Abstract
A haloalkane dehalogenase was purified to electrophoretic homogeneity from cell extracts of a 1-chlorobutane-utilizing strain, m15-3, which was identified as a Corynebacterium sp. The enzyme hydrolyzed C2 to C12 mono- and dihalogenated alkanes, some haloalcohols, and haloacids. The Km value of the enzyme for 1-chlorobutane was 0.18 mM. Its molecular weight was estimated to be 36,000 by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and 33,000 by gel filtration. The isoelectric point was pH 4.5. The optimum pH for enzyme activity was found to be 9.4, and the optimum temperature was 30 to 35 degrees C. The enzyme was stable for 1 h at temperatures ranging from 4 to 30 degrees C but was progressively less stable at 40 and 50 degrees C.
Full text
PDF

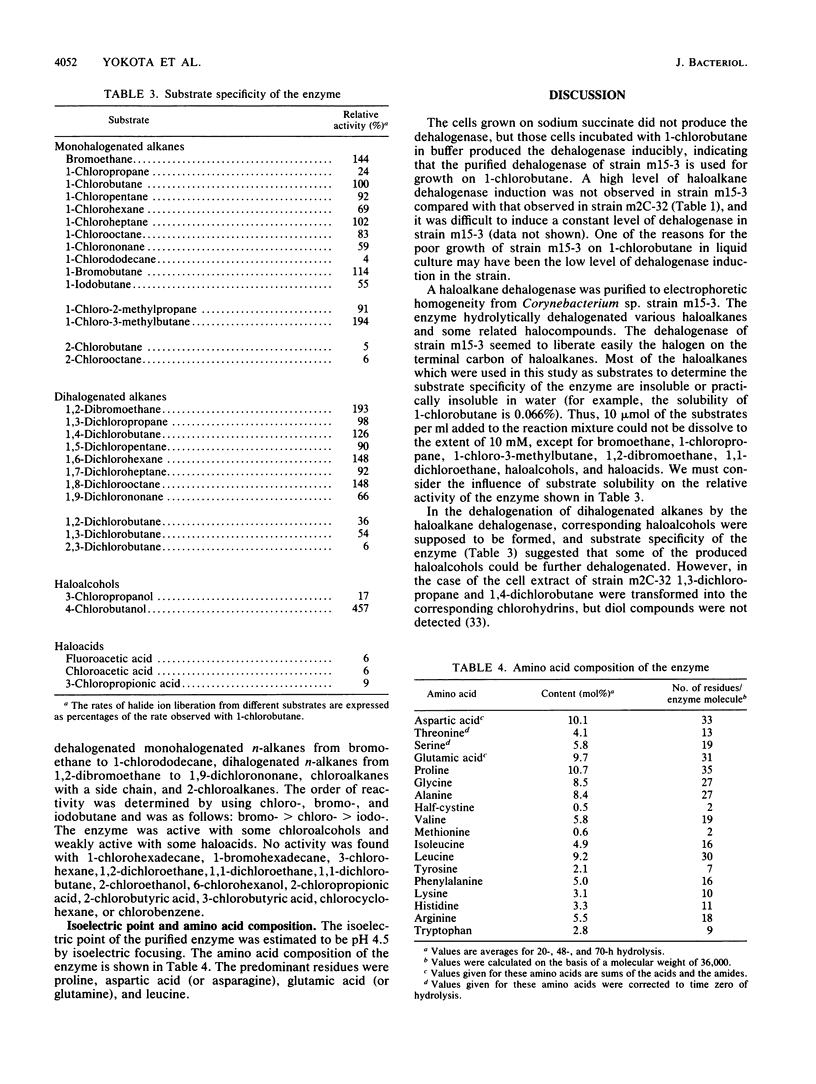


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouwer E. J., McCarty P. L. Transformations of 1- and 2-carbon halogenated aliphatic organic compounds under methanogenic conditions. Appl Environ Microbiol. 1983 Apr;45(4):1286–1294. doi: 10.1128/aem.45.4.1286-1294.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwer E. J., McCarty P. L. Transformations of halogenated organic compounds under denitrification conditions. Appl Environ Microbiol. 1983 Apr;45(4):1295–1299. doi: 10.1128/aem.45.4.1295-1299.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner W., Staub D., Leisinger T. Bacterial degradation of dichloromethane. Appl Environ Microbiol. 1980 Nov;40(5):950–958. doi: 10.1128/aem.40.5.950-958.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUMMINS C. S., HARRIS H. The chemical composition of the cell wall in some gram-positive bacteria and its possible value as a taxonomic character. J Gen Microbiol. 1956 Jul;14(3):583–600. doi: 10.1099/00221287-14-3-583. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DELEY J., SCHELL J. DEOXYRIBONUCLEIC ACID BASE COMPOSITION OF ACETIC ACID BACTERIA. J Gen Microbiol. 1963 Nov;33:243–253. doi: 10.1099/00221287-33-2-243. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- GOLDMAN P. THE ENZYMATIC CLEAVAGE OF THE CARBON-FLUORINE BOND IN FLUOROACETATE. J Biol Chem. 1965 Aug;240:3434–3438. [PubMed] [Google Scholar]
- Goldman P., Milne G. W., Keister D. B. Carbon-halogen bond cleavage. 3. Studies on bacterial halidohrolases. J Biol Chem. 1968 Jan 25;243(2):428–434. [PubMed] [Google Scholar]
- Janssen D. B., Scheper A., Dijkhuizen L., Witholt B. Degradation of halogenated aliphatic compounds by Xanthobacter autotrophicus GJ10. Appl Environ Microbiol. 1985 Mar;49(3):673–677. doi: 10.1128/aem.49.3.673-677.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuning S., Janssen D. B., Witholt B. Purification and characterization of hydrolytic haloalkane dehalogenase from Xanthobacter autotrophicus GJ10. J Bacteriol. 1985 Aug;163(2):635–639. doi: 10.1128/jb.163.2.635-639.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler-Staub D., Leisinger T. Dichloromethane dehalogenase of Hyphomicrobium sp. strain DM2. J Bacteriol. 1985 May;162(2):676–681. doi: 10.1128/jb.162.2.676-681.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little M., Williams P. A. A bacterial halidohydrolase. Its purification, some properties and its modification by specific amino acid reagents. Eur J Biochem. 1971 Jul 15;21(1):99–109. doi: 10.1111/j.1432-1033.1971.tb01445.x. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Marks T. S., Wait R., Smith A. R., Quirk A. V. The origin of the oxygen incorporated during the dehalogenation/hydroxylation of 4-chlorobenzoate by an Arthrobacter sp. Biochem Biophys Res Commun. 1984 Oct 30;124(2):669–674. doi: 10.1016/0006-291x(84)91607-3. [DOI] [PubMed] [Google Scholar]
- Motosugi K., Esaki N., Soda K. Purification and properties of a new enzyme, DL-2-haloacid dehalogenase, from Pseudomonas sp. J Bacteriol. 1982 May;150(2):522–527. doi: 10.1128/jb.150.2.522-527.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori T., Alexander M. Bacterial dehalogenation of halogenated alkanes and fatty acids. Appl Environ Microbiol. 1978 May;35(5):867–871. doi: 10.1128/aem.35.5.867-871.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staneck J. L., Roberts G. D. Simplified approach to identification of aerobic actinomycetes by thin-layer chromatography. Appl Microbiol. 1974 Aug;28(2):226–231. doi: 10.1128/am.28.2.226-231.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel T. M., McCarty P. L. Biotransformation of tetrachloroethylene to trichloroethylene, dichloroethylene, vinyl chloride, and carbon dioxide under methanogenic conditions. Appl Environ Microbiol. 1985 May;49(5):1080–1083. doi: 10.1128/aem.49.5.1080-1083.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS D. E., REISFELD R. A. DISC ELECTROPHORESIS IN POLYACRYLAMIDE GELS: EXTENSION TO NEW CONDITIONS OF PH AND BUFFER. Ann N Y Acad Sci. 1964 Dec 28;121:373–381. doi: 10.1111/j.1749-6632.1964.tb14210.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]




