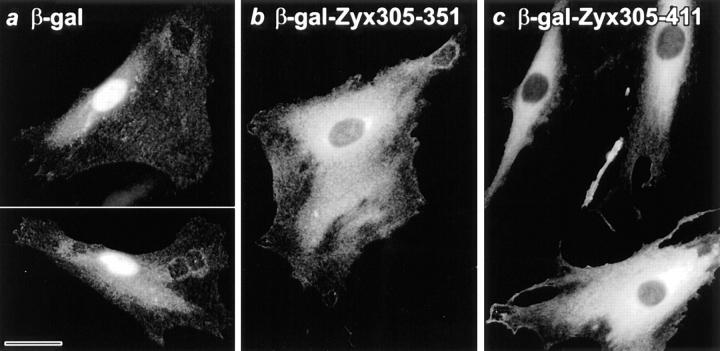
To evaluate whether the conserved leucine-rich sequence in zyxin would affect the subcellular localization of a nuclear protein, we examined whether these zyxin sequences would alter the distribution of β-gal, an Escherichia coli protein that somewhat surprisingly accumulates in cell nuclei when expressed in some eukaryotic cells (Kalderon et al., 1984). In REF-52 cells that express unmodified β-gal, the protein is strongly concentrated in cell nuclei, as detected by indirect immunofluorescence with antibodies directed against β-gal (Fig. 3 a); some protein is also detected within the cytoplasm. In contrast, in cells programmed to express β-gal–zyxin fusion proteins that display the conserved, leucine-rich region of zyxin, either amino acids 305–351 (Fig. 3 b) or amino acids 305–411 (Fig. 3 c), the β-gal is concentrated in the cytoplasm and appears to be excluded from cell nuclei. Thus, when tagged with zyxin sequences that include the conserved, leucine-rich region of zyxin, newly synthesized β-gal protein that would otherwise be destined for the nucleus exhibits a cytosolic distribution. A quantitative summary of these results is presented in Table I. Fusion of the zyxin sequences to β-gal could be altering the subcellular distribution of β-gal either by tethering the protein in the cytoplasm after synthesis, by blocking the nuclear import of β-gal or by triggering the export of any β-gal that was delivered to the nucleus.
Figure 3.
Fusion of sequences containing the conserved leucine-rich region of zyxin to β-gal is sufficient to redirect the expressed protein from the nucleus to the cytoplasm. REF-52 cells were microinjected with a eukaryotic expression construct engineered to express β-gal (a) or β-gal–zyxin fusion proteins containing zyxin's conserved leucine-rich region (b, amino acids 305–351; c, amino acids 305–411). Cells were prepared for indirect immunofluorescence using an anti– β-gal antibody 24 h after injection. Unmodified β-gal concentrates in the nuclei of REF-52 cells; fusion of zyxin sequences to β-gal results in exclusion of the protein from the nucleus and accumulation in the cytoplasm. Bar, 33 μM.
Table I.
Localization of Expressed Zyxin–β-gal Fusion Proteins in Rat Embryo Fibroblast (REF-52) Cells
| Construct | Nucleus (percentage of cells) | Cytoplasm (percentage of cells) | Number of cells | |||
|---|---|---|---|---|---|---|
| β-gal | 100 | 0 | 49 | |||
| β-gal-Zyx305-411 | 5 | 95 | 98 | |||
| β-gal-Zyx305-351 | 20 | 80 | 85 |
To test the ability of zyxin's conserved leucine-rich region to relocalize nuclear proteins to the cytoplasm, the distribution of β-gal with and without fused zyxin amino acid sequences was determined by immunofluorescence. Plasmids engineered to express β-gal or β-gal–zyxin fusion proteins were injected into REF-52 cells, and cells were stained with anti–β-gal antibodies 24 h after injected. Cells that expressed the construct were identified by fluorescence microscopy, and the distribution of fusion protein was scored. Results from three experiments are shown.
Zyxin's Conserved Leucine-rich Sequence Functions as a Nuclear Export Signal
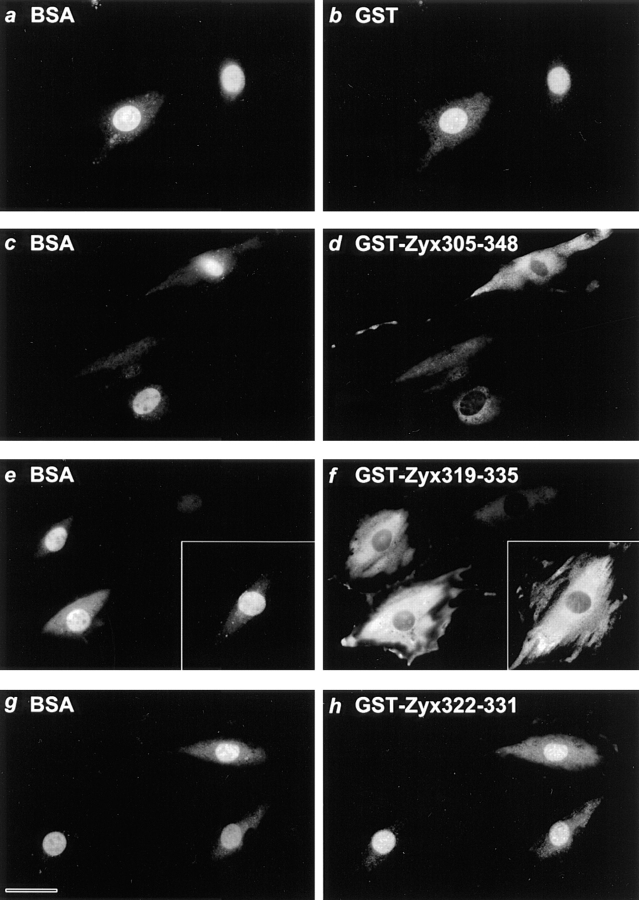
To test directly whether sequences contained in zyxin have the capacity to direct the specific export of a protein from the nucleus to the cytoplasm, we performed microinjection experiments in which fibroblast nuclei were co-injected with FITC-labeled BSA (FITC-BSA) and epitope-marked GST or GST that was tagged with short segments of zyxin sequence (Fig. 4). FITC-BSA was selected as a reporter of the injection site because it has been well characterized as a protein that is too large to diffuse freely through nuclear pores (Paine and Feldherr, 1972). As can be seen in Fig. 4, a and b, both nuclear-injected FITC-BSA and GST are retained in cell nuclei during the time course of the experiment. In striking contrast, when a GST–Zyx305-348 fusion protein is injected into cell nuclei, as evidenced by the nuclear localization of the co-injected FITC-BSA (Fig. 4 c), the zyxin-tagged GST is rapidly exported into the cytoplasm (Fig. 4 d). These results show that the microinjected GST is redistributed from the nucleus to the cytoplasm as a result of harboring the zyxin sequence tag.
Figure 4.
The conserved, leucine-rich sequence in zyxin is sufficient to direct export of nuclear-injected GST to the cytoplasm. REF-52 cell nuclei were co-injected with BSA and either GST (a and b) or GST–zyxin chimeras (c and d, GST-Zyx305-348; e and f, GST-Zyx319-335; g and h, GST-Zyx322-331). Within 30 to 45 min of injection, cells were prepared for indirect immunofluorescence using antibody directed against the FLAG epitope tag found within the GST leader peptide. Dual channel fluorescence microscopy was used to reveal the fluorochrome-labeled BSA localized at the injection site (a, c, e, and g) or the GST fusion proteins (b, d, f, and h). Comparable results were obtained using GST and GST-zyxin chimeras that were directly labeled with Texas red (data not shown). Bar, 33 μM.
To define further the zyxin sequences that are sufficient to direct the export of a nuclear protein, we examined the ability of smaller zyxin-derived sequences to facilitate nuclear export. We observed that GST–Zyx319-335 was efficiently exported from the nucleus to the cytoplasm (Fig. 4, e and f) whereas a shorter, 10-amino acid sequence found in GST–Zyx322-331 was not sufficient to direct nuclear export (Fig. 4, g and h). Experiments in which amino acids 322–331 are deleted from full length zyxin (Fig. 2) illustrated that this region of zyxin is necessary for zyxin to display a cytoplasmic distribution at steady state. However, additional sequence information contained within amino acids 319–335 is clearly required for efficient targeting of a protein for nuclear export. The results of these experiments are summarized in Table II. Similar results were obtained if the GST fusion proteins were directly labeled with a fluorochrome before microinjection (data not shown).
Table II.
Localization of Injected Zyxin-GST Fusion Proteins in Rat Embryo Fibroblast (REF-52) Cells
| Construct | Nucleus (percentage of cells) | Cytoplasm (percentage of cells) | Number of cells | |||
|---|---|---|---|---|---|---|
| GST | 100 | 0 | 130 | |||
| GST-Zyx305-348 | 20 | 80 | 36 | |||
| GST-Zyx319-335 | 15 | 85 | 120 | |||
| GST-Zyx322-331 | 100 | 0 | 115 |
To test the ability of zyxin's conserved, leucine-rich region to relocalize nuclear proteins to the cytoplasm, the distribution of nuclear injected GST, with and without fused zyxin aa sequences, was determined by immunofluorescence. GST or GST zyxin fusion proteins were expressed and purified from bacteria and co-injected into REF-52 cell nuclei along with fluorochrome-labeled BSA to mark successful nuclear injection. Within 30 to 45 min of injection, cells were prepared for indirect immunofluorescence using an antibody directed against the FLAG epitope tag found within the GST leader peptide. The percentage of cells that displayed nuclear or cytoplasmic localization of the fusion protein is shown; three independent experiments were performed.
Zyxin Shuttles between the Nucleus and Cytoplasmic Focal Contacts
To determine whether endogenous zyxin has the capacity to shuttle between the cytoplasm and the nucleus under normal physiological conditions, we employed a microinjection approach that would report the presence of zyxin within a cell's nucleus. To monitor the occurrence of zyxin within cell nuclei, we microinjected anti-zyxin antibody directly into cell nuclei. We reasoned that if zyxin moved from the cytoplasm into the nucleus during the time course of our experiment, we would observe one of two outcomes: either we would now detect zyxin in the nucleus because it would be trapped there by the anti-zyxin antibody or we would find that the anti-zyxin antibody was specifically depleted from nuclei because it had bound zyxin and had been exported as a complex with its NES-bearing ligand.
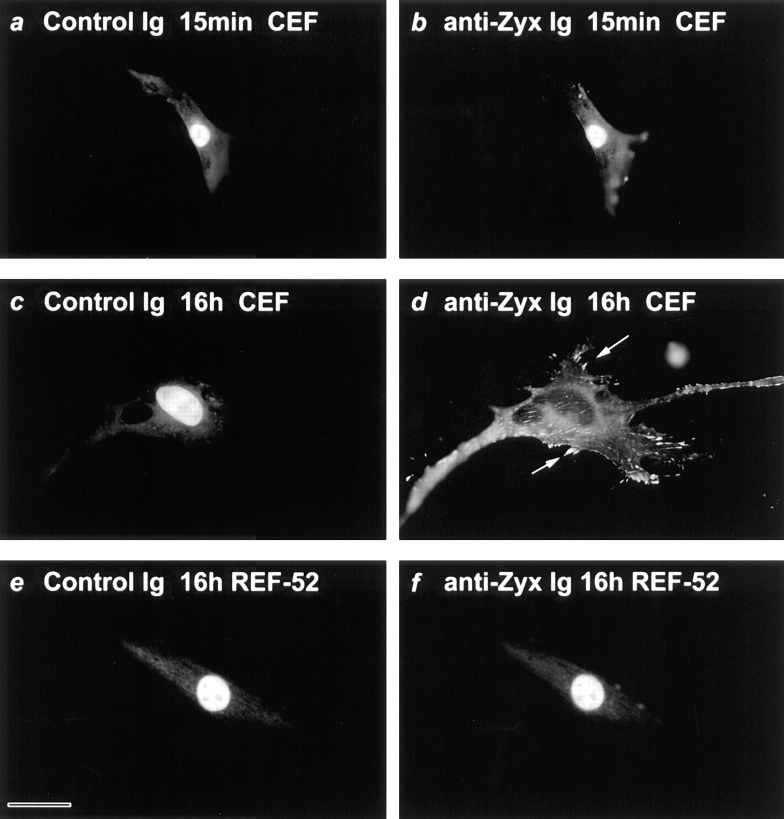
Chicken embryo fibroblast nuclei were co-injected with a chicken-specific anti-zyxin mouse monoclonal antibody and a control fluorochrome-labeled, noncross-reactive Ig that would serve as a marker for a successful nuclear injection. Immediately after microinjection, both antibodies are detected in cell nuclei (Fig. 5, a and b). However, examination of cells 16 h after injection revealed that the anti-zyxin antibody was specifically exported to the cytoplasm, where it labeled zyxin present at the focal contacts in chicken cells (Fig. 5 d). The noncross-reactive Ig is retained in the nuclei at the site of injection (Fig. 5 c), illustrating that nuclear envelope breakdown did not occur and that the anti-zyxin antibody was specifically depleted from the nucleus during the time course of the experiment. Because immunoglobulins do not passively exit the nucleus (Wen et al., 1995), the appearance of the anti-zyxin antibody in the cytoplasm demonstrates that zyxin has entered the nucleus, bound the anti-zyxin antibody, and facilitated the antibody's export.
Figure 5.
Zyxin shuttles between the nucleus and the focal contacts. Microinjected antibody was used to report the presence of zyxin within a cell's nucleus. Two antibodies, a chicken-specific anti-zyxin monoclonal antibody and a non-cross-reactive anti–goat Ig, were co-injected into the nuclei of fibroblast cells. Cells were fixed either 15 min (a and b) or 16 h (c–f) after injection. Dual channel immunofluorescence microscopy was used to reveal the location of the control anti–goat Ig (a, c, and e) and the chicken-specific anti-zyxin monoclonal antibody in chicken (b and d) or rat (f) fibroblast cells. The control Ig that is injected into cell nuclei remains there for the duration of the experiment (a, c, and e). The co-injected anti– chicken zyxin antibody is specifically exported to the cytoplasm when injected into the nuclei of chicken cells but not rat cells after 16 h (d and f ). Anti-zyxin antibody that is exported from the nuclei of chicken cells can be detected at the focal contacts (d, arrows). Bar, 33 μM.
In control experiments, we evaluated the specificity of the anti-zyxin antibody export and the dependence of the antibody export on ligand binding. We compared the behavior of the nuclear-injected anti-zyxin antibody with two different noncross-reactive Ig preparations that were co-injected into the cell nuclei with the anti-zyxin antibody. In both cases examined, the control antibody species was retained in cell nuclei during the time that the anti-zyxin antibody was depleted from cell nuclei. Neither of the control antibodies recognized any nuclear antigens, as monitored immunocytochemically (data not shown). Therefore, it is unlikely that the nuclear retention of the control antibody, in comparison with the anti-zyxin antibody, is due to ligand-dependent trapping of the control antibody in the nucleus as opposed to specific export of the anti-zyxin antibody. Consistent with the view that engagement of the anti-zyxin antibody with its protein ligand is required for the antibody export, injection of the antibody mixture into rat fibroblast cell nuclei yielded little or no export of either the control Ig (Fig. 5 e) or the chicken-specific anti-zyxin antibody (Fig. 5 f) after 16 h.
Discussion
A Nuclear Export Signal in Zyxin
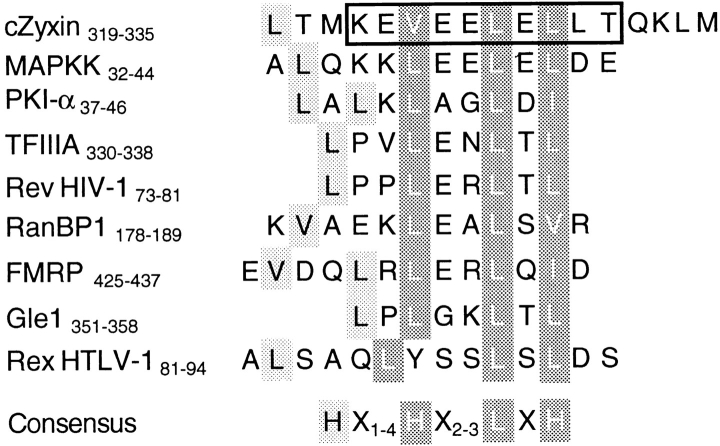
By transient transfection and microinjection studies, we have demonstrated that chicken zyxin contains a functional NES that is required to exclude zyxin from cell nuclei at steady state. Moreover, this sequence is sufficient to induce the export of nuclear proteins to the cytoplasm. The NES identified in zyxin shares key features with NESs that have previously been shown to trigger the rapid, active delivery of proteins and RNA–protein complexes from the nucleus to the cytoplasm (Fig. 6; Fischer et al., 1995; Wen et al., 1995; Bogerd et al., 1996; Eberhart et al., 1996; Fridell et al., 1996a ,b; Fukuda, et al., 1996; Kim et al., 1996; Murphy and Wente, 1996; Palmeri and Malim, 1996; Richards et al., 1996). The key hydrophobic residues shown to be important for NES function in other proteins are also found in zyxin's conserved leucine-rich region (Fig. 6, shaded residues; Bogerd et al., 1996; Kim et al., 1996).
Figure 6.
Zyxin's conserved, leucine-rich region displays conservation of the key hydrophobic residues required for NES function (Fischer et al., 1995; Wen et al., 1995; Bogerd et al., 1996; Eberhart et al., 1996; Fridell et al., 1996a ,b; Fukuda et al., 1996; Kim et al., 1996; Murphy and Wente, 1996; Palmeri and Malim, 1996; Richards et al., 1996). NESs from a variety of proteins have been characterized and shown to share greatest similarity in a particular arrangement of (shaded) hydrophobic residues. Mutational analysis of several NESs implicate the shaded, black-lettered residues as important and the shaded, white-lettered residues as critical for NES function (Bogerd et al., 1996; Kim et al., 1996). The consensus sequence was derived by comparing published NES sequences. H represents the hydrophobic amino acids leucine, isoleucine, or valine. As was shown in Figs. 1 and 2, deletion of the boxed 10-amino acid sequence from chicken zyxin was sufficient to disable zyxin's NES, resulting in nuclear accumulation of the expressed protein.
The results of our functional studies of the zyxin NES are consistent with work that defined the essential features of NESs from other proteins. Deletion of amino acid residues 322–331 (Fig. 6) from chicken zyxin, which represents the core of the NES but lacks at least one residue that has been shown to be important for NES function in other systems, was sufficient to disable zyxin's NES leading to nuclear accumulation of the expressed protein (Fig. 2). However, this region was not in itself sufficient to function as an NES (Fig. 4). The inclusion of seven additional amino acids from zyxin (amino acids 319–321 and 332–335) reconstituted the activity of the zyxin NES (Fig. 4). Given what is known about sequence requirements for NES function, it seems likely that leucine 319 is critical for the activity of the zyxin NES. Our results further emphasize that NESs, like nuclear import signals (Görlich and Mattaj, 1996) exhibit sequence diversity both in terms of the precise positioning and the chemistry of key residues in the consensus.
Regulated Shuttling of Zyxin to the Cell Nucleus?
The presence of a functional NES in zyxin suggested that zyxin might actually be able to shuttle between the nucleus and the focal contacts. However, since we do not detect zyxin in the nuclei of cells at steady state by immunocytochemical methods, it was important to test directly the hypothesis that zyxin transiently localizes to cell nuclei. We have used a nuclear injected antibody to report the presence of zyxin within cell nuclei. By that approach, we have demonstrated that zyxin is capable of shuttling from the cytoplasm into the nucleus and back again to the focal contacts, as evidenced by the accumulation of the nuclear injected anti-zyxin antibody at sites of cell–substratum adhesion.
Because zyxin is localized at sites of integrin-dependent attachment to the extracellular matrix where signals are transduced, it is tempting to speculate that zyxin's distribution might be responsive to changes in integrin activity. In this view, one would imagine that specific signals generated at sites of cell–substratum attachment would affect the ratio of nuclear to cytoplasmic zyxin. While this is a very attractive hypothesis, there is as yet no direct link between integrin function and the subcellular distribution of zyxin. Indeed, our efforts to induce a bulk redistribution of zyxin from the focal contacts to the nucleus by manipulation of cell adhesion or cell growth conditions failed to induce a discernible increase in nuclear zyxin. Likewise, induction of either A-kinase or C-kinase activity (Crawford, A., and M.C. Beckerle, unpublished results) failed to result in an accumulation of zyxin in cell nuclei.
It is of course possible that we have simply not yet identified the physiological signal that would result in a nuclear accumulation of zyxin. Alternatively, such a condition may not exist. It may be that only a small number of zyxin molecules reside in the nucleus, perhaps due to a short dwell time imposed by the presence of the NES, and these are not detectable above background by standard immunocytochemical methods. In this regard, it is of interest that MAP kinase kinase, a well characterized signaling molecule that exhibits a functional NES, is also detected exclusively in the cytoplasmic compartment (Fukuda et al., 1996).
Although wild-type zyxin is found within the cytoplasm at steady state, the deletion of the NES from zyxin clearly unmasks the protein's ability to accumulate in cell nuclei. The ability of zyxin to enter the nucleus is likely to be an active event. Zyxin is a 542-amino acid protein with a Stokes radius of 5.6 nm and a relative sedimentation coefficient of 3.0 s. Based on its hydrodynamic properties, zyxin behaves as an asymmetric 69-kD protein (Crawford and Beckerle, 1991; Sadler et al., 1992), too large to passively diffuse through the nuclear pore (Paine and Feldherr, 1972). Thus, it appears that some information contained within the zyxin sequence is involved in the active targeting of the protein to the nucleus, either acting directly as a nuclear import signal or by providing a binding site for a partner that displays such a signal.
Role of the NES in Zyxin
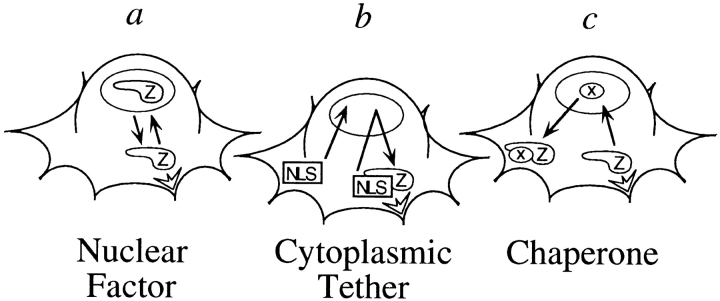
Proteins that display an NES are capable of regulated shuttling between the nucleus and the cytoplasm and that ability is likely to play a central role in each protein's biological function. Our demonstration that zyxin exhibits an NES and has the ability to transit from the nucleus to the adhesive membrane (Fig. 5) makes it an excellent candidate for participating in communication between these two compartments (Fig. 7). The mechanism by which zyxin's capacity to shuttle between the nucleus and the cytoplasm contributes to its function is not understood, but there are a number of intriguing possibilities. For example, one could imagine that zyxin's NES plays an important role in regulating zyxin's access to the nucleus (Fig. 7 a). Zyxin may itself play a role in the nucleus, perhaps even functioning in the regulation of gene expression. In this regard, it is interesting that many LIM domain proteins play well established roles in transcriptional control (Sadler et al., 1992; Sanchez-Garcia and Rabbitts, 1994; Gill, 1995), and the structure of the LIM domain appears to be compatible with a nucleic acid-binding function (Perez-Alvarado et al., 1994).
Figure 7.
Potential roles for zyxin's nuclear–cytoplasmic shuttling activity. Zyxin's capacity to shuttle between the nucleus and the focal contacts would allow it to convey information between these two cellular locations. Zyxin may play a nuclear role, possibly modulating the transcriptional machinery (a). Zyxin's shuttling activity could serve to sequester a nuclear protein at focal contacts where a particular signal would elicit its release from zyxin (b). Zyxin may act as a chaperone to escort nuclear factors to the cytoplasm and potentially localize them to sites of cell adhesion (c). Lastly, zyxin's nuclear cytoplasmic shuttling activity may be a mechanism to ensure cytoplasmic distribution of this protein after cell division. See text for additional discussion.
Alternatively, if the presence of zyxin in the cell nucleus were to have deleterious consequences for the cell, it is possible that the function of zyxin's NES is to ensure that the protein does not have access to nuclear components; although zyxin is sufficiently large that it would not be expected to passively diffuse into the nucleus, zyxin could gain access to the nucleus at the time of cell division, and the NES would ensure that the protein would rapidly be dispensed into the cytoplasm. It is also possible that zyxin's NES may be important for regulating the subcellular localization of a binding partner that has a nuclear function (Fig. 7 b). For example, zyxin might tether a transcriptional regulator at the focal contacts where signals are generated. When complexed to zyxin, any errant excursion of the regulatory protein into the nucleus would be very brief, owing to the presence of the NES on zyxin. Controlled release of such a regulatory molecule from zyxin would provide a mechanism to activate its nuclear function by regulating its subcellular distribution. In this view, zyxin would function in a fashion similar to the NES-bearing protein IκB, a molecule that inhibits a transcription factor by sequestering it in the cytoplasm. (Wen et al., 1995; Baldwin, 1996). Lastly, zyxin may transit to the nucleus and act as a chaperone to deliver a nuclear component, either protein or RNA, to the cytoplasm (Fig. 7 c).
Signaling from the Adhesive Cell Surface to the Nucleus
It has been clear for some time that sites of cell adhesion represent signaling hotspots on the cell surface, however the mechanism by which information at the cell surface is communicated to the cell nucleus has remained relatively obscure. Recently, three proteins have been demonstrated to reside both at adhesive junctions and in the nucleus. For example, the tight junction protein, ZO-1, accumulates in cell nuclei in a cell density-dependent fashion (Gottardi et al., 1996), and a subpopulation of the tyrosine kinase c-Abl is thought to shuttle between focal contacts and the nucleus in response to cell cycle cues (Lewis et al., 1996). Moreover, β-catenin, a protein found at cell–cell adherens junctions, has been shown to play a role in both linking the actin cytoskeleton to the cadherin class of homophilic cell adhesion receptors as well as directly transducing Wnt/ Wingless signals (Gumbiner, 1995; Huber et al., 1996a ; Orsulic and Peifer, 1996); Wnt/Wingless signaling results in the nuclear accumulation of a β-catenin-Lef-1 transcription factor complex that binds the promoter regions of several genes (Behrens, et al., 1996; Huber, et al., 1996b). With the discovery of zyxin's nuclear export signal and its capacity to shuttle between the nucleus and sites of cell adhesion to the extracellular matrix, zyxin emerges as a candidate for participation in the relay of information between the cell surface and the nucleus.
Abbreviations used in this paper
- β-gal
β-galactosidase
- GST
glutathione-S-transferase
- NES
nuclear export signal
Footnotes
Please address all correspondence to Mary C. Beckerle, Department of Biology, 201 South Biology Building, University of Utah, Salt Lake City, UT 84112-0840. Tel.: (801) 581-4485; Fax: (801) 581-4668; E-mail: beckerle @bioscience.utah.edu
The authors are particularly grateful to S. Tsukita for the anti-zyxin monoclonal antibody, to D. Gard and B. Stronach for helpful suggestions, and to K. Schmeichel for help with Fig. 7.
This research was supported by National Institutes of Health predoctoral award to D.A. Nix (CA09602) and by a National Institutes of Health grant (GM50877) to M.C. Beckerle. M.C. Beckerle is a recipient of a Faculty Research Award from the American Cancer Society. Support from the University of Utah Sequencing Facility and Biotechnology Core Facility (CA42014) is also gratefully acknowledged.
References
- Arber S, Halder G, Caroni P. Muscle LIM protein, a novel essential regulator of myogenesis, promotes myogenic differentiation. Cell. 1994;79:221–231. doi: 10.1016/0092-8674(94)90192-9. [DOI] [PubMed] [Google Scholar]
- Arber S, Hunter JJ, Ross JJ, Hongo M, Sansig G, Borg J, Perriard J, Chien KR, Caroni P. MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy, and heart failure. Cell. 1997;88:393–403. doi: 10.1016/s0092-8674(00)81878-4. [DOI] [PubMed] [Google Scholar]
- Baldwin AS., Jr The NF-κ B and I κB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Beckerle MC. Identification of a new protein localized at sites of cell– substrate adhesion. J Cell Biol. 1986;103:1679–1687. doi: 10.1083/jcb.103.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckerle, M.C. 1997. Zyxin: zinc fingers at sites of cell adhesion. Bioessays. In press. [DOI] [PubMed]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature (Lond) 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Bogerd HP, Fridell RA, Benson RE, Hua J, Cullen BR. Protein sequence requirements for function of the human T-cell leukemia virus type 1 Rex nuclear export signal delineated by a novel in vivo randomization-selection assay. Mol Cell Biol. 1996;16:4207–4214. doi: 10.1128/mcb.16.8.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Kinch MS, Lin TH, Burridge K, Juliano RL. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J Biol Chem. 1994;269:26602–26605. [PubMed] [Google Scholar]
- Chun JS, Jacobson BS. Requirement for diacylglycerol and protein kinase C in HeLa cell-substratum adhesion and their feedback amplification of arachidonic acid production for optimum cell spreading. Mol Biol Cell. 1993;4:271–281. doi: 10.1091/mbc.4.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Redistribution of activated pp60c-src to integrin-dependent cytoskeletal complexes in thrombin-stimulated platelets. Mol Cell Biol. 1993;13:1863–1871. doi: 10.1128/mcb.13.3.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science (Wash DC) 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Cobb BS, Schaller MD, Leu TH, Parsons JT. Stable association of pp60src and pp59fyn with the focal adhesion-associated protein tyrosine kinase, pp125FAK. Mol Cell Biol. 1994;14:147–155. doi: 10.1128/mcb.14.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AW, Beckerle MC. Purification and characterization of zyxin, an 82,000-dalton component of adherens junctions. J Biol Chem. 1991;266:5847–5853. [PubMed] [Google Scholar]
- Crawford AW, Michelsen JW, Beckerle MC. An interaction between zyxin and α-actinin. J Cell Biol. 1992;116:1381–1393. doi: 10.1083/jcb.116.6.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AW, Pino JD, Beckerle MC. Biochemical and molecular characterization of the chicken cysteine-rich protein, a developmentally regulated LIM-domain protein that is associated with the actin cytoskeleton. J Cell Biol. 1994;124:117–127. doi: 10.1083/jcb.124.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart DE, Malter HE, Feng Y, Warren ST. The fragile X mental retardation protein is a ribonucleoprotein containing both nuclear localization and nuclear export signals. Hum Mol Genet. 1996;5:1083–1091. doi: 10.1093/hmg/5.8.1083. [DOI] [PubMed] [Google Scholar]
- Fischer U, Huber J, Boelens WC, Mattaj IW, Luhrmann R. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell. 1995;82:475–483. doi: 10.1016/0092-8674(95)90436-0. [DOI] [PubMed] [Google Scholar]
- Fridell RA, Fischer U, Luhrmann R, Meyer BE, Meinkoth JL, Malim MH, Cullen BR. Amphibian transcription factor IIIA proteins contain a sequence element functionally equivalent to the nuclear export signal of human immunodeficiency virus type 1 Rev. Proc Natl Acad Sci USA. 1996a;93:2936–2940. doi: 10.1073/pnas.93.7.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridell RA, Benson RE, Hua J, Bogerd HP, Cullen BR. A nuclear role for the fragile X mental retardation protein. EMBO (Eur Mol Biol Organ) J. 1996b;15:5408–5414. [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Gotoh I, Gotoh Y, Nishida E. Cytoplasmic localization of mitogen-activated protein kinase kinase directed by its NH2-terminal, leucine-rich short amino acid sequence, which acts as a nuclear export signal. J Biol Chem. 1996;271:20024–20028. doi: 10.1074/jbc.271.33.20024. [DOI] [PubMed] [Google Scholar]
- Gertler FB, Niebuhr K, Reinhard M, Wehland J, Soriano P. Mena, a relative of VASP and DrosophilaEnabled, is implicated in the control of microfilament dynamics. Cell. 1996;87:227–239. doi: 10.1016/s0092-8674(00)81341-0. [DOI] [PubMed] [Google Scholar]
- Gill GN. The enigma of LIM domains. Structure (Lond) 1995;3:1285–1289. doi: 10.1016/s0969-2126(01)00265-9. [DOI] [PubMed] [Google Scholar]
- Golsteyn, R.M., M.C. Beckerle, T. Koay, and E. Friederich. 1997. J. Cell Science. In press. [DOI] [PubMed]
- Görlich D, Mattaj IW. Nucleocytoplasmic transport. Science (Wash DC) 1996;271:1513–1518. doi: 10.1126/science.271.5255.1513. [DOI] [PubMed] [Google Scholar]
- Gottardi CJ, Arpin M, Fanning AS, Louvard D. The junction-associated, ZO-1, localizes to the nucleus before the maturation and during the remodeling of cell-cell contacts. Proc Natl Acad Sci USA. 1996;93:10779–10784. doi: 10.1073/pnas.93.20.10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. Signal transduction of β-catenin. Curr Opin Cell Biol. 1995;7:634–640. doi: 10.1016/0955-0674(95)80104-9. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Harlow, E., and D. Lane. 1988. Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. 726 pp.
- Hobert O, Schilling JW, Beckerle MC, Ullrich A, Jallal B. SH3 domain-dependent interaction of the proto-oncogene product Vav with the focal contact protein zyxin. Oncogene. 1996;12:1577–1581. [PubMed] [Google Scholar]
- Huber O, Bierkamp C, Kemler R. Cadherins and catenins in development. Curr Opin Cell Biol. 1996a;8:685–691. doi: 10.1016/s0955-0674(96)80110-4. [DOI] [PubMed] [Google Scholar]
- Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, Kemler R. Nuclear localization of β-catenin by interaction with transcription factor LEF-1. Mech Dev. 1996b;59:3–10. doi: 10.1016/0925-4773(96)00597-7. [DOI] [PubMed] [Google Scholar]
- Kalderon D, Roberts BL, Richardson WD, Smith AE. A short amino acid sequence able to specify nuclear localization. Cell. 1984;39:499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Kim FJ, Beeche AA, Hunter JJ, Chin DJ, Hope TJ. Characterization of the nuclear export signal of human T-cell lymphotropic virus type 1 Rex reveals that nuclear export is mediated by position-variable hydrophobic interactions. Mol Cell Biol. 1996;16:5147–5155. doi: 10.1128/mcb.16.9.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarche N, Tapon N, Stowers L, Burbelo PD, Aspenstrom P, Bridges T, Chant J, Hall A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- Lewis JM, Baskaran R, Taagepera S, Schwartz MA, Wang JYJ. Integrin regulation of c-Abl tyrosine kinase activity and cytoplasmic-nuclear transport. Proc Natl Acad Sci USA. 1996;93:15174–15179. doi: 10.1073/pnas.93.26.15174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macalma T, Otte J, Hensler ME, Bockholt SM, Louis HA, Kalff-Suske M, Grzeschik KH, von der Ahe D, Beckerle MC. Molecular characterization of human zyxin. J Biol Chem. 1996;271:31470–31478. doi: 10.1074/jbc.271.49.31470. [DOI] [PubMed] [Google Scholar]
- McClure, D.B., M.J. Hightower, and W.C. Topp. 1982. Effect of SV40 transformation of the growth factor requirements of the rat embryo cell line REF52 in serum free medium. In Growth of Cells in Hormonally Defined Media. G.H. Sato, A.B. Pardee, and D.A. Sirbasku, editors. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. 345–364.
- McNamee HP, Ingber DE, Schwartz MA. Adhesion to fibronectin stimulates inositol lipid synthesis and enhances PDGF-induced inositol lipid breakdown. J Cell Biol. 1993;121:673–678. doi: 10.1083/jcb.121.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R, Wente SR. An RNA-export mediator with an essential nuclear export signal. Nature (Lond) 1996;383:357–360. doi: 10.1038/383357a0. [DOI] [PubMed] [Google Scholar]
- Orsulic S, Peifer M. An in vivo structure–function study of armadillo, the β-catenin homologue, reveals both separate and overlapping regions of the protein required for cell adhesion and for wingless signaling. J Cell Biol. 1996;134:1283–1300. doi: 10.1083/jcb.134.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otey CA. pp125FAK in the focal adhesion. Int Rev Cytol. 1996;167:161–183. doi: 10.1016/s0074-7696(08)61347-9. [DOI] [PubMed] [Google Scholar]
- Paine PL, Feldherr CM. Nucleocytoplasmic exchange of macromolecules. Exp Cell Res. 1972;74:81–98. doi: 10.1016/0014-4827(72)90483-1. [DOI] [PubMed] [Google Scholar]
- Palmeri D, Malim MH. The human T-cell leukemia virus type 1 posttranscriptional trans-activator Rex contains a nuclear export signal. J Virol. 1996;70:6442–6445. doi: 10.1128/jvi.70.9.6442-6445.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavalko FM, Burridge K. Disruption of the actin cytoskeleton after microinjection of proteolytic fragments of α-actinin. J Cell Biol. 1991;114:481–491. doi: 10.1083/jcb.114.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Alvarado GC, Miles C, Michelsen JW, Louis HA, Winge DR, Beckerle MC, Summers MF. Structure of the COOH-terminal LIM domain from the cysteine rich protein CRP. Nat Struct Biol. 1994;1:388–398. doi: 10.1038/nsb0694-388. [DOI] [PubMed] [Google Scholar]
- Reinhard M, Jouvenal K, Tripier D, Walter U. Identification, purification, and characterization of a zyxin-related protein that binds the focal adhesion and microfilament protein VASP (vasodilator-stimulated phosphoprotein) Proc Natl Acad Sci USA. 1995;92:7956–7960. doi: 10.1073/pnas.92.17.7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards SA, Lounsbury KM, Carey KL, Macara IG. A nuclear export signal is essential for the cytosolic localization of the Ran binding protein, RanBP1. J Cell Biol. 1996;134:1157–1168. doi: 10.1083/jcb.134.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler I, Crawford AW, Michelsen JW, Beckerle MC. Zyxin and cCRP: two interactive LIM domain proteins associated with the cytoskeleton. J Cell Biol. 1992;119:1573–1587. doi: 10.1083/jcb.119.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., E.F. Fritsch, and T. Maniatis. 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. 1.38–1.46.
- Sanchez-Garcia I, Rabbitts TH. The LIM domain: a new structural motif found in zinc-finger-like proteins. Trends Genet. 1994;10:315–320. doi: 10.1016/0168-9525(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Schmeichel KL, Beckerle MC. The LIM domain is a modular protein-binding interface. Cell. 1994;79:211–219. doi: 10.1016/0092-8674(94)90191-0. [DOI] [PubMed] [Google Scholar]
- Schmeichel KL, Beckerle MC. Molecular dissection of a LIM domain. Mol Biol Cell. 1997;8:219–230. doi: 10.1091/mbc.8.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y, Mobley JL, Finkelstein LD, Chan AS. A role for phosphatidylinositol 3-kinase in the regulation of β1 integrin activity by the CD2 antigen. J Cell Biol. 1995;131:1867–1880. doi: 10.1083/jcb.131.6.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stronach BE, Siegrist SE, Beckerle MC. Two muscle-specific LIM proteins in Drosophila. . J Cell Biol. 1996;134:1179–1195. doi: 10.1083/jcb.134.5.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuori K, Ruoslahti E. Activation of protein kinase C precedes α5 β1 integrin-mediated cell spreading on fibronectin. J Biol Chem. 1993;268:21459–21462. [PubMed] [Google Scholar]
- Wang LF, Miao SY, Zong SD, Bai Y, Koide SS. Gene encoding a mammalian epididymal protein. Biochem Mol Biol Int. 1994;34:1131–1136. [PubMed] [Google Scholar]
- Wen W, Meinkoth JL, Tsien RY, Taylor SS. Identification of a signal for rapid export of proteins from the nucleus. Cell. 1995;82:463–473. doi: 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- Wu RY, Gill GN. LIM domain recognition of a tyrosine-containing tight turn. J Biol Chem. 1994;269:25085–25090. [PubMed] [Google Scholar]
- Zhu X, Assoian RK. Integrin-dependent activation of MAP kinase: a link to shape-dependent cell proliferation. Mol Biol Cell. 1995;6:273–282. doi: 10.1091/mbc.6.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]