Abstract
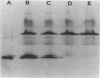
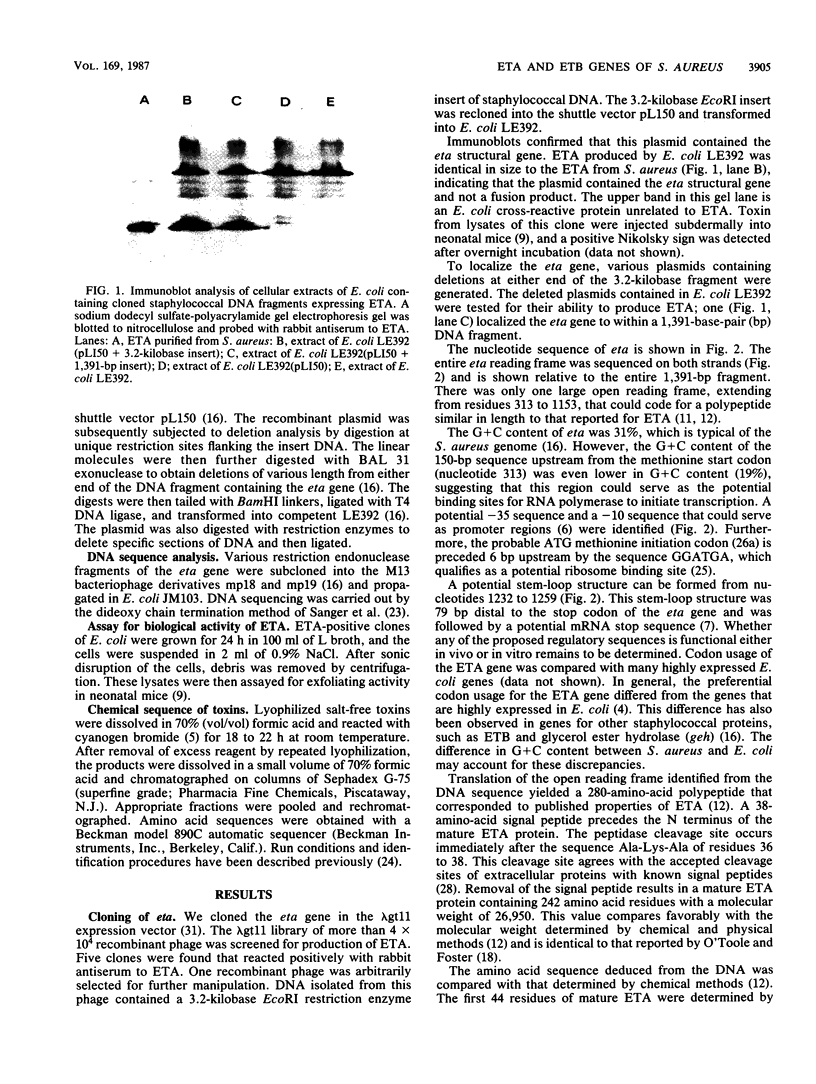
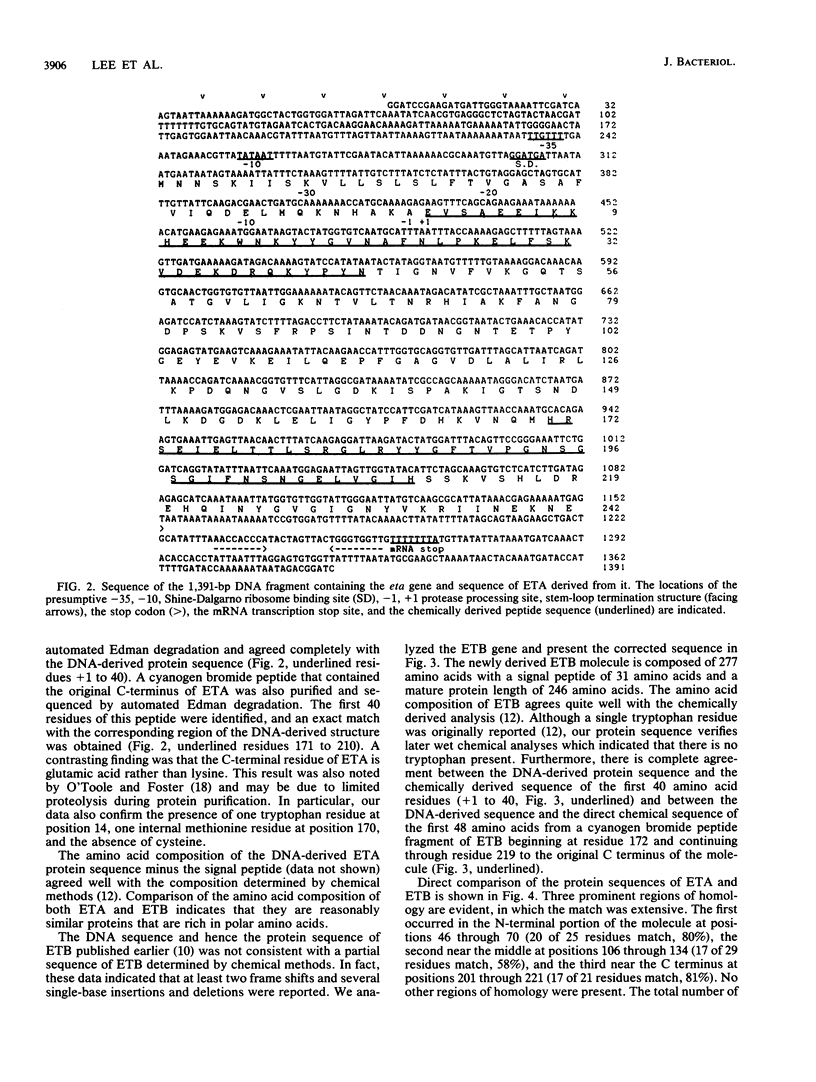
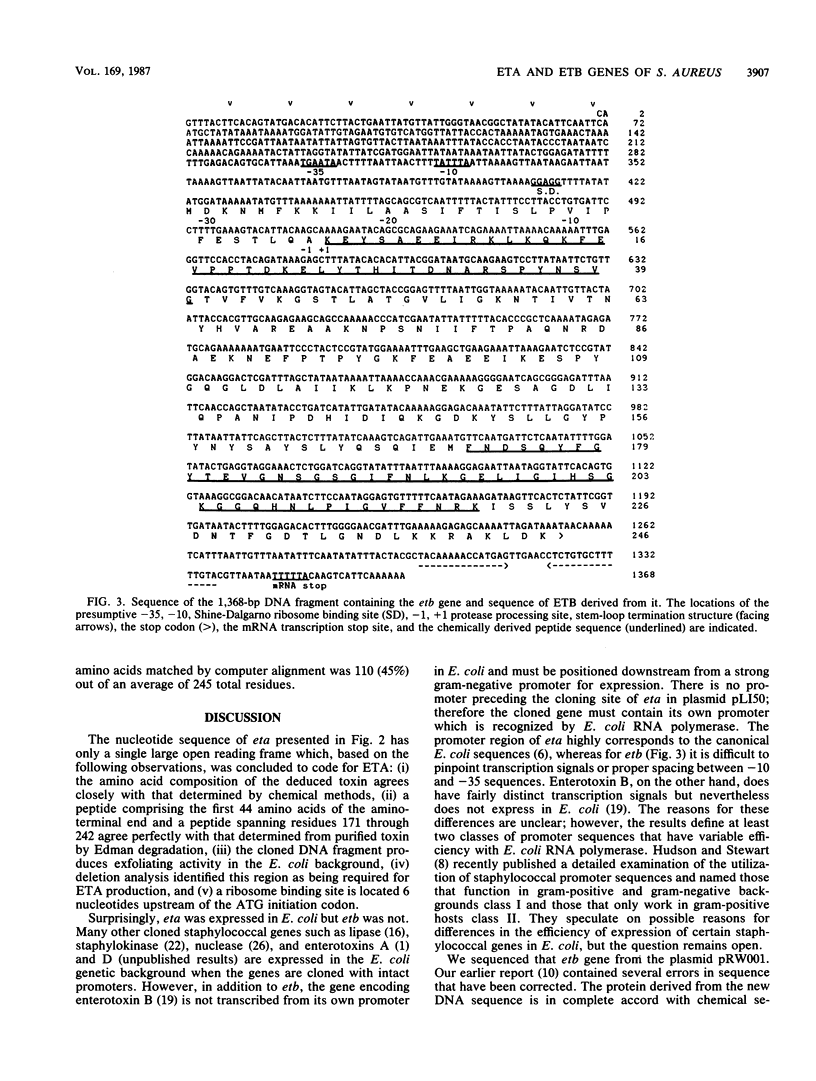
The DNA encoding the exfoliative toxin A gene (eta) of Staphylococcus aureus was cloned into bacteriophage lambda gt11 and subsequently into plasmid pLI50 on a 1,391-base-pair DNA fragment of the chromosome. Exfoliative toxin A is expressed in the Escherichia coli genetic background, is similar in length to the toxin purified from culture medium, and is biologically active in an animal assay. The nucleotide sequence of the DNA fragment containing the gene was determined. The protein deduced from the nucleotide sequence is a polypeptide of 280 amino acids. The mature protein is 242 amino acids. The DNA sequence of the exfoliative toxin B gene was also determined. Corrections indicate that the amino acid sequence of exfoliative toxin B is in accord with chemical sequence data.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betley M. J., Löfdahl S., Kreiswirth B. N., Bergdoll M. S., Novick R. P. Staphylococcal enterotoxin A gene is associated with a variable genetic element. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5179–5183. doi: 10.1073/pnas.81.16.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Dyer D. W., Iandolo J. J. Rapid isolation of DNA from Staphylococcus aureus. Appl Environ Microbiol. 1983 Jul;46(1):283–285. doi: 10.1128/aem.46.1.283-285.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes W. M., Platt T., Rosenberg M. Termination of transcription in E. coli. Cell. 1983 Apr;32(4):1029–1032. doi: 10.1016/0092-8674(83)90287-8. [DOI] [PubMed] [Google Scholar]
- Jackson M. P., Iandolo J. J. Cloning and expression of the exfoliative toxin B gene from Staphylococcus aureus. J Bacteriol. 1986 May;166(2):574–580. doi: 10.1128/jb.166.2.574-580.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. P., Iandolo J. J. Sequence of the exfoliative toxin B gene of Staphylococcus aureus. J Bacteriol. 1986 Aug;167(2):726–728. doi: 10.1128/jb.167.2.726-728.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. D., Spero L., Cades J. S., de Cicco B. T. Purification and characterization of different types of exfoliative toxin from Staphylococcus aureus. Infect Immun. 1979 Jun;24(3):679–684. doi: 10.1128/iai.24.3.679-684.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyhani M., Rogolsky M., Wiley B. B., Glasgow L. A. Chromosomal synthesis of staphylococcal exfoliative toxin. Infect Immun. 1975 Jul;12(1):193–197. doi: 10.1128/iai.12.1.193-197.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Iandolo J. J. Lysogenic conversion of staphylococcal lipase is caused by insertion of the bacteriophage L54a genome into the lipase structural gene. J Bacteriol. 1986 May;166(2):385–391. doi: 10.1128/jb.166.2.385-391.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole P. W., Foster T. J. Molecular cloning and expression of the epidermolytic toxin A gene of Staphylococcus aureus. Microb Pathog. 1986 Dec;1(6):583–594. doi: 10.1016/0882-4010(86)90043-4. [DOI] [PubMed] [Google Scholar]
- O'Toole P. W., Foster T. J. Nucleotide sequence of the epidermolytic toxin A gene of Staphylococcus aureus. J Bacteriol. 1987 Sep;169(9):3910–3915. doi: 10.1128/jb.169.9.3910-3915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranelli D. M., Jones C. L., Johns M. B., Mussey G. J., Khan S. A. Molecular cloning of staphylococcal enterotoxin B gene in Escherichia coli and Staphylococcus aureus. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5850–5854. doi: 10.1073/pnas.82.17.5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogolsky M., Warren R., Wiley B. B., Nakamura H. T., Glasgow L. A. Nature of the genetic determinant controlling exfoliative toxin production in Staphylococcus aureus. J Bacteriol. 1974 Jan;117(1):157–165. doi: 10.1128/jb.117.1.157-165.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum E. D., Tyrone S. Chromosomal determinants for exfoliative toxin production in two strains of staphylococci. Infect Immun. 1976 Nov;14(5):1259–1260. doi: 10.1128/iai.14.5.1259-1260.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sako T., Sawaki S., Sakurai T., Ito S., Yoshizawa Y., Kondo I. Cloning and expression of the staphylokinase gene of Staphylococcus aureus in Escherichia coli. Mol Gen Genet. 1983;190(2):271–277. doi: 10.1007/BF00330650. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. J., Spero L. The complete amino acid sequence of staphylococcal enterotoxin C1. J Biol Chem. 1983 May 25;258(10):6300–6306. [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortle D. A genetic system for analysis of staphylococcal nuclease. Gene. 1983 May-Jun;22(2-3):181–189. doi: 10.1016/0378-1119(83)90102-6. [DOI] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweten R. K., Iandolo J. J. Purification and partial characterization of a putative precursor to staphylococcal enterotoxin B. Infect Immun. 1981 Dec;34(3):900–907. doi: 10.1128/iai.34.3.900-907.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R., Rogolsky M., Wiley B. B., Glasgow L. A. Isolation of extrachromosomal deoxyribonucleic acid for exfoliative toxin production from phage group II Staphylococcus aureus. J Bacteriol. 1975 Apr;122(1):99–105. doi: 10.1128/jb.122.1.99-105.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]