Abstract
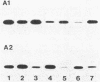
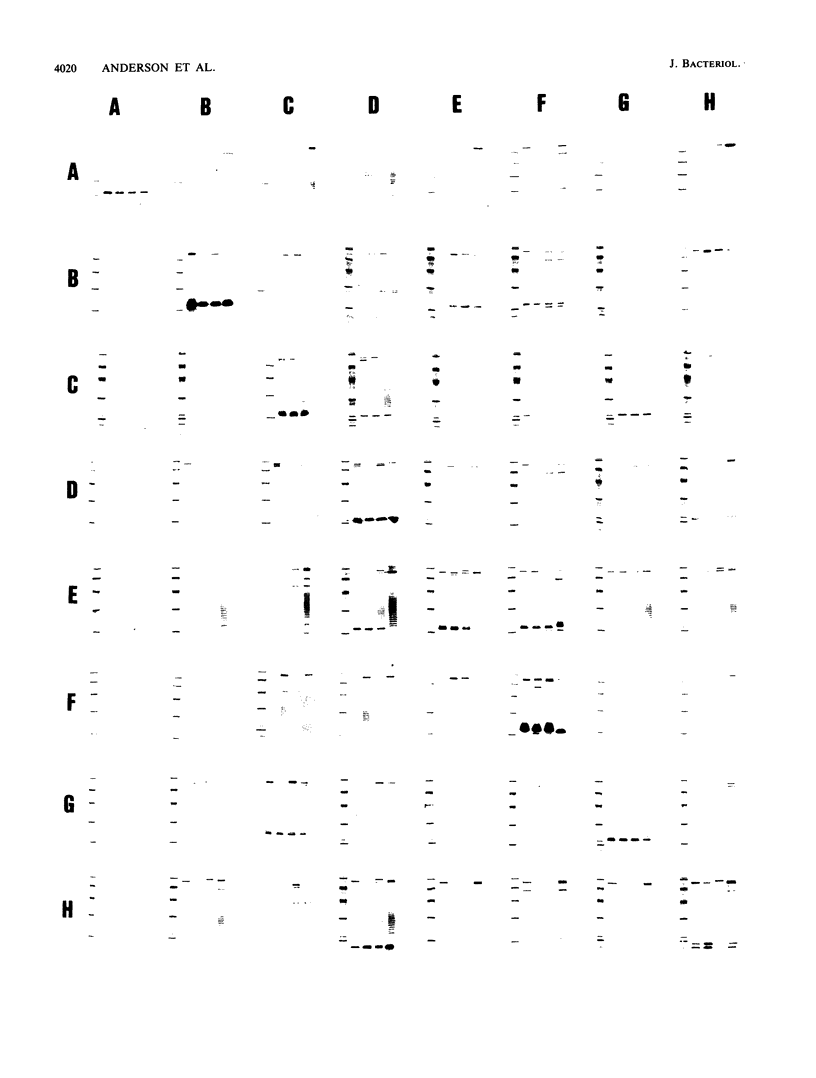
The roles of the fimbrial subunit and the putative basal protein antigens in the serological classification of Bacteroides nodosus have been examined by Western blot (immunoblot)-antibody binding studies of fimbriae isolated from a wide range of strains representative of different serogroups and serotypes. Fimbrial subunits were recognized by antiserum against the homologous serogroup but not generally by heterologous antisera, whereas recognition of the basal antigen was independent of serological classification. Secondary cross-reaction patterns among fimbrial subunits indicated that some serogroups may be more closely related than others. Examples include serogroups C and G and serogroups D and H. Similar analyses of isolates classified within serotypes A1 and A2, with serotype-specific antisera, showed that this subdivision is also determined by the fimbrial subunit and that significant variation does occur even at this level. These studies suggest that the various serogroups and serotypes of B. nodosus comprise a series of overlapping sets of antigenically related strains.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. J., Kristo C. L., Egerton J. R., Mattick J. S. Variation in the structural subunit and basal protein antigens of Bacteroides nodosus fimbriae. J Bacteriol. 1986 May;166(2):453–460. doi: 10.1128/jb.166.2.453-460.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesley J. E., Day S. E., Betts M. P., Thorley C. M. Immunocytochemical labelling of Bacteroides nodosus pili using an immunogold technique. J Gen Microbiol. 1984 Jun;130(6):1481–1487. doi: 10.1099/00221287-130-6-1481. [DOI] [PubMed] [Google Scholar]
- Claxton P. D., Ribeiro L. A., Egerton J. R. Classification of Bacteroides nodosus by agglutination tests. Aust Vet J. 1983 Nov;60(11):331–334. doi: 10.1111/j.1751-0813.1983.tb02834.x. [DOI] [PubMed] [Google Scholar]
- Egerton J. R., Roberts D. S., Parsonson I. M. The aetiology and pathogenesis of ovine foot-rot. I. A histological study of the bacterial invasion. J Comp Pathol. 1969 Apr;79(2):207–215. doi: 10.1016/0021-9975(69)90007-3. [DOI] [PubMed] [Google Scholar]
- Egerton J. R. Significance of Fusiformis nodosus serotypes in resistance of vaccinated sheep to experimental foot-rot. Aust Vet J. 1974 Feb;50(2):59–62. doi: 10.1111/j.1751-0813.1974.tb05252.x. [DOI] [PubMed] [Google Scholar]
- Egerton J. R. Surface and somatic antigens of Fusiformis nodosus. J Comp Pathol. 1973 Jan;83(1):151–159. doi: 10.1016/0021-9975(73)90038-8. [DOI] [PubMed] [Google Scholar]
- Elleman T. C., Hoyne P. A., McKern N. M., Stewart D. J. Nucleotide sequence of the gene encoding the two-subunit pilin of Bacteroides nodosus 265. J Bacteriol. 1986 Jul;167(1):243–250. doi: 10.1128/jb.167.1.243-250.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Every D. Purification of pili from Bacteroides nodosus and an examination of their chemical, physical and serological properties. J Gen Microbiol. 1979 Dec;115(2):309–316. doi: 10.1099/00221287-115-2-309. [DOI] [PubMed] [Google Scholar]
- Every D., Skerman T. M. Protection of sheep against experimental footrot by vaccination with pili purified from Bacteroides nodosus. N Z Vet J. 1982 Oct;30(10):156–158. doi: 10.1080/00480169.1982.34921. [DOI] [PubMed] [Google Scholar]
- Hagblom P., Segal E., Billyard E., So M. Intragenic recombination leads to pilus antigenic variation in Neisseria gonorrhoeae. Nature. 1985 May 9;315(6015):156–158. doi: 10.1038/315156a0. [DOI] [PubMed] [Google Scholar]
- Handley B. G., Ng M. L., Schwartzkoff C. L. Electrophoretic mobility of Bacteroides nodosus pilus antigens. Aust Vet J. 1986 Feb;63(2):62–63. doi: 10.1111/j.1751-0813.1986.tb02928.x. [DOI] [PubMed] [Google Scholar]
- Henrichsen J. Twitching motility. Annu Rev Microbiol. 1983;37:81–93. doi: 10.1146/annurev.mi.37.100183.000501. [DOI] [PubMed] [Google Scholar]
- Hindmarsh F., Fraser J. Serogroups of Bacteroides nodosus isolated from ovine footrot in Britain. Vet Rec. 1985 Feb 16;116(7):187–188. doi: 10.1136/vr.116.7.187. [DOI] [PubMed] [Google Scholar]
- Lee S. W., Alexander B., McGowan B. Purification, characterization, and serologic characteristics of Bacteroides nodosus pili and use of a purified pili vaccine in sheep. Am J Vet Res. 1983 Sep;44(9):1676–1681. [PubMed] [Google Scholar]
- Lehr C., Jayappa H. G., Goodnow R. A. Serologic and protective characterization of Moraxella bovis pili. Cornell Vet. 1985 Oct;75(4):484–492. [PubMed] [Google Scholar]
- Lepper A. W., Hermans L. R. Characterisation and quantitation of pilus antigens of Moraxella bovis by ELISA. Aust Vet J. 1986 Dec;63(12):401–405. [PubMed] [Google Scholar]
- Marrs C. F., Schoolnik G., Koomey J. M., Hardy J., Rothbard J., Falkow S. Cloning and sequencing of a Moraxella bovis pilin gene. J Bacteriol. 1985 Jul;163(1):132–139. doi: 10.1128/jb.163.1.132-139.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick J. S., Bills M. M., Anderson B. J., Dalrymple B., Mott M. R., Egerton J. R. Morphogenetic expression of Bacteroides nodosus fimbriae in Pseudomonas aeruginosa. J Bacteriol. 1987 Jan;169(1):33–41. doi: 10.1128/jb.169.1.33-41.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKern N. M., O'Donnell I. J., Stewart D. J., Clark B. L. Primary structure of pilin protein from Bacteroides nodosus strain 216: comparison with the corresponding protein from strain 198. J Gen Microbiol. 1985 Jan;131(1):1–6. doi: 10.1099/00221287-131-1-1. [DOI] [PubMed] [Google Scholar]
- Ottow J. C. Ecology, physiology, and genetics of fimbriae and pili. Annu Rev Microbiol. 1975;29:79–108. doi: 10.1146/annurev.mi.29.100175.000455. [DOI] [PubMed] [Google Scholar]
- Pugh G. W., Jr, Hughes D. E., Booth G. D. Experimentally induced infections bovine keratoconjunctivitis: effectiveness of a pilus vaccine against exposure to homologous strains of Moraxella bovis. Am J Vet Res. 1977 Oct;38(10):1519–1522. [PubMed] [Google Scholar]
- Sastry P. A., Finlay B. B., Pasloske B. L., Paranchych W., Pearlstone J. R., Smillie L. B. Comparative studies of the amino acid and nucleotide sequences of pilin derived from Pseudomonas aeruginosa PAK and PAO. J Bacteriol. 1985 Nov;164(2):571–577. doi: 10.1128/jb.164.2.571-577.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J. A., Gradin J. L. Serotypic and biochemical characterization of Bacteroides nodosus isolates from Oregon. Can J Comp Med. 1980 Oct;44(4):440–446. [PMC free article] [PubMed] [Google Scholar]
- Stewart D. J. An electron microscopic study of Fusiformis nodosus. Res Vet Sci. 1973 Jan;14(1):132–134. [PubMed] [Google Scholar]
- Stewart D. J., Clark B. L., Emery D. L., Peterson J. E., Fahey K. J. A Bacteroides nodosus immunogen, distinct from the pilus, which induces cross-protective immunity in sheep vaccinated against footrot. Aust Vet J. 1983 Mar;60(3):83–85. doi: 10.1111/j.1751-0813.1983.tb05877.x. [DOI] [PubMed] [Google Scholar]
- Stewart D. J., Clark B. L., Peterson J. E., Griffiths D. A., Smith E. F. Importance of pilus-associated antigen in Bacteroides nodosus vaccines. Res Vet Sci. 1982 Mar;32(2):140–147. [PubMed] [Google Scholar]
- Thorley C. M., Egerton J. R. Comparison of alum-absorbed or non-alum-absorbed oil emulsion vaccines containing either pilate or non-pilate Bacteroides nodosus cells in inducing and maintaining resistance of sheep to experimental foot rot. Res Vet Sci. 1981 Jan;30(1):32–37. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P. D., Short J., Thomson R. O., Roberts D. S. The fine structure of Fusiformis nodosus with special reference to the location of antigens associated with immunogenicity. J Gen Microbiol. 1973 Aug;77(2):351–361. doi: 10.1099/00221287-77-2-351. [DOI] [PubMed] [Google Scholar]