Abstract
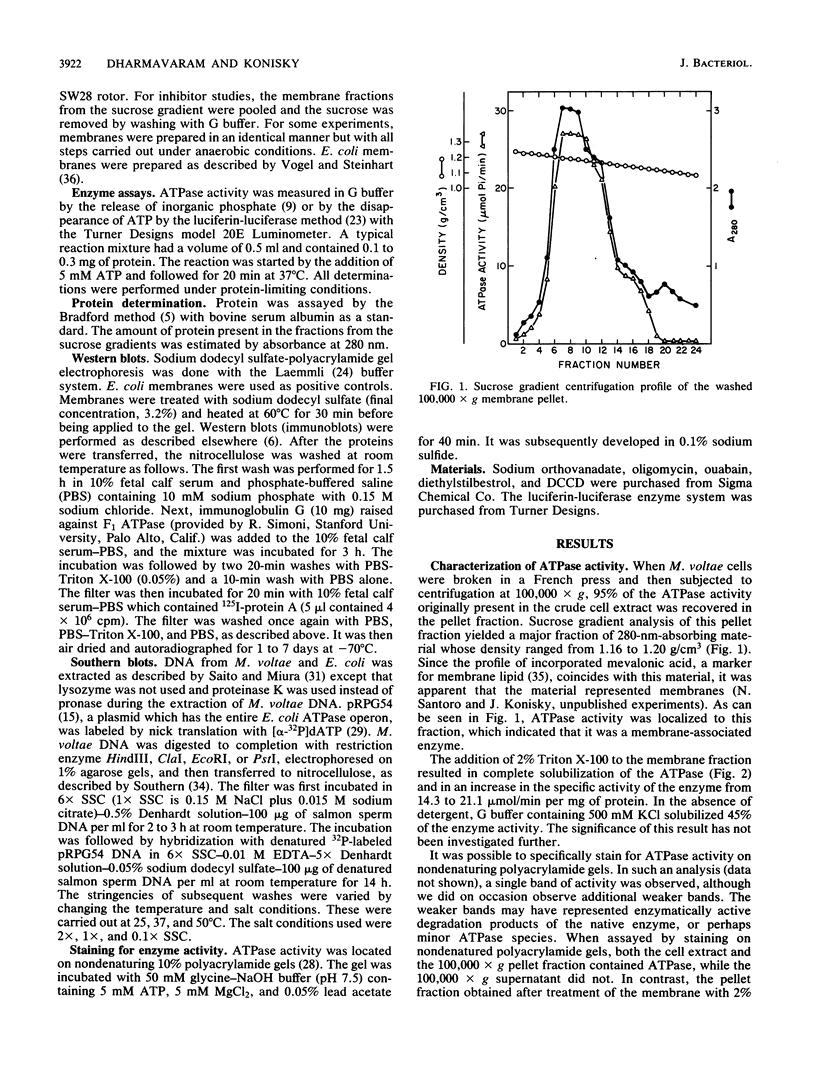
Membrane-bound ATPase activity was detected in the methanogen Methanococcus voltae. The ATPase was inhibited by vanadate, a characteristic inhibitor of E1E2 ATPases. The enzyme activity was also inhibited by diethylstilbestrol. However, it was insensitive to N,N'-dicyclohexylcarbodiimide, ouabain, and oligomycin. The enzyme displayed a high preference for ATP as substrate, was dependent on Mg2+, and had a pH optimum of approximately 7.5. The enzyme was completely solubilized with 2% Triton X-100. The enzyme was insensitive to oxygen and was stabilized by ATP. There was no homology with the Escherichia coli F0F1 ATPase at the level of DNA and protein. The membrane-bound M. voltae ATPase showed properties similar to those of E1E2 ATPases.
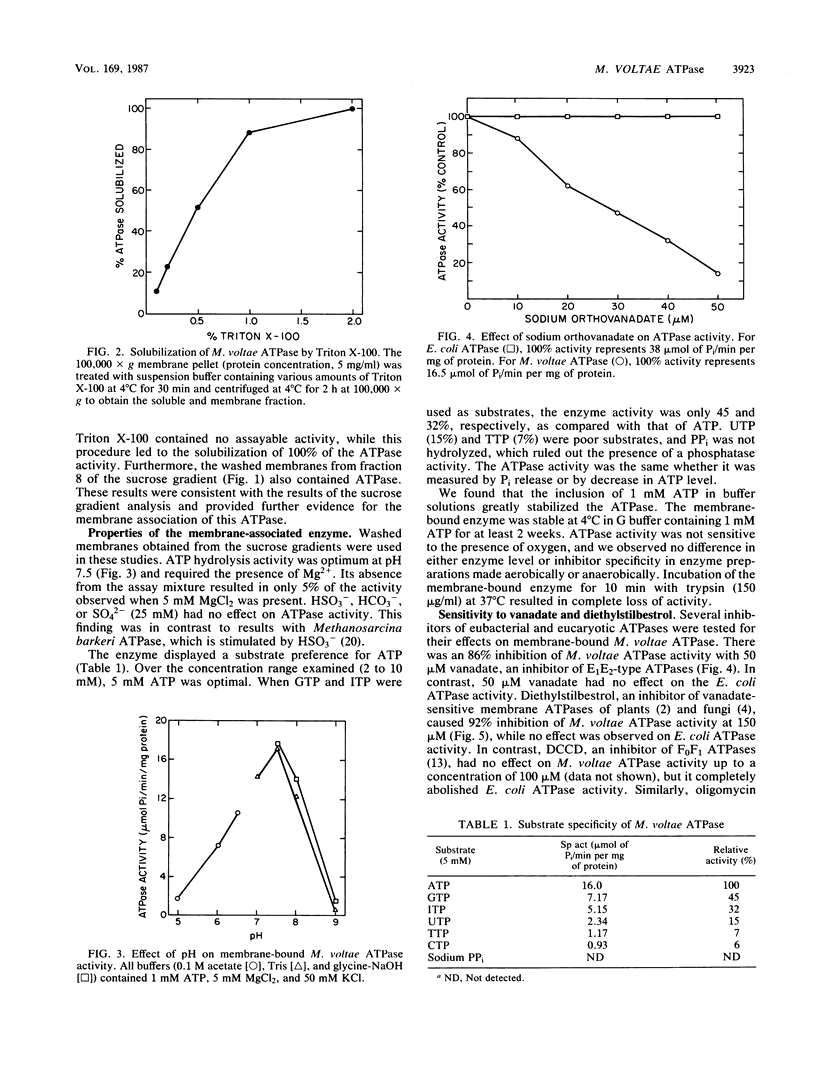
Full text
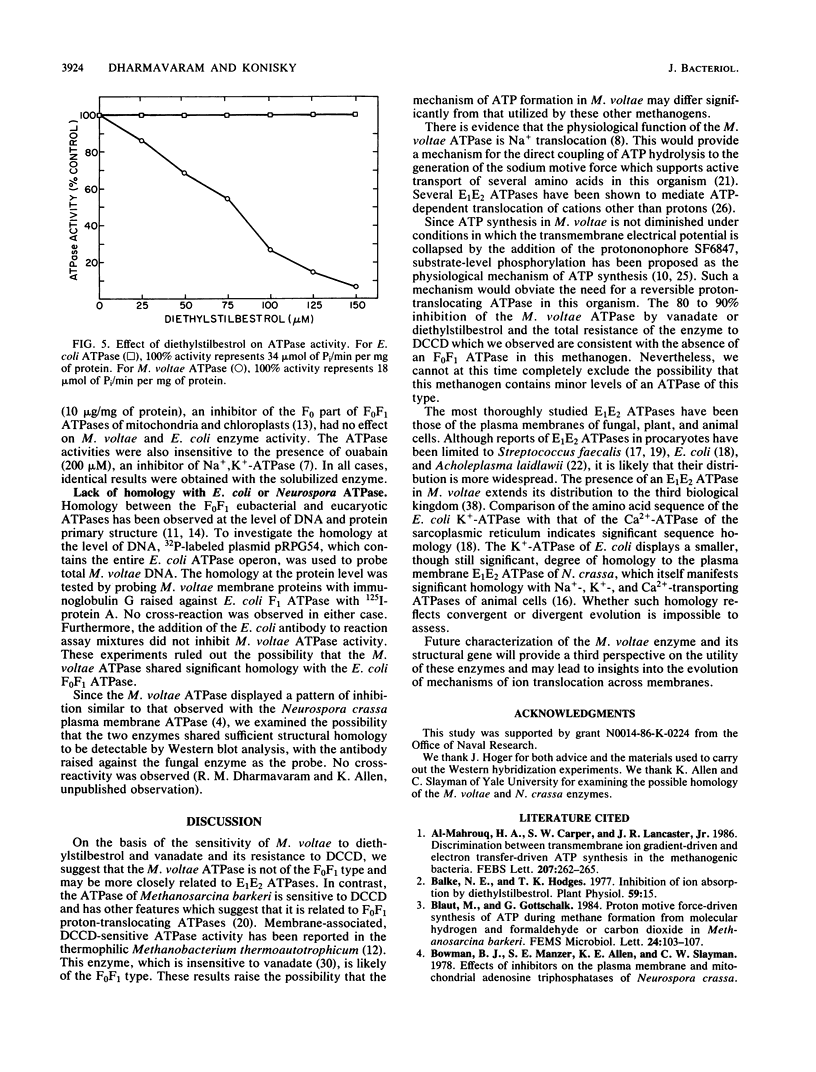
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowman B. J., Mainzer S. E., Allen K. E., Slayman C. W. Effects of inhibitors on the plasma membrane and mitochondrial adenosine triphosphatases of Neurospora crassa. Biochim Biophys Acta. 1978 Sep 11;512(1):13–28. doi: 10.1016/0005-2736(78)90214-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cantley L., Carilli C. T., Farley R. A., Perlman D. M. Location of binding sites on the (Na,K)-ATPase for fluorescein-5'-isothiocyanate and ouabain. Ann N Y Acad Sci. 1982;402:289–291. doi: 10.1111/j.1749-6632.1982.tb25749.x. [DOI] [PubMed] [Google Scholar]
- Crider B. P., Carper S. W., Lancaster J. R. Electron transfer-driven ATP synthesis in Methanococcus voltae is not dependent on a proton electrochemical gradient. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6793–6796. doi: 10.1073/pnas.82.20.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayhoff M. O., Schwartz R. M. Evidence on the origin of eukaryotic mitochondria from protein and nucleic acid sequences. Ann N Y Acad Sci. 1981;361:92–104. doi: 10.1111/j.1749-6632.1981.tb46513.x. [DOI] [PubMed] [Google Scholar]
- Doddema H. J., Hutten T. J., van der Drift C., Vogels G. D. ATP hydrolysis and synthesis by the membrane-bound ATP synthetase complex of Methanobacterium thermoautotrophicum. J Bacteriol. 1978 Oct;136(1):19–23. doi: 10.1128/jb.136.1.19-23.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. W., Lloyd D. Mitochondrial adenosine triphosphatase of the fission yeast, Schizosaccharomyces pombe 972h-. Changes in activity and oligomycin-sensitivity during the cell cycle of catabolite-repressed and -de-repressed cells. Biochem J. 1977 Jan 15;162(1):39–46. doi: 10.1042/bj1620039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai M., Kanazawa H. Structure and function of proton-translocating adenosine triphosphatase (F0F1): biochemical and molecular biological approaches. Microbiol Rev. 1983 Sep;47(3):285–312. doi: 10.1128/mr.47.3.285-312.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Brusilow W. S., Simoni R. D. Gene order and gene-polypeptide relationships of the proton-translocating ATPase operon (unc) of Escherichia coli. Proc Natl Acad Sci U S A. 1982 Jan;79(2):320–324. doi: 10.1073/pnas.79.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager K. M., Mandala S. M., Davenport J. W., Speicher D. W., Benz E. J., Jr, Slayman C. W. Amino acid sequence of the plasma membrane ATPase of Neurospora crassa: deduction from genomic and cDNA sequences. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7693–7697. doi: 10.1073/pnas.83.20.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heefner D. L., Harold F. M. ATP-driven sodium pump in Streptococcus faecalis. Proc Natl Acad Sci U S A. 1982 May;79(9):2798–2802. doi: 10.1073/pnas.79.9.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse J. E., Wieczorek L., Altendorf K., Reicin A. S., Dorus E., Epstein W. Sequence homology between two membrane transport ATPases, the Kdp-ATPase of Escherichia coli and the Ca2+-ATPase of sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4746–4750. doi: 10.1073/pnas.81.15.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugentobler G., Heid I., Solioz M. Purification of a putative K+-ATPase from Streptococcus faecalis. J Biol Chem. 1983 Jun 25;258(12):7611–7617. [PubMed] [Google Scholar]
- Inatomi K. Characterization and purification of the membrane-bound ATPase of the archaebacterium Methanosarcina barkeri. J Bacteriol. 1986 Sep;167(3):837–841. doi: 10.1128/jb.167.3.837-841.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinks D. C., Silvius J. R., McElhaney R. N. Physiological role and membrane lipid modulation of the membrane-bound (Mg2+, na+)-adenosine triphosphatase activity in Acholeplasma laidlawii. J Bacteriol. 1978 Dec;136(3):1027–1036. doi: 10.1128/jb.136.3.1027-1036.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmich G. A., Randles J., Brand J. S. Assay of picomole amounts of ATP, ADP, and AMP using the luciferase enzyme system. Anal Biochem. 1975 Nov;69(1):187–206. doi: 10.1016/0003-2697(75)90580-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerner H. R., Avron M. Dihydroxyacetone Kinase Activity in Dunaliella parva. Plant Physiol. 1977 Jan;59(1):15–17. doi: 10.1104/pp.59.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountfort D. O., Mörschel E., Beimborn D. B., Schönheit P. Methanogenesis and ATP synthesis in a protoplast system of Methanobacterium thermoautotrophicum. J Bacteriol. 1986 Nov;168(2):892–900. doi: 10.1128/jb.168.2.892-900.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen P., Salton M. R. Antigenic and enzymatic architecture of Micrococcus lysodeikticus membranes established by crossed immunoelectrophoresis. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3711–3715. doi: 10.1073/pnas.72.9.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Santoro N., Konisky J. Characterization of bromoethanesulfonate-resistant mutants of Methanococcus voltae: evidence of a coenzyme M transport system. J Bacteriol. 1987 Feb;169(2):660–665. doi: 10.1128/jb.169.2.660-665.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönheit P., Beimborn D. B. ATP synthesis in Methanobacterium thermoautotrophicum coupled to CH4 formation from H2 and CO2 in the apparent absence of an electrochemical proton potential across the cytoplasmic membrane. Eur J Biochem. 1985 May 2;148(3):545–550. doi: 10.1111/j.1432-1033.1985.tb08874.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sprott G. D., Shaw K. M., Jarrell K. F. Isolation and chemical composition of the cytoplasmic membrane of the archaebacterium Methanospirillum hungatei. J Biol Chem. 1983 Mar 25;258(6):4026–4031. [PubMed] [Google Scholar]
- Vogel G., Steinhart R. ATPase of Escherichia coli: purification, dissociation, and reconstitution of the active complex from the isolated subunits. Biochemistry. 1976 Jan 13;15(1):208–216. doi: 10.1021/bi00646a032. [DOI] [PubMed] [Google Scholar]
- Whitman W. B., Ankwanda E., Wolfe R. S. Nutrition and carbon metabolism of Methanococcus voltae. J Bacteriol. 1982 Mar;149(3):852–863. doi: 10.1128/jb.149.3.852-863.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Magrum L. J., Fox G. E. Archaebacteria. J Mol Evol. 1978 Aug 2;11(3):245–251. doi: 10.1007/BF01734485. [DOI] [PubMed] [Google Scholar]


