Abstract
Objective To evaluate the benefits and harms of antithrombin III in critically ill patients.
Design Systematic review and meta-analysis of randomised trials.
Data sources CENTRAL, Medline, Embase, International Web of Science, LILACS, the Chinese Biomedical Literature Database, and CINHAL (to November 2006); hand search of reference lists, contact with authors and experts, and search of registers of ongoing trials.
Review methods Two reviewers independently selected parallel group randomised clinical trials comparing antithrombin with placebo or no intervention and extracted data related to study methods, interventions, outcomes, bias risk, and adverse events. Disagreements were resolved by discussion. Trials in any type of critically ill patients in intensive care were eligible. All trials, irrespective of blinding or language status, that compared any antithrombin III regimen with no intervention or placebo were included. Trials were considered to be at low risk of bias if they had adequate randomisation procedure, blinding, and used intention to treat analysis. Risk ratios with 95% confidence intervals were estimated with fixed and random effects models according to heterogeneity.
Main outcome measures Mortality, length of stay in intensive care or hospital, quality of life, severity of sepsis, respiratory failure, duration of mechanical ventilation, incidence of surgical intervention, intervention effect among various populations, and adverse events (such as bleeding).
Results 20 trials randomly assigning 3458 patients met inclusion criteria. Eight trials had low risk of bias. Compared with placebo or no intervention, antithrombin III did not reduce overall mortality (relative risk 0.96, 95% confidence interval 0.89 to 1.03). No subgroup analyses on risk of bias, populations of patients, or with and without adjuvant heparin yielded significant results. Antithrombin III increased the risk of bleeding events (1.52, 1.30 to 1.78). Heterogeneity was observed in only a few analyses.
Conclusion Antithrombin III cannot be recommended for critically ill patients based on the available evidence.
Introduction
Mortality in critically ill patients is substantial and can reach as high as 30-50% in those with severe sepsis and septic shock.1 Each year in the United States around 750 000 patients become critically ill and are admitted to intensive care units.2 Critical illness results in uncontrolled inflammation and vascular damage even when the cause of the illness is not infection (sepsis)—for example, trauma, malignancy, complications of pregnancy, poisoning, allergic reactions, and liver failure.3 During critical illness, systemic activation of coagulation can occur, which can result in disseminated intravascular coagulation. This is characterised by simultaneous widespread microvascular thrombosis and profuse bleeding from various sites.4
Antithrombin III is a potent anticoagulant with anti-inflammatory properties. It is thought to have an inhibitory effect on the proinflammatory and procoagulant processes.5 6 The blood concentration of antithrombin III falls by 20-40% in critically ill (septic) patients and correlates with severity of disease.5 7 Hence, theoretically replenishing concentrations might benefit critically ill patients. Furthermore, its interaction with heparin, which is a standard treatment for patients with disseminated intravascular coagulation, seems important.w1
Four small meta-analyses assessed the use of antithrombin III in critically ill patients. Two assessed patients with severe sepsis, one included three randomised clinical trials,w2 and one included eight trials (both randomised and non-randomised).8 One recent meta-analysis examined the effect of antithrombin III on mortality among 364 patients with disseminated intravascular coagulation in three trials.w3 Finally, a Cochrane systematic review with two randomised controlled trials assessed use of antithrombin III for respiratory distress syndrome in preterm infants.9 These meta-analyses had selected populations. All found non-significant effects on mortality, with wide confidence intervals (that is, “absence of evidence”). Only one of these meta-analyses was a systematic review.9
Information from the Danish Medicinal Agency (www.medstat.dk) suggests that the extrapolated annual costs of antithrombin III in the US and the European Union are around £70m (€100m, $145m). The clinical benefit of supplementation in critically ill patients, however, is still controversial and its efficacy debated.
Methods
Study selection
We searched the Cochrane central register of controlled trials (CENTRAL), Medline (1950 to November 2006), Embase (1980 to November 2006), International Web of Science (1945 to November 2006), LILACS (1984 to March 2005), the Chinese Biomedical Literature Database (to March 2005), and CINHAL (1982 to March 2005). We used the search terms: antithrombin, antithrombin-3, sepsis, and critically ill. Table 1 gives details of the search strategy. We limited searches to controlled or randomised controlled trials in humans. There were no language restrictions. We also hand searched the reference lists of included trials and relevant reviews for additional trials. We contacted the main authors of included trials and experts to ask for any missed, unreported, or ongoing trials. We searched for ongoing clinical trials and unpublished trials on http://controlled-trials.com, http://clinicaltrials.gov, and http://centerwatch.com (Centre Watch Clinical Trials Listing Service) up to March 2005.
Table 1.
Search strategy
| Database | Search date | Search strategy | Search result |
|---|---|---|---|
| Cochrane central register of controlled trials on Cochrane library | Nov 2006 | 1: Antithrombin, 2: Antithrombin III, 3: AT III, 4: ATIII, 5: antithrombi*, 1 OR 2 OR 3 OR 4 OR 5 | 1367 |
| Medline | 1950-Nov 2006 | 1: Antithrombins [MeSH], 2: Antithrombin III [MeSH], 3: antithrombin*, 4: AT III OR ATIII OR antithrombin III, 5: ((((1)) OR (2)) OR (3)) OR (4), 6: random* OR controlled OR blind* OR placebo OR “controlled ? trial”, 7: ((6)) AND (5). Limits: humans | 1479 |
| Embase | 1980-Nov 2006 | 1: random*, 2: controlled, 3: blind*, 4: placebo, 5: antithrombi*, 6: antithrombin III, 7: ATIII, 8: AT III, 9: (antithrombi*) or (AT III) or (ATIII) or (antithrombin III), 10: (placebo) or (blind*) or (controlled) or (random*), 11: ((placebo) or (blind*) or (controlled) or (random*)) and ((antithrombi*) or (AT III) or (ATIII) or (antithrombin III)) | 4801 |
| Science Citation Index Expanded | 1945-Nov 2006 | 1: antithrombin*, 2: antithrombin III, 3: ATIII, 4: 3 OR 2 OR 1, 5: random*, 6: controlled, 7: placebo, 8: blind*, 9: 8 OR 7 OR 6 OR 5, 10: 9 AND 4 | 1128 |
| Latin American Caribbean Health Sciences Literature (LILACS), Chinese Biomedical Literature Database, and CINHAL | March 2005 | 1: Antithrombins, 2: Antithrombin III, 3: antithrombin*, 4: AT III OR ATIII OR antithrombin III, 5: ((((1)) OR (2)) OR (3)) OR (4), 6: random* OR controlled OR blind* OR placebo OR “controlled ? trial”, 7: ((6)) AND (5). Limits: humans | No randomised trials of relevance |
Trials were included only if they were clearly randomised trials, reported in a full paper article, and provided data on mortality. The included patients were loosely defined as being critically ill—that is, sepsis, septic shock, disseminated intravascular coagulation, and other critical illnesses as proposed by the authors. There was no restriction of dose and duration of treatment with antithrombin III. We excluded trials assessing administration of antithrombin III for the reduction of cardiovascular events in invasive treatment of acute myocardial infarction.
Data abstraction
Two investigators (AA and JW) independently screened the titles and abstracts for relevant articles identified by the search strategy. They also abstracted trial data based on a predefined detailed data abstraction form. Disagreements were resolved by discussion. Authors of the included trials were approached for relevant additional information on outcome measures and risk of bias.
We identified the primary author’s name, year of publication, inclusion and exclusion criteria, number of randomised patients, type of participants (for example, trauma, septic, etc), dose and duration of antithrombin III, and duration of follow-up. We evaluated the validity and design characteristics of each trial and major potential sources of bias (such as adequate random sequence generation, adequate allocation concealment, adequate blinding, and use of intention to treat analysis). Trials were defined as low risk of bias if they fulfilled all of the above criteria.10 Our primary outcome was mortality. For this outcome we used the longest follow-up data from each trial. Secondary outcomes included length of stay in intensive care or hospital, quality of life, severity of sepsis, respiratory failure (mechanically assisted ventilation), duration of mechanical ventilation, incidence of surgical intervention, intervention effect among various populations and adverse events (such as bleeding).
Statistical analysis
We used random and fixed effect models for all meta-analyses. In the case of heterogeneity, we reported results from the random effects model. We analysed data by intention to treat and included all patients. We calculated the relative risk with 95% confidence intervals for dichotomous variables and the weighted mean difference for continuous outcomes. Heterogeneity was explored by Cochrane’s Q2 test and I2.11 I2 can be interpreted as the proportion of total variation observed between the trials attributable to differences between trials rather than sampling error (chance). I2 >75% is considered as a heterogeneous meta-analysis.
We carried out subgroup analyses in different populations (for example, trauma, newborns, etc), in patients with and without adjuvant heparin, in trials with short (less than one week) and long duration of treatment, in trials with short (less than the median follow-up) and long follow-up, and in trials with high and low risk of bias. If any results were significant, we estimated the difference between the estimates of the subgroups according to tests of interaction.12 P<0.05 indicates that the effects of treatment differ between the tested subgroups.
To assess publication bias and other types of bias we created funnel plots for mortality in which were plotted the log risk ratios against their standard errors as proposed by Egger et al.13
Estimation of risk ratio in our meta-analysis did not include trials with no events in both intervention groups. Because such trials might be important, however, we performed an exploratory analysis adding an imagined trial with one death in each study group and with a total number of participants being equal to the total number of participants in all the trials with no events.
In a single trial, interim analyses increase the risk of type I errors. To avoid this, monitoring boundaries can be applied to decide whether a single randomised trial could be terminated early because the P value was sufficiently small.14 Because there is no reason why the standards for a meta-analysis should be less rigorous than those for a single trial, analogous monitoring boundaries can be applied to meta-analyses; these are called trial sequential monitoring boundaries. Trial sequential analysis provides the necessary sample size for our meta-analysis and boundaries that determine whether the evidence in our meta-analysis is reliable and conclusive.15
Results
Study search results
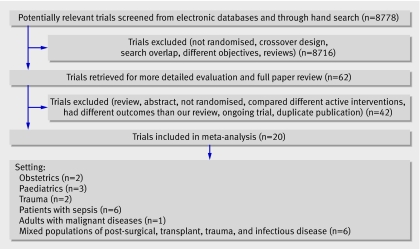
We identified 8778 references through electronic and hand searches (fig 1). After screening titles and abstracts, we excluded 8716 clearly irrelevant references and retrieved 62 references, all written in English, for further assessment. Twenty trials (described in 21 references with one duplicate publication) fulfilled our inclusion criteria (table 2). These trials were published from 1991 to 2003. We received additional data for six trials through correspondence with authors. We excluded 40 trials because they were reported as abstracts, were ongoing trials (data unavailable), had unclear trial design, were not randomised studies, or did not provide mortality data (table 3).
Fig 1 Identification of trials for inclusion
Table 2.
Characteristics of 20 included randomised trials on use of antithrombin III in critically ill patients
| Study | No | Population/trial description | Intervention/characteristics* | Heparin (both groups) | Follow-up (days) | ITT/bias risk† |
|---|---|---|---|---|---|---|
| Albert et al,w14 1992 | 32 | ICU patients with <70% AT III level, Primary end point: mortality. No sample size calculation | Infusion twice daily as long as AT III <90%. Total amount: 3500-17 000 IU. AT III before treatment unknown. No placebo | Yes | 90 | No/high |
| Baudo et al,w15 1992 | 29 | Liver transplantation in cirrhotic patients. Primary outcome: coagulatory variables and bleeding. No sample size calculation | Substitution preoperatively until AT III >100%, infusion 1000 IU/h during entire surgery. Total amount: 1000-4000 IU before surgery; 1740-12 000 IU during surgery. No placebo | No | NA | Yes/high |
| Baudo et al,w16 1998 | 120 | ICU patients with <70% AT III. Primary outcomes: mortality, 30 day survival, MOF score, FFP, and PC requirements. Sample size calculated | Fixed dose of 4000 AT III and 2000 IU/12 hours, 5 days. Total amount: 24 000 IU. AT III (%) before treatment: 52.9 (SD 14.5) IU in placebo group; 52.8 (15.5) IU in AT III group. Placebo: albumin | No | 30 | No/low |
| Diaz-Cremadez et al,w17 1994 | 195 | ICU patients with <70% AT III, no DIC. Primary outcome: mortality. No sample size calculation | Initial dose 60 U/kg + 10 U/kg every 6 hours. Total amount: 11.165 (SD 5.980) IU. AT III (%) before treatment: 48.3 (SD 12.2) IU in placebo group: 52 (11.7) IU in AT III group. Placebo: albumin | Yes | NA | No/high |
| Eisele et al,w2 1998 | 42 | Septic and critically ill patients. Primary outcome: 30 day mortality. No sample size calculation | Loading dose: 3000 IU + 1500 IU/12 h, 5 days. Total amount: 18 000 IU. AT III (%) before treatment: 49.0 (SD 19.1) IU in placebo group; 45.7 (14.4) in AT II group. Placebo: unknown | No | 30 | Yes/high |
| Fourrier et al,w18 1993 | 35 | Critically ill with septic shock and DIC. Primary outcome: 28 day mortality. Sample size calculated | Loading dose over 3 h (3 ml/kg) + 3 ml/kg over 21 h, then 90-120 IU/kg/day for 3 days. Total amount: average 6000 IU. AT III (%) before treatment: 44 (SD 16) IU in placebo group; 52 (20) IU in AT III group. Placebo: albumin | No | 28 | Yes/low |
| Fulia et al,w19 2003 | 60 | Infants, gestational age <30 weeks, <40% AT III. Primary outcome: mortality and intraventricular haemorrhage. No sample size calculation | Loading dose: 2 ml/kg (100 U/kg), then 1 ml/kg (50 U/kg)/8 h for 48 hours. Total amount: unknown. AT III (%) before treatment (mg/dl): 7.93 (SD 0.59) in placebo group; 8.22 (0.62) in AT III group. Placebo: glucose | Yes | 8 | Yes/low |
| Grenander et al,w20 2001 | 28 | Traumatic brain injury patients. Primary outcome: coagulatory variables, 90 days mortality. No sample size calculation | 60 IU/kg initially. 20 IU/kg 8 and 16 h later, total 100 IU/kg during 24 h (adjusted to nearest 500 IU). Total dose: 8269 (SD 1562) IU. AT III (%) before treatment: 0.87 (0.12) in control; 1.06 (0.46) in AT III group. No placebo | Yes | 90 | No/high |
| Haire et al,w21 1998 | 49 | Patients with malignant disease admitted for HSCT. Primary outcome: mortality, severity of illness score, length of hospital stay. Sample size calculated | 70 IU/kg <24 h of organ dysfunction detection + 50 IU/kg 8, 16, 48, and 72 h later. Mean total dose: 20 520 IU. No values of AT III before treatment. Placebo: albumin | No | 41 | Yes/low |
| Harper et al,w22 1991 | 50 | ICU population with <70% AT III. Primary outcome: mortality, coagulatory parameters. No sample size calculation | Aim: AT III >120. AT III until discharge from ICU, twice daily. No information on total amount or values before treatment. No placebo | No | 10 | Yes/high |
| Inthorn et al,w23 1997 | 40 | Severe sepsis, ICU population. Primary outcome: 14 and 90 days mortality, hospital discharge. Sample size calculated | Continuous AT III infusion over 14 days, Aim: AT III >120%. Total amount: 6000 IU on first day and 4000 IU on subsequent days. AT III (%) before treatment: 58 (SD 11) in control; 50.5 (3.2) in AT III group. No placebo | Yes | 90 | Yes/high |
| Kobayashi et al,w4 2003 | 29 | Severe pre-eclamptic shock, gestational age 24-36 weeks. Primary outcome: mortality, week of delivery, coagulatory parameters, gestosis index improvement. Sample size calculated | 1500 U/day once daily for 7 consecutive days. Total amount: 10.500 IU. No values of AT III before treatment. Placebo: unknown | Yes | 90 | Yes/low |
| Langley et al,w24 2003 | 25 | Hepatic coma with sepsis or risk of organ failure. Primary outcome: mortality, AT III activity. Sample size calculated | Initial dose: 3000 IU + 1000 IU/6 h unless normal AT III levels. Total amount: 3000-23 000 IU (mean 7000 (SD 5000 U). AT III (%) before treatment: 26 (4) in control; 26 (3) in AT III group. No placebo | No | 15 | Yes/high |
| Maki et al,w5 2000 | 66 | Severe pre-eclampsia (gestational age 24-35 weeks). Primary outcomes: mortality, gestosis index improvement, IUGR, coagulatory parameters. No sample size calculation | 3000 IU once daily for 7 consecutive days. Total amount: 21 000 IU. AT III (%) before treatment: 82.3 (SD 19.4) in placebo group; 72.3 (25.7) in AT III group. Placebo: albumin | No | 60 | Yes/low |
| Mitchell et al,w6 2003 | 109 | Children with ALL. Primary outcome: prevalence of thrombotic events, bleeding events. Sample size calculated | Once weekly for 4 weeks. Aim: ATIII levels between 3.0-4.0 units/ml. No data on total amount or values of AT III before treatment. No placebo | Yes | 28 | No/low |
| Schmidt et al,w25 1998 | 122 | Premature infants with RDS in neonatal ICU. Primary outcome: mortality. Sample size calculated | Loading dose 100 U/kg followed by 50 U/kg every 6 h for 48 h. No data on total amount of AT III. AT III (%) before treatment: 32 (SD 8) in placebo group; 33 (8) in AT III group. Placebo: albumin | Yes | 90 | Yes/low |
| Schorr et al,w26 2000 | 50 | Secondary peritonitis, surgical population in ICU. Primary outcome: 90 day mortality. Sample size calculated | Continuous infusion (200 IU-800 IU/h) + 2 intraperitoneal installations of FFS, aim: AT III >140%. Total amount: 26.196 (SD 299) IU. No data values of AT III before treatment. No placebo | No | 90 | Yes/high |
| Smith-Erichsen et al,w27 1996 | 83 | Critically ill and trauma patients in ICU. Primary outcome: plasma protease changes, mortality, days in ICU and hospital. No sample size calculation | Aim: AT III activity of 100% (SD 10%) for 3 consecutive days, max 14 days treatment. No data on total amount or values of AT III before treatment. No placebo | No | 34 | Yes/high |
| Warren et al,w1 2002 | 2314 | ICU critically ill patients with sepsis or septic shock. Primary outcome: mortality (28 and 90 days). Sample size calculated | Loading dose of 6000 IU over 30 min + continuous infusion of 6000 IU/day for 4 days. Total amount: 30 000 IU. No exact data on AT III before treatment. Placebo: albumin | Yes | 90 | Yes/low |
| Waydhas et al,w28 1998 | 40 | Trauma patients in ICU. Primary outcome: organ dysfunction, mortality, length of stay in ICU and hospital. No sample size calculation | Aim: AT III at 140%, first day via infusion, next 2 days once daily. Total amount: 20 000 IU (16.125-22.875 IU). AT III (%) before treatment: 44% (38-55) in placebo group; 45% (38-57) in AT III group. Placebo: albumin | Yes | 34 | Yes/high |
MOF score=multi-organ failure score; PC=platelet concentrates; FFS=fresh frozen serum; ICU=intensive care unit; AT III=antithrombin III; RDS=respiratory distress syndrome; DIC=disseminated intravascular coagulation; ALL=acute lymphoblastic leukaemia; IU=international unit; HSCT=haematopoietic stem cell transplantation; NA=not available; ITT=intention to treat analysis; IUGR=intrauterine growth retardation.
*Total amounts of AT III for each trial are average figures provided by authors of trials.
†Trials were defined as low bias risk if they adequately fulfilled all of random sequence generation, allocation concealment, blinding, and intention-to-treat analysis.
Table 3.
Characteristics of excluded articles
| Source (year) | Population | Design | Intervention | Reason for exclusion | |
|---|---|---|---|---|---|
| Balk,w7 1995 | Septic patients | Randomised, double blinded | AT III v placebo | Published only as an abstract | |
| Banfi,w29 1996 | Critically ill in ICU | Observational, retrospective | AT III | No randomisation | |
| Blauhut,w30 1985 | Critically ill in ICU | 3 group parallel randomised trial | AT III v AT III plus heparin v heparin | Comparison of 3 different interventions | |
| Brangenberg,w31 1997 | Preterm infants, gestational age 25-32 weeks | Observational study | AT III single dose | No randomisation | |
| Danielsson,w32 1997 | Burn injury ≥20% | Observational study | AT III | No randomisation | |
| du Cheyron D,w33 2006 | Critically ill, septic | Retrospective | AT III plus heparin, effect on filter lifespan of CRRT | No randomisation, different outcomes | |
| Fertmann,w34 2006 | Pancreas-kidney transplantation | Retrospective | AT III | No randomisation | |
| Fourrier,w35 1990 | Meningococcaemia (type B) and purpura fulminans | Observational, case report | AT III | No randomisation | |
| Hanada,w36 1985 | Children with DIC | Observational, case report | AT III and heparin v continuous heparin infusion | No randomisation | |
| Haussmann,w37 2006 | Critically ill children | Prospective case series, two phases | Defibrotide plus ATIII v no treatment | No randomisation | |
| Hellgren,w38 1984 | DIC | Pilot study, case report | AT III | No randomisation | |
| Hoffmann,w39 2004 | Severe sepsis | Randomised | See Inthorn 1997w23 | Based on a previously published trial | |
| Hoffmann,w13 2006 | Critically ill, severe sepsis | Randomised | See Warren 2001w1 | Based on a previously published trial | |
| Ilias,w40 2000 | Critically ill in ICU | Open, randomised | Intermittent bolus AT III v continuous infusion | Comparison of two different AT III regimens | |
| Inthorn,w41 1998 | Severe sepsis | Randomised | Se Inthorn 1997w23 | Based on a previously published trial | |
| Jochum,w42 1995 | Severe sepsis | Review and randomised, open trial | AT III plus heparin v heparin | Review plus reference to unpublished trial | |
| Kienast,w43 2006 | Sepsis with or without DIC | Randomised | See Warren 2001w1 | Based on previously published trial | |
| Korninger,w8 1987 | Peritoneovenous shunt operation because of intractable ascites | Randomised | AT III v placebo | Published as abstract only | |
| Kowal-Vern,w44 2000 | Thermal injuries, 2nd or 3rd degree burn ≥20% of body surface | Observational, open | AT III v no treatment | Allocation to group at patient’s choice | |
| Leitner,w45 2006 | Healthy male volunteers, infusion of endotoxin | Randomised | Two different regimens of AT III | No critically ill patient, comparison of two | AT III regimens |
| Maki,w46 1987 | Obstetric complications | Randomised | AT III v gabexate mesilate | Comparison of different interventions | |
| Messori,w47 2002 | Critically ill, sepsis, DIC | Observational retrospective | AT III | No randomisation | |
| Mitchell,w48 2003 | Children with ALL | Randomised | See Mitchell 2003w48 | Based on previously published trial | |
| Moriss,w49 1997 | Severe organ dysfunction after bone marrow transplantation | Observational study | AT III | No randomisation | |
| Muntean,w9 1989 | Premature infants (mean gestational age 31, range 26-34) | Randomised, open | AT III v control | Published as abstract only | |
| Nishiyama,w50 2006 | Abdominal aortic aneurysm | Randomised | AT III plus heparin v placebo | Not critically ill, different objectives | |
| Palareti,w10 1995 | Sepsis, post-surgical critically ill | Randomised | AT III v placebo | Published as abstract only | |
| Paternoster,w11 2000 | Pre-eclamptic population | Randomised | AT III v control | Published as brief communication only | |
| Paternoster,w51 2004 | Pre-eclamptic population | Randomised | Two regimens of AT III | Comparison of two AT III regimens | |
| Scherer,w52 1994 | Liver transplantation | Observational study | AT III v prothrombin complex substitution | No randomisation | |
| Scherer,w53 1997 | Liver cirrhosis scheduled for liver transplantation | Randomised | Two different AT III regimens v control | Different objectives, no data on mortality, comparison of 3 regimens | |
| Schuster,w12 1997 | Sepsis without DIC | Randomised | AT III plus heparin v albumin plus heparin | Published as abstract only | |
| Seitz,w54 1989 | Septic shock | Uncontrolled, open | AT III plus FFP plus heparin v heparin | No randomisation | |
| Shimada,w55 1994 | Hepatic resection (hepatocellular carcinoma) | Randomised | AT III v control group | Different objectives, no data on mortality | |
| Terao,w56 1989 | Pre-eclamptic population | Randomised | AT III v control group | Different objectives, no data on mortality | |
| van Beek,w57 1994 | Sepsis and <0.45 AT III | Retrospective | AT III v control group | No randomisation | |
| van Leeuwen,w58 2003 | Critically ill, severe sepsis | Randomised | See Warren 2001w1 | Based on previously published trial | |
| Vinazzer,w59 1995 | Severe sepsis, DIC | Review and case report | Various AT III trials | Review plus reference to unpublished trial | |
| Von Kries,w60 1985 | Newborns with DIC | Case report | AT III plus heparin | No randomisation | |
| Wiedermann,w3 2006 | Critically ill | Randomised | See Warren 2001w1 | Based on previously published trial | |
| D’angelo,w61 2005 | Pre-eclamptic, onset before 30th week of gestation | Randomised | AT III v control, no heparin | Ongoing, no data available |
AT III=antithrombin III; CRRT=continuous renal replacement therapy; ALL=acute lymphoblastic leukaemia; FFP=fresh frozen plasma; DIC=disseminated intravascular coagulation; ICU=intensive care unit.
Characteristics of included trials and assessment of risk of bias
The trials included 3458 patients, and the sample sizes varied from 25 to 2314. Patients had sepsis (n=13) or were from paediatric (n=3), obstetric (n=2), and trauma (n=2) specialties. The dose regimen of antithrombin III varied from a single bolus to 14 days of administration, aiming for above normal antithrombin III activity. Follow-up ranged from 7 to 90 days. All trials were carried out in high income countries.
Of the included trials, nine reported adequate allocation sequence generation; 12 adequate allocation concealment; 10 adequate double blinding; 14 performed intention to treat analysis, or provided sufficient data to allow us to do so, and the remaining trials excluded drop outs from the final analyses. Only eight trials reported adequate generation of allocation sequence, allocation concealment, blinding, and intention to treat analyses. These were classified as trials with low risk of bias (table 2).
Effects of antithrombin III
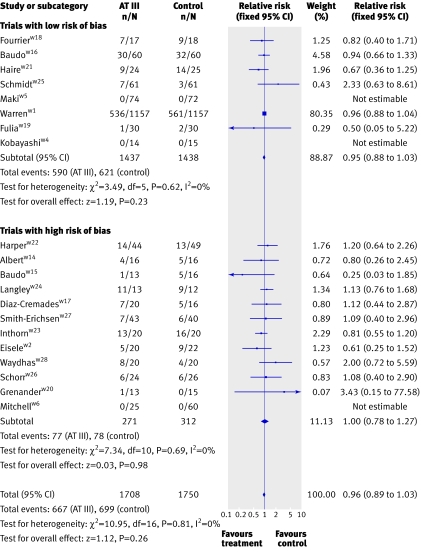
Combination of data from all 20 trials showed no significant effect of antithrombin III on mortality, with 667 (39.1%) deaths in the intervention group compared with 699 (39.9%) in the control group (pooled relative risk 0.96, 95% confidence interval 0.89 to 1.03). There was no heterogeneity between trials (I2=0%) (fig 2).
Fig 2 Forest plot of mortality (subgroup analyses on risk of bias). Weight is relative contribution of each study to overall estimate of treatment effect on log scale assuming random effects model
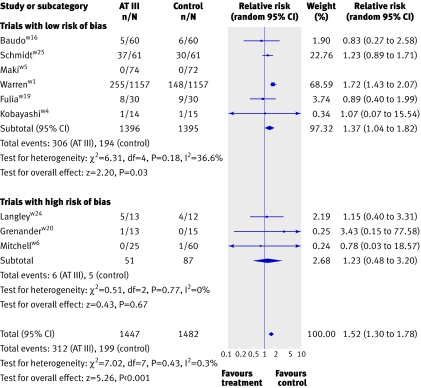
Compared with no intervention or placebo, antithrombin III did not significantly influence the incidence of respiratory failure (0.93, 0.76 to 1.14), duration of mechanical ventilation (weighted mean difference 2.2 days, (−1.2 days to 5.6 days), need for surgical intervention (relative risk 1.04, 0.85 to 1.27), and length of stay in hospital (weighted mean difference −1.9 days, −11.4 days to 7.7 days) or in intensive care unit (0.0 days, −1.8 days to 1.8 days). One trial found no significant effect of antithrombin III on quality of life, and most scores used to assess severity of sepsis were also non-significant.6 Antithrombin III significantly increased the risk of bleeding events (1.52, 1.30 to 1.78, I2=0.3%) (fig 3).
Fig 3 Forest plot for bleeding events
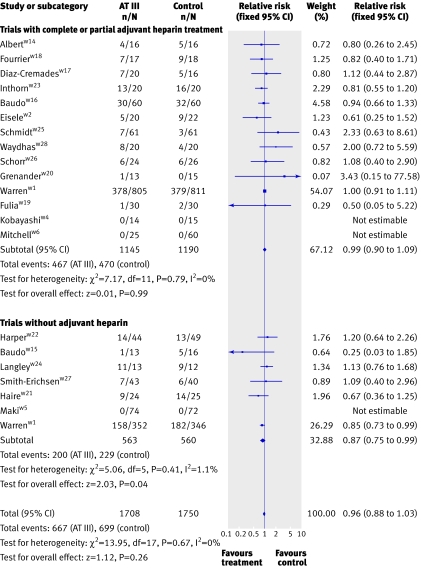
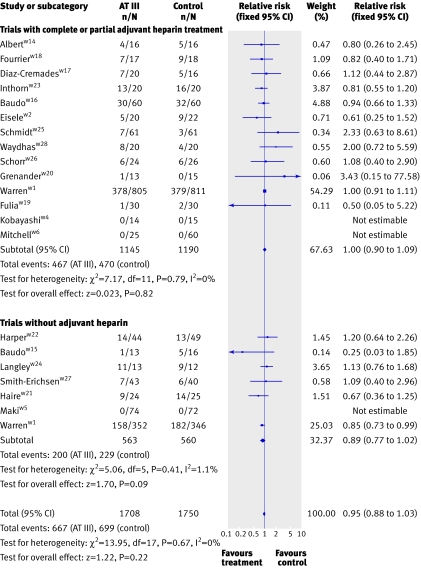
There was no significant effect on mortality or other outcome measures (P>0.05) in all analyses of different subgroup populations (sepsis, paediatrics, obstetrics, and trauma) showed. Additionally, subgroup analyses of trials with short and long duration of treatment, short or long follow-up, and high or low risk of bias showed no significant effect on the examined outcome measures. In the subgroup of patients who received antithrombin III without adjuvant heparin, antithrombin III was associated with a significant effect (0.87, 0.75 to 0.99) but with heterogeneity (I2=1.1, P=0.41). When we used a random effects model because of the observed heterogeneity, however, the significant effect was no longer present (0.87, 0.77 to 1.02). Antithrombin III showed no significant effect in patients with adjuvant heparin (0.99, 0.90 to 1.09) (fig 4)
Fig 4 Forest plot of overall mortality based on heparin administration with fixed relative risk (Warren 2001w1 data split based on heparin administration)
We have also presented the forest plot for overall mortality with random (rather than fixed) relative risks because heterogeneity (I2) >0 (fig 5).
Fig 5 Forest plot on overall mortality based on heparin administration, with random relative risk (Warren 2001w1 data split based on heparin administration)
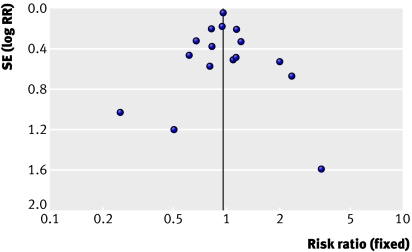
The funnel plot analysis showed no evidence of bias (P=0.37) (fig 6). In three trials with 260 patients there were no deaths in either study group.w4-w6 Exploratory analysis in which we added an imagined trial with one death and 130 participants in each study group had no noticeable effect on the result.
Fig 6 Funnel plot
Trial sequential analyses
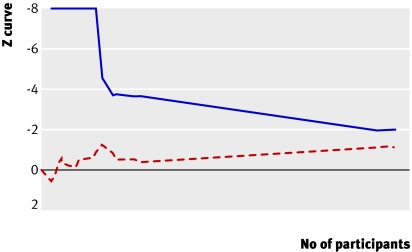
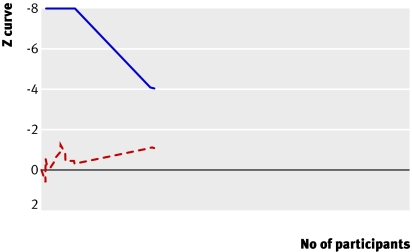
To demonstrate or reject an a priori anticipated intervention effect of 10% relative risk reduction 3317 should be randomised. As more patients than that were included in our meta-analysis without the result becoming significant, a relative risk reduction of 10% or more on mortality is unlikely (fig 7). To determine the required sample size we assumed a 48.5% mortality in the control group and a relative risk reduction of 5% (mortality and relative risk reduction among trials with low bias risk in our meta-analysis). Our calculations showed that we would need a sample of 14 294 (with 80% power and α 0.05) to detect a plausible treatment effect for antithrombin III on mortality, corresponding to a relative risk reduction of 5% (figs 7 and 8). We could, however, obtain solid evidence with fewer patients if eventually the cumulative meta-analysis’ z curve crosses the trial sequential monitoring boundary constructed for a required information size of 14 294 randomised patients. Currently 3458 patients have been randomised and no boundaries have been crossed, indicating that the cumulative evidence is inconclusive for a 5% relative risk reduction. Only 24.2% of the required number is actually available to reject a 5% relative risk reduction of mortality.
Fig 7 Trial sequential analysis (cumulative meta-analysis) assessing effect of antithrombin III in critically ill patients. Cumulative z curve (red dashed line) does not cross sequential monitoring boundary constructed for sample size of 3317 patients in meta-analysis (blue line) with relative risk reduction of 10% (from 48.5% to 43.2%) (α=0.05) and power of 80% (β=0.20). With total number of accrued participants in randomised trials being 3458, we can reject relative risk reduction of 10% at 80% power
Fig 8 Trial sequential analysis (cumulative meta-analysis) assessing effect of antithrombin III in critically ill patients with sample size adjusted for low bias heterogeneity. Cumulative z curve (red dashed line) does not cross trial sequential monitoring boundary (blue line) constructed for sample size of 14 294 patients, corresponding to relative risk reduction of 5% (from 48.5% to 46.2%) with α=0.05 and 80% power (β=0.20). Only 24% of required information size has been reached so far
Discussion
Treatment of critically ill patients with antithrombin III does not significantly affect mortality and length of stay in hospital or in intensive care but is associated with a significantly increased risk of bleeding events. In patients who do not receive heparin there might be a benefit, though this should be explored in further randomised trials. In the overall mortality analysis, one low bias risk trialw1 dominates the analysis, contributing 80% of the available information. Not surprisingly the results of our meta-analysis are in accordance with the results from that trial.
Strength and limitations
We carried out a comprehensive search with no language limitations to identify randomised controlled trials. Two investigators independently abstracted data and obtained data from or confirmed data with all corresponding authors of included trials. This reduces the risk of publication, ascertainment, and information bias. We incorporated mortality data from 20 randomised controlled trials in our meta-analysis (adding 11 trials more than previously reported). We excluded six trials that reported mortality data in 285 patients because they were published only as abstracts or brief communications.w7-w12 Data from these trials showed 20% mortality in both groups, similar to that seen in the included trials (available from author) and there was no beneficial effect when including these studies in the overall mortality analysis based on bias risk (risk ratio 0.96, 95% confidence interval fixed 0.89 to 1.04).
Furthermore, we evaluated the strength of the available evidence through trial sequential analysis, a method adapted from interim monitoring boundaries in single randomised controlled trials applied to cumulative meta-analysis.15 This analysis supports our findings and shows that if antithrombin III has any effect on mortality, it is small.
The weaknesses of our conclusions are closely linked with the weaknesses in the individual trials. Only eight of the 20 included trials reported adequate randomisation, double blinding, and intention to treat analysis. These aspects may be essential to minimise the risk of bias. The impact of bias, however, seems to vary across interventions and diseases.10 We found no significant association between risk of bias and trial estimates of intervention effects in subgroup analyses. Furthermore, the funnel plot did not indicate publication bias and other bias. These findings, combined with the absence of heterogeneity observed in most meta-analyses, support the robustness of our results but do not exclude the possibility of bias.
Although there was minimal heterogeneity among trial results on mortality, we are aware that we pooled heterogeneous trials regarding patients, setting, and treatment regimen. However, all included conditions were associated with low concentrations of antithrombin III, can result in disseminated intravascular coagulation, and have similar inflammatory pathways. Therefore, we think that there is a good biological reason to perform a broad meta-analysis, which increases the generalisability and usefulness considerably. Further, a broad meta-analysis increases power, reduces the risk of erroneous conclusions, and facilitates exploratory analyses, which can generate hypotheses for future research (such as adjuvant heparin).16
Effects of antithrombin III in subgroups
The included patients were heterogeneous and the dose and duration of treatment varied. The analysed outcomes, however, had minimal heterogeneity indicating robust results. Furthermore, all subgroup analyses but one had non-significant results, analogous to the overall analyses on mortality (data available from author). Thus, antithrombin III in a particular dose and duration or in a particular group among the included types of patients does not seem to reduce mortality.
In one subgroup that did not receive adjuvant heparin, antithrombin III seemed to reduce mortality significantly (0.87, 0.75 to 0.99) with a fixed effect model. When we used a random effects model, however, as defined in our protocol, the effect was no longer significant (0.89, 0.77 to 1.02). This might or might not support the previously generated hypothesis that antithrombin III is beneficial in patients who do not receive adjuvant heparin.w1 This subgroup analysis, however, has an important weakness. We split one trialw1 into two “separate trials” with and without concomitant use of heparin. Subsequently we pooled these results in subgroups with the other trials. This procedure probably violates the randomisation, which may cast considerable doubt on the isolated significant result in the fixed effect model. If this trial was not split, the subgroup without adjuvant heparin becomes insignificant. Accordingly, antithrombin III cannot be recommended for patients without adjuvant heparin, but it may be relevant to explore this further in future trials. The negative interaction of antithrombin III and heparin has been recognised on a molecular level,7 and a randomised trial evaluating effectiveness of antithrombin III without heparin was already proposed after the KyberSept study.w13
Nevertheless, if antithrombin III is used in clinical practice adjuvant heparin should be avoided as the potentially harmful interactions are unclear.2 4
Trial sequential analysis
The results of trial sequential analysis rule out an effect of antithrombin III on mortality larger than 10% relative risk reduction because the number of randomised patients in our meta-analysis exceeds the required sample size for detecting an intervention effect of this size. Trial sequential analysis, however, also revealed absence of evidence of an intervention effect of a relative risk reduction of 5%, as suggested by the low bias risk trials. We included 3458 critically ill patients in the cumulative meta-analysis, and neither the trial sequential monitoring boundary nor the traditional z=1.96 (P<0.05) was crossed. The number of patients randomised is much smaller than the sample size of 14 294 we calculated was required, which is the number of patients needed to reliably reject an effect of antithrombin III on mortality based on the 5% relative risk reduction and a control mortality of about 50%. Our use of trial sequential monitoring boundaries applied to cumulative meta-analysis, adapted from interim monitoring boundaries of a single trial, showed that the current evidence for a clinically relevant effect of antithrombin III is still inconclusive.
Conclusion
In our systematic review, we have shown that antithrombin III seems ineffective in any population of critically ill patients regarding mortality and it even increases the risk of bleeding events. Its use in critically ill patients cannot be recommended based on the available evidence nor in patients without adjuvant heparin, but it may be relevant to explore this further in future trials.
What is already known on this topic
Antithrombin III is a costly intervention that is widely used for critically ill patients
Four minor meta-analyses of randomised and non-randomised trials in selected populations have previously shown inconclusive evidence on mortality
What this study adds
Trial sequential analysis showed that there is evidence of absence of beneficial effects (10% mortality reduction or more) of antithrombin III in critically ill patients
Antithrombin III increases the risk of bleeding
Supplementary Material
We thank J Albert, C Waydhas, S Opral, T Kobayashi, and F Fourrier for providing valuable additional data.
Contributors: AA and AM conceived and designed the study. AA and JW acquired analysed and interpreted data. All authors drafted and revised the review. AA and JW wrote to authors of papers for additional information. AA is guarantor.
Funding: None.
Competing interests: None declared.
Ethical approval: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, et al, Recombinant human protein C worldwide evaluation in severe sepsis (PROWESS) study group. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 2001;344:699-709. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303-10. [DOI] [PubMed] [Google Scholar]
- 3.Periti P. Current treatment of sepsis and endotoxaemia. Expert Opin Pharmacother 2000;1:1203-17. [DOI] [PubMed] [Google Scholar]
- 4.Levi M, de Jonge E, van der Poll T. New treatment strategies for disseminated intravascular coagulation based on current understanding of the pathophysiology. Ann Med 2004;36:41-9. [DOI] [PubMed] [Google Scholar]
- 5.Opal SM, Kessler CM, Roemisch J, Knaub S. Antithrombin, heparin, and heparan sulfate. Crit Care Med 2002;30(5 suppl):S325-31. [DOI] [PubMed] [Google Scholar]
- 6.Rublee D, Opal SM, Schramm W, Keinecke HO, Knaub S. Quality of life effects of antithrombin III in sepsis survivors: results from the KyberSept trial. Crit Care 2002;6:349-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiedermann CJ, Römisch J. The anti-inflammatory actions of antithrombin—a review. Acta Med Austriaca 2002;29:89-92. [DOI] [PubMed] [Google Scholar]
- 8.Freeman BD, Zehnbauer BA, Buchman TG. A meta-analysis of controlled trials of anticoagulant therapies in patients with sepsis. Shock 2003;20:5-9. [DOI] [PubMed] [Google Scholar]
- 9.Bassler D, Millar D, Schmidt B. Antithrombin for respiratory distress syndrome in preterm infants. Cochrane Database Syst Rev 2006;(4):CD005383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gluud LL. Bias in clinical intervention research. Am J Epidemiol 2006;163:493-501. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [DOI] [PubMed] [Google Scholar]
- 12.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika 1983;70:659-63. [Google Scholar]
- 15.Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analyses may establish when firm evidence is reached in cumulative meta-analyses. J Clin Epidemiol doi: 10.1016/j.jclinepi.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Gotzsche PC. Why we need a broad perspective on meta-analysis. It may be crucially important for patients. BMJ 2000;321:585-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.