Abstract
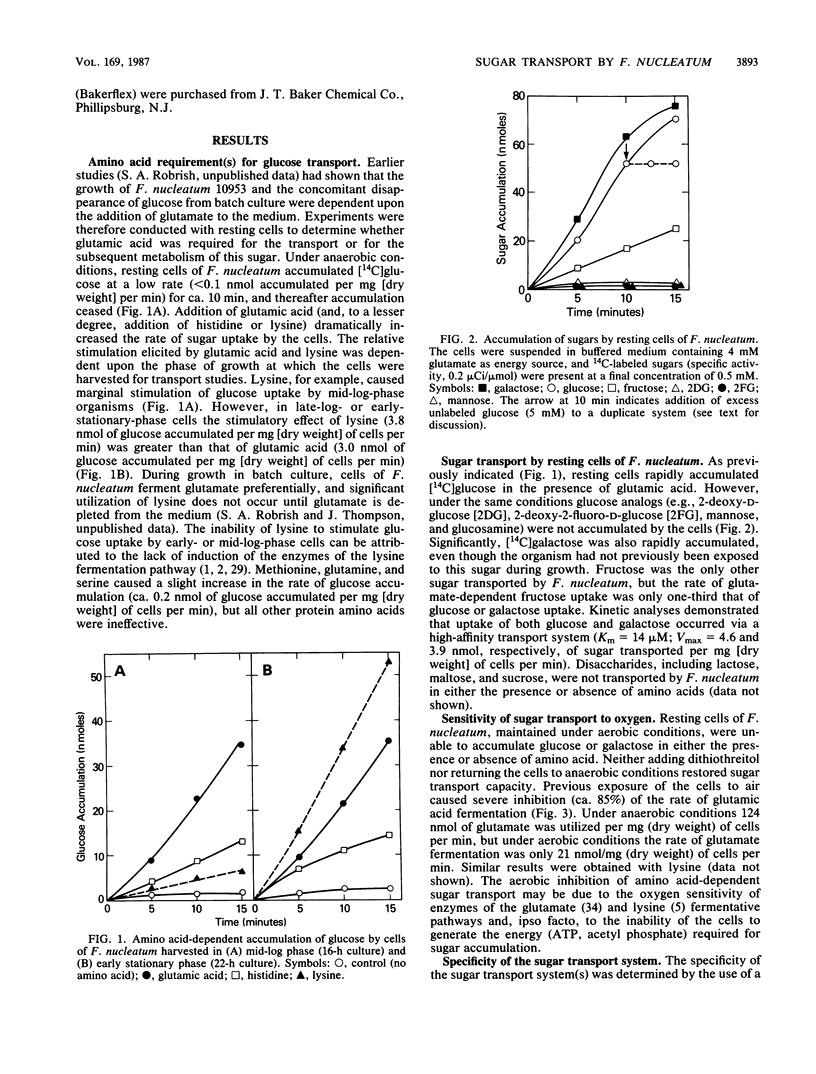
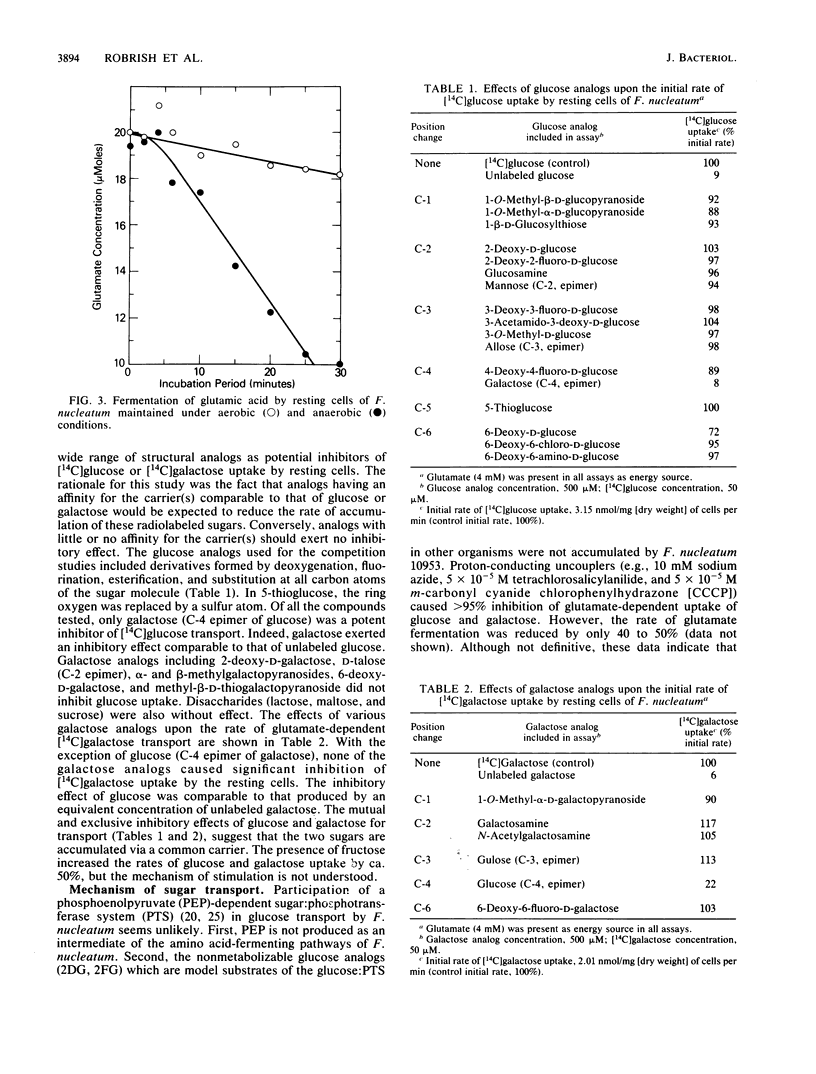
Resting cells of Fusobacterium nucleatum 10953 (grown previously in a medium containing glucose) failed to accumulate glucose under aerobic or anaerobic conditions. However, the addition of glutamic acid, lysine, or histidine to anaerobic suspensions of cells caused the immediate and rapid accumulation of glucose. Except for the amino acid-dependent transport of galactose and fructose (the latter being transported at approximately one-third the rate of glucose), no other sugars tested were accumulated by the resting cells. Amino acid-dependent uptake of sugar(s) by F. nucleatum was abolished by exposure of cells to air, and under aerobic conditions the rates of fermentation of glutamic acid and lysine were less than 15% of the rates determined anaerobically. The energy necessary for active transport of the sugars (acetyl phosphate and ATP) is derived from the anaerobic fermentation of glutamic acid, lysine, or histidine. Competition studies revealed that glucose and galactose were mutual and exclusive inhibitors of transport, and it is suggested that the two sugars (Km = 14 microM) are translocated via a common carrier. The products of amino acid-dependent sugar transport were recovered from resting cells as ethanol-precipitable, high-molecular-weight polymers. Polymer formation by F. nucleatum, during growth in medium containing glucose or galactose, was confirmed by electron microscopy.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker H. A. Amino acid degradation by anaerobic bacteria. Annu Rev Biochem. 1981;50:23–40. doi: 10.1146/annurev.bi.50.070181.000323. [DOI] [PubMed] [Google Scholar]
- Barker H. A., Kahn J. M., Hedrick L. Pathway of lysine degradation in Fusobacterium nucleatum. J Bacteriol. 1982 Oct;152(1):201–207. doi: 10.1128/jb.152.1.201-207.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessell E. M., Foster A. B., Westwood J. H. The use of deoxyfluoro-D-glucopyranoses and related compounds in a study of yeast hexokinase specificity. Biochem J. 1972 Jun;128(2):199–204. doi: 10.1042/bj1280199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckel W., Barker H. A. Two pathways of glutamate fermentation by anaerobic bacteria. J Bacteriol. 1974 Mar;117(3):1248–1260. doi: 10.1128/jb.117.3.1248-1260.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirpich T. P., Zappia V., Costilow R. N., Barker H. A. Lysine 2,3-aminomutase. Purification and properties of a pyridoxal phosphate and S-adenosylmethionine-activated enzyme. J Biol Chem. 1970 Apr 10;245(7):1778–1789. [PubMed] [Google Scholar]
- Coles R. S., Jr Glucose utilization by resting cells of Fusobacterium polymorphum. Arch Oral Biol. 1977;22(2):87–90. doi: 10.1016/0003-9969(77)90083-8. [DOI] [PubMed] [Google Scholar]
- Curtis M. A., Wittenberger C. L., Thompson J. Proline requirement for glucose utilization by Peptostreptococcus anaerobius ATCC 27337. Infect Immun. 1987 Feb;55(2):352–357. doi: 10.1128/iai.55.2.352-357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkler W. A., Jr, Clayman E. B., Shaefer D. F. Haemolysis of human erythrocytes by the Fusobacterium nucleatum associated with periodontal disease. Arch Oral Biol. 1983;28(8):735–739. doi: 10.1016/0003-9969(83)90109-7. [DOI] [PubMed] [Google Scholar]
- Fox D. K., Meadow N. D., Roseman S. Phosphate transfer between acetate kinase and enzyme I of the bacterial phosphotransferase system. J Biol Chem. 1986 Oct 15;261(29):13498–13503. [PubMed] [Google Scholar]
- GIBBONS R. J., KAPSIMALIS B. Synthesis of intracellular iodophilic polysaccharide by Streptococcus mitis. Arch Oral Biol. 1963 May-Jun;8:319–329. doi: 10.1016/0003-9969(63)90024-4. [DOI] [PubMed] [Google Scholar]
- Hitz W. D., Card P. J., Ripp K. G. Substrate recognition by a sucrose transporting protein. J Biol Chem. 1986 Sep 15;261(26):11986–11991. [PubMed] [Google Scholar]
- Hofstad T. Pathogenicity of anaerobic gram-negative rods: possible mechanisms. Rev Infect Dis. 1984 Mar-Apr;6(2):189–199. doi: 10.1093/clinids/6.2.189. [DOI] [PubMed] [Google Scholar]
- JACKINS H. C., BARKER H. A. Fermentative processes of the fusiform bacteria. J Bacteriol. 1951 Feb;61(2):101–114. doi: 10.1128/jb.61.2.101-114.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J., Gibbons R. J. A practical scheme for identification of the most numerous oral gram negative anaerobic rods. Arch Oral Biol. 1965 Jul-Aug;10(4):723–725. doi: 10.1016/0003-9969(65)90017-8. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Gibbons R. J. Amino acid fermentation by Fusobacterium nucleatum. Arch Oral Biol. 1968 Feb;13(2):191–202. doi: 10.1016/0003-9969(68)90051-4. [DOI] [PubMed] [Google Scholar]
- Melton T., Kundig W., Hartman P. E., Meadow N. 3-Deoxy-3-fluoro-D-glucose-resistant Salmonella typhimurium mutants defective in the phosphoenolpyruvate:glycose phosphotransferase system. J Bacteriol. 1976 Dec;128(3):794–800. doi: 10.1128/jb.128.3.794-800.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Smibert R. M., Good I. J., Burmeister J. A., Palcanis K. G., Ranney R. R. Bacteriology of experimental gingivitis in young adult humans. Infect Immun. 1982 Nov;38(2):651–667. doi: 10.1128/iai.38.2.651-667.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Smibert R. M., Hash D. E., Burmeister J. A., Ranney R. R. Bacteriology of severe periodontitis in young adult humans. Infect Immun. 1982 Dec;38(3):1137–1148. doi: 10.1128/iai.38.3.1137-1148.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiocho F. A., Vyas N. K. Novel stereospecificity of the L-arabinose-binding protein. Nature. 1984 Aug 2;310(5976):381–386. doi: 10.1038/310381a0. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees W. D., Holman G. D. Hydrogen bonding requirements for the insulin-sensitive sugar transport system of rat adipocytes. Biochim Biophys Acta. 1981 Aug 20;646(2):251–260. doi: 10.1016/0005-2736(81)90331-x. [DOI] [PubMed] [Google Scholar]
- Singer R. E., Buckner B. A. Butyrate and propionate: important components of toxic dental plaque extracts. Infect Immun. 1981 May;32(2):458–463. doi: 10.1128/iai.32.2.458-463.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Street I. P., Armstrong C. R., Withers S. G. Hydrogen bonding and specificity. Fluorodeoxy sugars as probes of hydrogen bonding in the glycogen phosphorylase-glucose complex. Biochemistry. 1986 Oct 7;25(20):6021–6027. doi: 10.1021/bi00368a028. [DOI] [PubMed] [Google Scholar]
- Thompson J. In vivo regulation of glycolysis and characterization of sugar: phosphotransferase systems in Streptococcus lactis. J Bacteriol. 1978 Nov;136(2):465–476. doi: 10.1128/jb.136.2.465-476.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren A. Polysaccharide accumulation in Fusiformis necrophorus. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Oct;82B(5):635–643. doi: 10.1111/j.1699-0463.1974.tb00230.x. [DOI] [PubMed] [Google Scholar]
- Whistler R. L., Lake W. C. Inhibition of cellular transport processes by 5-thio-D-glucopyranose. Biochem J. 1972 Dec;130(4):919–925. doi: 10.1042/bj1300919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth G., Buckel W. A sodium ion gradient as energy source for Peptostreptococcus asaccharolyticus. Arch Microbiol. 1985 Jul;142(2):128–135. doi: 10.1007/BF00447055. [DOI] [PubMed] [Google Scholar]




