Abstract
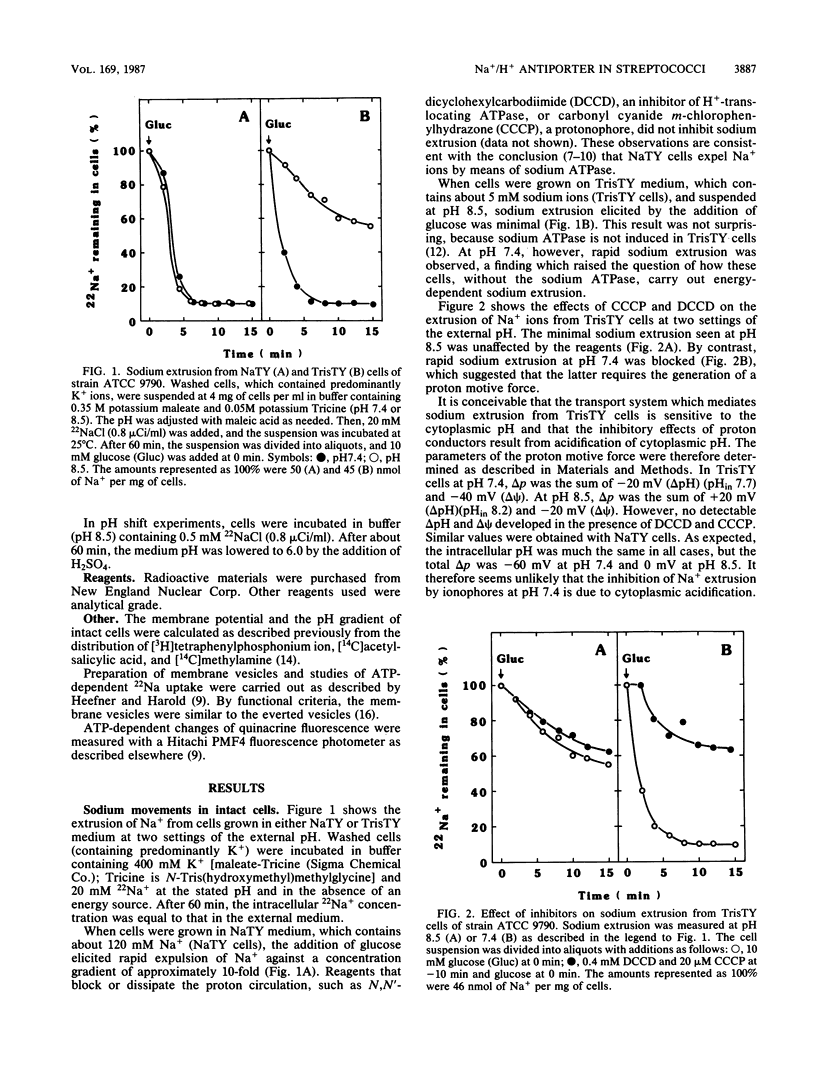
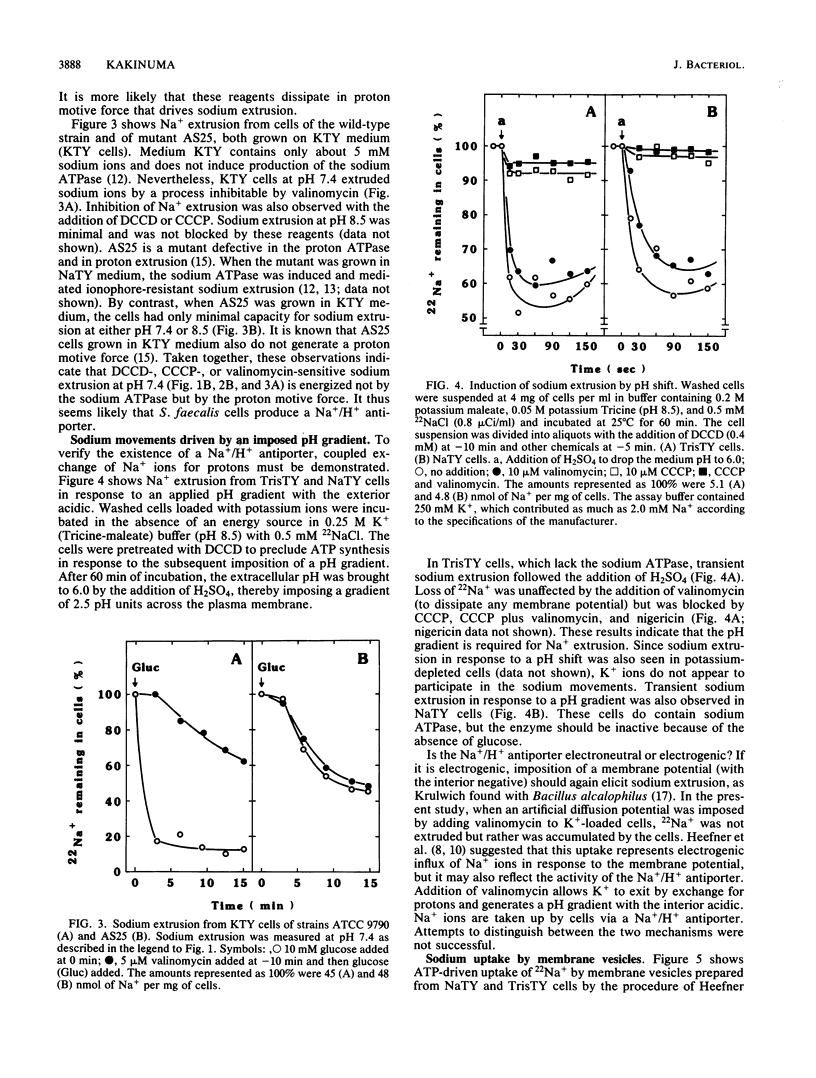
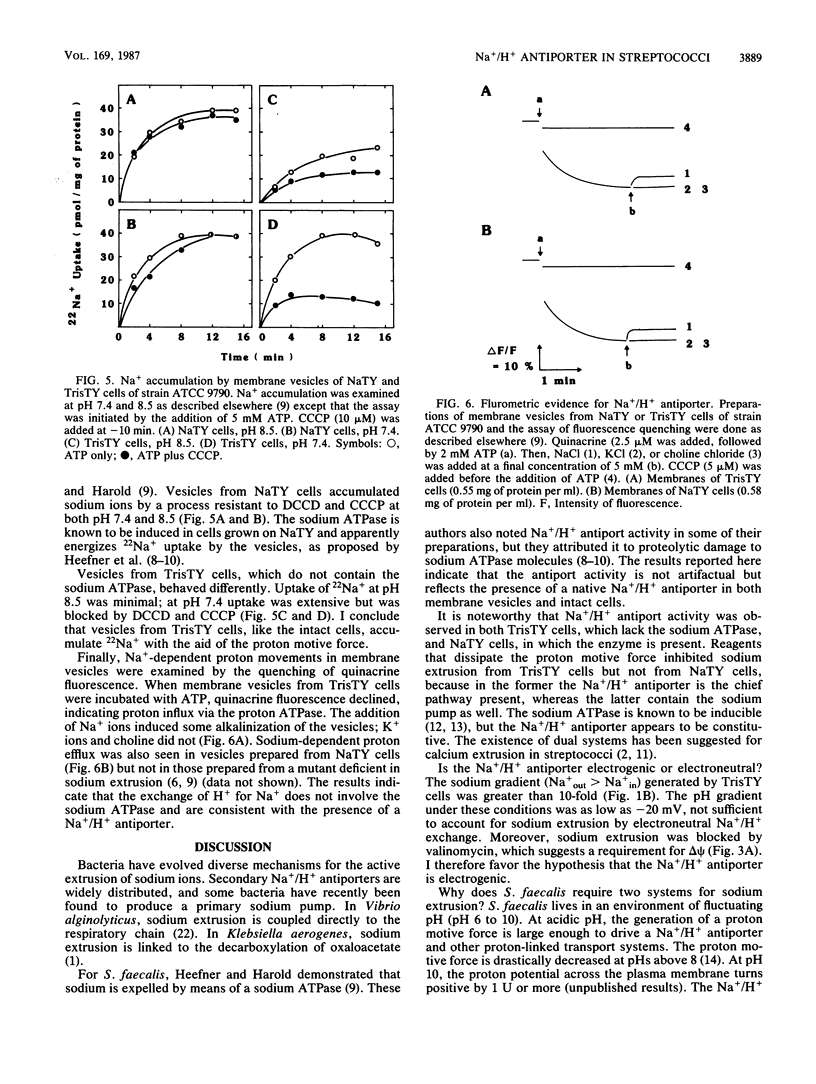
Streptococcus faecalis, like other bacteria, accumulates potassium ions and expels sodium ions. This paper is concerned with the pathway of sodium extrusion. Earlier studies (D.L. Heefner and F.M. Harold, Proc. Natl. Acad. Sci. USA 79:2798-2802, 1982) showed that sodium extrusion is effected by a primary, ATP-linked sodium pump. I report here that cells grown under conditions in which sodium ATPase is not induced can still expel sodium ions. This finding suggested the existence of an alternate pathway. Sodium extrusion by the alternate pathway requires the cells to generate a proton motive force. This conclusion rests on the following observations. (i) Sodium extrusion required glucose. (ii) Sodium extrusion was observed at neutral pH, which allows the cells to generate a proton motive force, but not at alkaline pH, which reduces the proton motive force to zero. (iii) Sodium extrusion was inhibited by the addition of dicyclohexylcarbodiimide and of proton-conducting ionophores. (iv) In response to an artificial pH gradient (with the exterior acid), energy-depleted cells exhibited a transient sodium extrusion which was unaffected by treatments that dissipated the membrane potential and which was blocked by proton conductors. I propose that streptococci have two independent systems for sodium extrusion: an inducible sodium ATPase and a constitutive sodium/proton antiporter.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dimroth P. A new sodium-transport system energized by the decarboxylation of oxaloacetate. FEBS Lett. 1980 Dec 29;122(2):234–236. doi: 10.1016/0014-5793(80)80446-7. [DOI] [PubMed] [Google Scholar]
- Driessen A. J., Konings W. N. Calcium transport in membrane vesicles of Streptococcus cremoris. Eur J Biochem. 1986 Aug 15;159(1):149–155. doi: 10.1111/j.1432-1033.1986.tb09845.x. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Kakinuma Y. Primary and secondary transport of cations in bacteria. Ann N Y Acad Sci. 1985;456:375–383. doi: 10.1111/j.1749-6632.1985.tb14888.x. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Papineau D. Cation transport and electrogenesis by Streptococcus faecalis. II. Proton and sodium extrusion. J Membr Biol. 1972;8(1):45–62. doi: 10.1007/BF01868094. [DOI] [PubMed] [Google Scholar]
- Heefner D. L., Harold F. M. ATP-driven sodium pump in Streptococcus faecalis. Proc Natl Acad Sci U S A. 1982 May;79(9):2798–2802. doi: 10.1073/pnas.79.9.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heefner D. L., Harold F. M. ATP-linked sodium transport in Streptococcus faecalis. I. The sodium circulation. J Biol Chem. 1980 Dec 10;255(23):11396–11402. [PubMed] [Google Scholar]
- Heefner D. L., Kobayashi H., Harold F. M. ATP-linked sodium transport in Streptococcus faecalis. II. Energy coupling in everted membrane vesicles. J Biol Chem. 1980 Dec 10;255(23):11403–11407. [PubMed] [Google Scholar]
- Heefner D. L. Transport of H+, K+, Na+ and Ca++ in Streptococcus. Mol Cell Biochem. 1982 Apr 30;44(2):81–106. doi: 10.1007/BF00226893. [DOI] [PubMed] [Google Scholar]
- Houng H. S., Lynn A. R., Rosen B. P. ATP-driven calcium transport in membrane vesicles of Streptococcus sanguis. J Bacteriol. 1986 Nov;168(2):1040–1044. doi: 10.1128/jb.168.2.1040-1044.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakinuma Y., Harold F. M. ATP-driven exchange of Na+ and K+ ions by Streptococcus faecalis. J Biol Chem. 1985 Feb 25;260(4):2086–2091. [PubMed] [Google Scholar]
- Kinoshita N., Unemoto T., Kobayashi H. Sodium-stimulated ATPase in Streptococcus faecalis. J Bacteriol. 1984 Jun;158(3):844–848. doi: 10.1128/jb.158.3.844-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Murakami N., Unemoto T. Regulation of the cytoplasmic pH in Streptococcus faecalis. J Biol Chem. 1982 Nov 25;257(22):13246–13252. [PubMed] [Google Scholar]
- Kobayashi H., Unemoto T. Streptococcus faecalis mutants defective in regulation of cytoplasmic pH. J Bacteriol. 1980 Sep;143(3):1187–1193. doi: 10.1128/jb.143.3.1187-1193.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Van Brunt J., Harold F. M. ATP-linked calcium transport in cells and membrane vesicles of Streptococcus faecalis. J Biol Chem. 1978 Apr 10;253(7):2085–2092. [PubMed] [Google Scholar]
- Krulwich T. A. Na+/H+ antiporters. Biochim Biophys Acta. 1983 Dec 30;726(4):245–264. doi: 10.1016/0304-4173(83)90011-3. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. The role of Na+ in transport processes of bacterial membranes. Biochim Biophys Acta. 1979 Dec 20;559(4):377–397. doi: 10.1016/0304-4157(79)90011-x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Vectorial chemistry and the molecular mechanics of chemiosmotic coupling: power transmission by proticity. Biochem Soc Trans. 1976;4(3):399–430. doi: 10.1042/bst0040399. [DOI] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Schuldiner S. pH homeostasis in bacteria. Biochim Biophys Acta. 1981 Dec;650(2-3):151–166. doi: 10.1016/0304-4157(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Tokuda H., Unemoto T. Characterization of the respiration-dependent Na+ pump in the marine bacterium Vibrio alginolyticus. J Biol Chem. 1982 Sep 10;257(17):10007–10014. [PubMed] [Google Scholar]



