Abstract
Background
Ocular mucosa is exposed constantly to the external environment, and chronic exposure to air pollution may affect the ocular surface.
Objective
We assessed the effect of air pollution on the ocular surface by combining determinations of individual exposure and conjunctival impression cytology.
Methods
A panel study was conducted with 29 volunteers recruited in two locations with different pollution levels: São Paulo (n = 13) and Divinolândia (n = 16). We assessed mean individual levels of nitrogen dioxide (NO2) exposure for 7 days, using a passive sampler. Impression cytology samples were obtained from inferior tarsal conjunctiva. Comparisons between the two groups in terms of NO2 exposure and goblet-cell counts were performed using the Student t-test. Correlations between goblet-cells counts and corresponding individual NO2 exposure levels were determined using Spearman’s correlation.
Results
Individuals living in São Paulo received a significantly (p = 0.005) higher dose of NO2 (mean 32.47; SD 9.83) than those living in Divinolândia (mean 19.33; SD 5.24). There was a steady increase in goblet-cell counts, proportional to NO2 exposure (Spearman’s correlation = 0.566, p = 0.001), with a dose–response pattern.
Conclusions
A positive and significant association between exposure to air pollution and goblet-cell hyperplasia in human conjunctiva was detected. The combination of simple measurements of exposure and impression cytology was an effective and noninvasive approach for characterizing human response to ambient levels of air pollution.
Keywords: air pollutants, conjunctiva, environmental, goblet-cell, impression cytology, nitrogen dioxide
Acute adverse health effects of ambient levels of air pollution have been demonstrated in humans, mostly in terms of respiratory and cardiovascular events (Barnett et al. 2006; Ito et al. 2005; Medina-Ramón et al. 2006; Pope et al. 2002; Samoli et al. 2003; Souza et al. 1998).
Ocular mucosa is exposed constantly to the external environment. Indeed individuals living in areas with high concentrations of pollutants frequently report ocular symptoms (Saxena et al. 2003; Versura et al. 1999), and previous studies have detected tear film abnormalities and subclinical changes of the ocular surface in individuals who lived in cities with high levels of air pollution (Gupta et al. 2002; Saxena et al. 2003). In such scenario, changes in ocular mucosa may indicate potential damage to the eyes and represent a convenient biomarker of the adverse health effects induced by air pollution if objective estimators of ocular surface changes in individuals living in urban areas are proportional to the degree of exposure. In the present study we explored this concept by conducting a panel study combining determinations of individual exposure to air pollution, expressed in terms of nitrogen dioxide (NO2) and measuring ocular surface changes by impression cytology.
Materials and Methods
Study population
The study involved 29 volunteers, who were recruited in two locations, with different pollution levels: São Paulo (n = 13) and Divinolândia (n = 16). São Paulo is the largest city in Latin America, with high levels of air pollution, mainly as a result of traffic emissions. Divinolândia is a small city in the countryside of the state of São Paulo, where half the population lives in rural settings and where there is no significant industrial activity. The volunteers were recruited among the employees of two public hospitals: at the General Clinics Hospital of the University of São Paulo Medical School and the Regional Hospital in Divinolândia. The hospital in São Paulo is located downtown at an intersection of broad avenues with heavy traffic, whereas in Divinolândia the hospital is located in a relatively isolated spot surrounded by farms. The research protocol was approved by the ethics committees of both institutions, and all subjects gave their informed consent before enrollment in the study. The following inclusion criteria were adopted: a) to be part of the fixed staff of the hospitals, b) to have been living in the study area for at least 5 years, and c) to accept participating in the study after reading and signing an informed consent form. The following parameters were adopted to exclude volunteers: a) smoking, b) possibility of traveling to other areas during the monitoring period (7 days), c) contact with chemical solutions such as organic solvents, sodium hypochloride, and formalin, and d) individuals with history of use of contact lenses, ophthalmic surgery, and preexisting ophthalmic conditions. All volunteers used gas cooking and no houses had home heating.
Exposure assessment
NO2 was used as an indicator of exposure to air pollution. We employed a passive sampler that included a cellulose filter (Energetica, Rio de Janeiro, Brazil) impregnated with an absorbant solution—trietanolamine 2%, 0.05% o-methoxyphenol, and 0.025% sodium metabisulfite (Lodge 1989), which was enclosed within a small plastic tube with one of its extremities open to ambient air. The nitrite produced during sampling was determined colorimetrically by reacting the exposed absorbing reagent with sulfanilamide and 8-anilino-1-naphthalene-sulfonic acid (ANSA) at a wavelength of 550 nm.
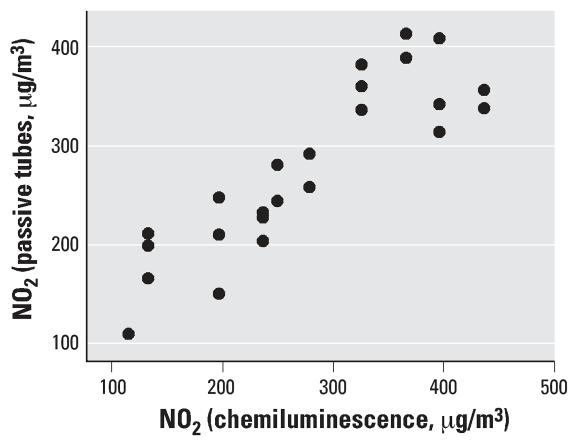
Before the study we evaluated the efficiency and sensitivity of this measuring system by placing the filters near an automatic chemiluminescence monitor of the State Sanitation Agency of São Paulo (CETESB 2007). Our method has an accuracy of 98.6% and a precision of 80%. The lower detection limit is an accumulated exposure of 100 μg/m3, and no evidence of saturation was observed up to 450 μg/m3 of accumulated exposure (defined as the average concentration of 24 hr of NO2 exposure per number of days). The accumulated NO2 concentration obtained by the two methods was compared across a time window ranging from 3 to 10 days. A good agreement between the two methods was observed, as depicted in Figure 1.
Figure 1.
Graphic representation of simultaneous determinations of accumulated ambient concentrations of NO2, measured by the passive sampler and corresponding values determined by the Sanitation Agency of the São Paulo (CETESB 2007).
Passive samplers were fixed in the pocket of the studied individuals, who were instructed to keep them at all times during their daily activities and by the bedside during the night for 7 days.
We used a cumulative measure of 1 week of NO2 exposure and divided such measure by 7, which gave us a mean individual level of exposure that was compared with goblet-cell counts.
Impression cytology of conjunctival mucosa
Impression cytology was used to obtain samples from the superficial epithelial cell layers of inferior tarsal conjunctiva. Impression cytology samples were collected after the 7-day monitoring of NO2. All samples were collected in the morning (0900–1100 hours).
Semicircular filters approximately 15 mm diameter in size (cellulose ester filter 22-μm pore; Millipore Corp., Bedford, MA, USA) were applied to the inferior tarsal conjunctiva after instillation of one drop of topical anesthetic (tetracaine) in each eye, and the excess fluid was wiped away. The paper fragments were applied for approximately 10 sec, and after gentle pressure with the blunt end of the forceps, the fragments were peeled off and immediately immersed in tubes containing absolute ethanol. After fixation, specimens were rehydrated in 70% ethyl alcohol, then placed successively in periodic acid–Schiff reagent, sodium metabisulfite, Gill’s hematoxylin and Scott’s tap water. Specimens were then rinsed with 95% alcohol and absolute alcohol. Xylene was used to make the filter paper transparent. Before mounting, the filter paper was placed with epithelial cells facing up. Slides were examined by light microscopy and goblet cells were counted in 10 high-power fields (HPF) with a 400× magnification (Singh et al. 2005). The same investigator (A.B.) performed the goblet-cell counts and was blinded to the place of origin of the samples.
Statistical analysis
Comparisons of NO2 exposure and goblet-cell counts between the two groups (São Paulo and Divinolândia) were performed using the Student t-test, with corrections for inequality of variances. Correlations between goblet-cell counts and corresponding individual NO2 exposure level were determined using Spearman’s correlation. The effect of air pollutants on goblet-cell counts was also measured by analysis of variance (ANOVA) and Bonferroni’s post hoc test. The level of significance was set at 5%. Goblet-cell counts were performed in Argentina, and NO2 data were simultaneously computed in São Paulo. The two data sets were combined only after the analysis of the samples was concluded.
Results
There were no significant differences among the groups in São Paulo (SP) and Divinolândia (D) regarding age (SP: mean 37.15, SD 8.2; D: mean 32.13, SD 9.2; p = 0.136); sex (SP: female, 85%, male, 15%; D: female, 94%, male, 6%); and passive smoking (SP, 37.5%; D, 38.5%).
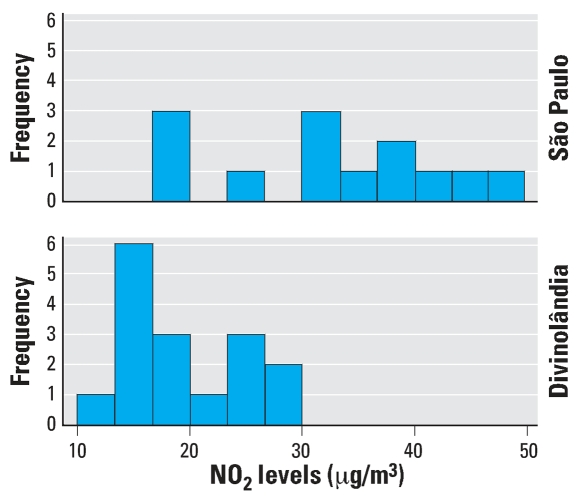
Individuals living in São Paulo received a significantly (p = 0.005) higher dose of NO2 (mean 32.47, SD 9.83) than those in Divinolândia (mean 19.33, SD 5.24), as expected based on the characteristics of each city (Figure 2).
Figure 2.
Individual NO2 exposure levels in each group.
Mean (and corresponding SD) number of goblet cells per 10 HPF was 243.37 ± 132.67 in Divinolândia and 325.80 ± 147.90 in São Paulo. The difference between the two groups was not significant (p = 0.102, Student t-test). The difference between goblet-cell counts was not statistically significant in our sample because of the presence in the Divinolândia group of an outlier with a goblet-cell count of about 600 cells in 10 HPF. If this outlier were excluded, individual differences would be significant (p = 0.029, Student t-test).
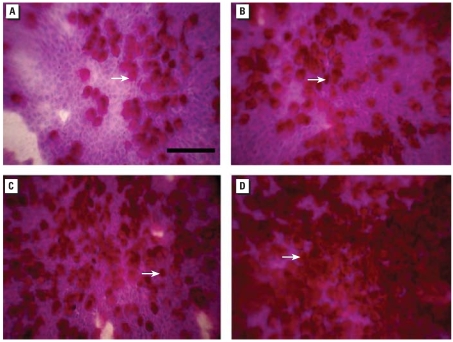
We classified subjects’ exposures to NO2 into four categories on the basis of quartiles of NO2 levels: Q1, < 17 μg/m3; Q2, 18–21 μg/m3; Q3, 22–33 μg/m3; and Q4, > 33 μg/m3, regardless of the city of residence. The mean counts of goblet cells, distributed by quartiles of NO2 were Q1, 186 cells; Q2, 253 cells; Q3, 312 cells; and Q4, 402 cells. There was a 216% increase in the number of goblet cells from the first to the fourth quartile. Figure 3 shows the microscopic aspect of impression cytology samples, and a marked increase in goblet-cell hyperplasia can be observed in subjects exposed to higher levels of NO2.
Figure 3.
Impression cytology photographs of representative samples. The arrows indicate goblet cells. Corresponding goblet-cell counts and NO2 exposure levels are: (A) 194 cells × 10HPF NO2 13.10 μg/m3; (B) 357 goblet cells × 10HPF NO2 18.04 μg/m3; (C) 409 goblet cells × 10HPF NO2 26.89 μg/m3; (D) 575 goblet cells × 10HPF NO2 45.31 μg/m3. Scale bar = 100 μm.
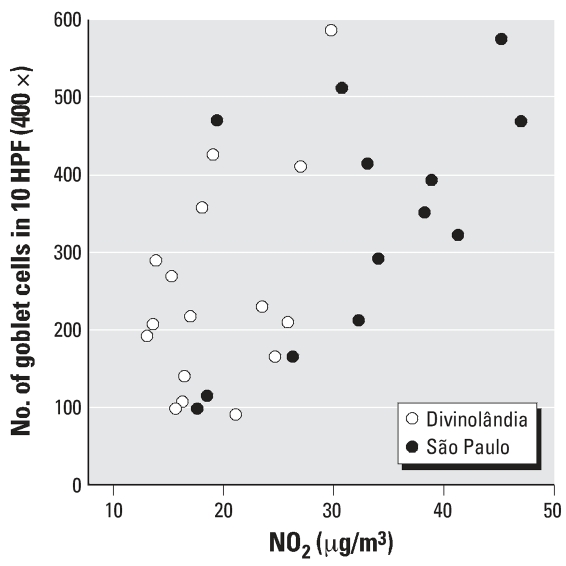
Goblet-cell counts increased proportionately to NO2 exposure in a dose–response pattern despite the variability observed (Figure 4). Indeed, Spearman’s correlation between NO2 and goblet cells per 10 HPF was positive (0.566) and significant (p = 0.001). Using ANOVA to measure the effect of air pollutants on goblet-cell counts, we detected a significant difference among groups (p = 0.036). The Bonferroni post hoc test indicated that goblet-cell counts of the first and fourth quartiles of NO2 exposure were significantly different.
Figure 4.
Individual cell counts plotted as a function of NO2 exposure levels, indicated by study location.
Discussion
Goblet-cell hyperplasia is a stereotyped response of mucosal surfaces when chronically exposed to air pollution, as demonstrated in rodents (Pires-Neto et al. 2006; Saldiva et al. 1992) and humans (Souza et al. 1998). In this present study, we report that conjunctival mucosa follows this same path. In addition, we demonstrated a positive and significant correlation between gradients of exposure and intensity of goblet-cell hyperplasia.
Conjunctival goblet cells are slow cycling cells that may proliferate in response to chronic inflammatory stimuli (Dartt 2004; Pellegrini et al. 1999; Wei et al. 1995). Goblet-cell hyperplasia is detected in chronic allergic eye disease such as vernal keratocon-junctivitis, atopic keratoconjunctivitis, and giant papillary conjunctivitis (Mc Dermott et al. 2005). Studies have shown that chronic exposure to ambient air pollution is associated with subclinical changes of the ocular surface and the tear film (Gupta et al. 2002; Saxena et al. 2003), but goblet-cell hyperplasia related to NO2 has not been documented previously. There is no accurate characterization of goblet-cell turnover in conjunctival mucosa; however, there is substantial information on mucous hyperplasia of the airways resulting from exposure to sulfur dioxide (SO2) and ozone across a wide range of exposure protocols (Fanucchi et al. 2006; Harkema et al. 1999; Saldiva et al. 1992; Seltzer et al. 1984; White et al. 1986). Generally, mucous hyperplasia occurs in the respiratory epithelium after weeks or months and is considered a marker of airway remodeling induced by persistent injurious stimuli. The observed mucous hyperplasia probably reflects exposure to air pollution that occurred within a much larger time window than the 1-week assessment that was conducted.
We considered NO2 a proxy estimator of exposure to air pollution, not the single causative agent. The passive sampler we employed for the determination of NO2 exposure was simple, did not restrict daily activities and exhibited a good correlation with the state monitoring system (Figure 1). In addition, previous studies our group conducted in the last decade observed consistent and significant correlations between NO2 and other primary pollutants such as PM10 (particulate matter with an aerodynamic diameter ≤ 10 μm), carbon monoxide, and SO2 (Braga et al. 2001; Conceição et al. 2001; de Paula Santos et al. 2005; Farhat et al. 2005; Lin et al. 2003, 1999; Martins et al. 2002; Pereira et al. 1998; Saldiva et al. 1995). Thus, NO2 should be considered, within the context of the current study, a marker of combustion-derived pollutants produced by vehicular emissions and other sources such as biomass burning.
In the present study the cumulative measure of 1 week of NO2 exposure was considered a representative measure of chronic exposure, and the study was conducted simultaneously in both cities to minimize climatic influence.
The range of exposure to NO2 experienced by our study population was between 10 and 50 μg/m3 (24-hr mean of 1-week exposure). Such levels are within those observed in several locations throughout the world, indicating that the individuals evaluated in this study were not exposed to extremely high levels of air pollution. Figure 4 suggests that the relationship between NO2 and goblet-cell hyperplasia is linear and without a threshold or plateau. Although our sample was small, it is tempting to speculate that exposure to low levels of air pollution may induce subclinical damage, which ultimately leads to an adaptive response of the conjunctival epithelium. Our findings reinforce the concept that populations exposed to ambient levels of air pollution are subjected to possible chronic injury, which includes, as discussed in the present study, the ocular surface.
Figure 4 shows a substantial degree of variability in the responses to similar levels of air pollution. This finding suggests that some individuals have greater susceptibility to airborne toxic agents. Our study was not designed to explore this point, but it would be interesting to explore in further studies whether ocular mucosa may be a convenient scenario to study the determinants—genetic or epigenetic—of the susceptibility of mucosal surfaces to air pollution.
In the present study we observed a positive and significant association between exposure to air pollution and goblet-cell hyperplasia in human conjunctiva. The combination of simple measurements of exposure and impression cytology was shown to be an effective and non-invasive approach to characterize human response to ambient levels of air pollution.
Footnotes
We especially thank M.C. Machado and E. Giantomassi for their support and help in selecting the subjects and allowing us the use of the facilities at Divinolândia General Hospital (CONDERG), Divinolândia, Brazil.
References
- Barnett AG, Williams GM, Schwartz J, Best TL, Neller AH, Petroeschevsky AL, et al. The effects of air pollution on hospitalizations for cardiovascular disease in elderly people in Australian and New Zealand cities. Environ Health Perspect. 2006;114:1018–1023. doi: 10.1289/ehp.8674. [Online 13 March 2006] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga AL, Saldiva PH, Pereira LA, Menezes JJ, Conceicao GM, Lin CA, et al. Health effects of air pollution exposure on children and adolescents in Sao Paulo, Brazil. Pediatr Pulmonol. 2001;31(2):106–113. doi: 10.1002/1099-0496(200102)31. 2<106::AID-PPUL1017>3.0.CO;2-M [Online 22 January 2001] [DOI] [PubMed] [Google Scholar]
- CETESB (Companhia de Tecnologia de Saneamento Ambiental) Relatório da Qualidade do Ar no Estado de São Paulo 2006. São Paulo. 2007. [[accessed 6 July 2007]]. Available: http://www.cetesb.sp.gov.br/Ar/ar_geral.asp.
- Conceição GM, Miraglia SG, Kishi HS, Saldiva PH, Singer JM. Air pollution and child mortality: a time-series study in Sao Paulo, Brazil. Environ Health Perspect. 2001;109(suppl 3):347–350. doi: 10.1289/ehp.109-1240551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartt DA. Control of mucin production by ocular surface epithelial cells. Exp Eye Res. 2004;78(2):173–185. doi: 10.1016/j.exer.2003.10.005. [Online 27 November 2003] [DOI] [PubMed] [Google Scholar]
- de Paula Santos U, Braga AL, Giorgi DM, Pereira LA, Grupi CJ, Lin CA, et al. Effects of air pollution on blood pressure and heart rate variability: a panel study of vehicular traffic controllers in the city of Sao Paulo, Brazil. Eur Heart J. 2005;26(2):193–200. doi: 10.1093/eurheartj/ehi035. [Online 3 December 2004] [DOI] [PubMed] [Google Scholar]
- Fanucchi MV, Plopper CG, Evans MJ, Hyde DM, Van Winkle LS, Gershwin LJ, Schelegle ES. Cyclic exposure to ozone alters distal airway development in infant rhesus monkeys. Am J Physiol Lung Cell Mol Physiol. 2006;291(4):L644–L650. doi: 10.1152/ajplung.00027.2006. [DOI] [PubMed] [Google Scholar]
- Farhat SC, Paulo RL, Shimoda TM, Conceicao GM, Lin CA, Braga AL, et al. Effect of air pollution on pediatric respiratory emergency room visits and hospital admissions. Braz J Med Biol Res. 2005;38(2):227–235. doi: 10.1590/S0100-879X2005000200011. [DOI] [PubMed] [Google Scholar]
- Gupta SK, Gupta V, Joshi S, Tandon R. Subclinically dry eyes in urban Delhi: an impact of air pollution? Ophthalmologica. 2002;216(5):368–71. doi: 10.1159/000066183. [DOI] [PubMed] [Google Scholar]
- Harkema JR, Hotchkiss JA, Barr EB, Bennett CB, Gallup M, Lee JK, Basbaum C. Long-lasting effects of chronic exposure on rat nasal epithelium. Am J Respir Cell Mol Biol. 1999;20(3):517–29. doi: 10.1165/ajrcmb.20.3.3227. [DOI] [PubMed] [Google Scholar]
- Ito K, De Leon SF, Lippmann M. Associations between ozone and daily mortality: analysis and meta-analysis. Epidemiology. 2005;16(4):446–457. doi: 10.1097/01.ede.0000165821.90114.7f. [DOI] [PubMed] [Google Scholar]
- Lin CA, Amador Pereira LA, de Souza Conceição GM, Kishi HS, Milani R, Jr, Ferreira Braga AL, Nascimento Saldiva PH. Association between air pollution and ischemic cardiovascular emergency room visits. Environ Res. 2003;92(1):57–63. doi: 10.1016/S0013-9351(02)00054-3. [Online 23 March 2003] [DOI] [PubMed] [Google Scholar]
- Lin CA, Martins MA, Farhat SC, Pope CA, 3rd, Conceicao GM, Anastacio VM, et al. Air pollution and respiratory illness of children in Sao Paulo, Brazil. Paediatr Perinat Epidemiol. 1999;13(4):475–488. doi: 10.1046/j.1365-3016.1999. 00210.x [Online 04 January 2002] [DOI] [PubMed] [Google Scholar]
- Lodge JP. Intersociety Committee. 3. Chelsea, MI: Lewis Publishers; 1989. Methods of air sampling and analysis. In: Analysis of Atmospheric Nitrogen Dioxide (24H–Average) pp. 399–402. [Google Scholar]
- Martins LC, Latorre Mdo R, Saldiva PH, Braga AL. Air pollution and emergency room visits due to chronic lower respiratory diseases in the elderly: an ecological time-series study in Sao Paulo, Brazil. J Occup Environ Med. 2002;44(7):622–627. doi: 10.1097/00043764-200207000-00006. [DOI] [PubMed] [Google Scholar]
- McDermott AM, Perez V, Huang AJW, Pflugfelder SC, Stern ME, Baudouin C, et al. Pathways of corneal and ocular surface inflammation: a perspective from the Cullen Symposium. Ocular Surf. 2005;3(4):S131–S138. doi: 10.1016/s1542-0124(12)70238-0. [DOI] [PubMed] [Google Scholar]
- Medina-Ramón M, Zanobetti A, Schwartz J. The effect of ozone and PM10 on hospital admissions for pneumonia and chronic obstructive pulmonary disease: a national multicity study. Am J Epidemiol. 2006;163(6):579–588. doi: 10.1093/aje/kwj078. [Online 27 January 2006] [DOI] [PubMed] [Google Scholar]
- Pellegrini G, Golisano O, Paterna P, Lambiase A, Bonini S, Rama P, et al. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol. 1999;145(4):769–782. doi: 10.1083/jcb.145.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira LA, Loomis D, Conceicao GM, Braga AL, Arcas RM, Kishi HS, et al. Association between air pollution and intrauterine mortality in Sao Paulo, Brazil. Environ Health Perspect. 1998;106:325–329. doi: 10.1289/ehp.98106325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires-Neto RC, Lichtenfels AJFC, Soares RS, Macchione M, Saldiva PHN, Dolhnikoff M. Effects of São Paulo air pollution on the upper airways of mice. Environ Res. 2006;101(3):356–361. doi: 10.1016/j.envres.2005.12.018. [Online 7 February 2006] [DOI] [PubMed] [Google Scholar]
- Pope CA, III, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287(9):1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldiva PHN, King M, Delmonte VLC, Macchione M, Parada MAC, Daliberto ML, et al. Respiratory alterations due to urban air pollution an experimental study in rats. Environ Res. 1992;57:19–33. doi: 10.1016/s0013-9351(05)80016-7. [DOI] [PubMed] [Google Scholar]
- Saldiva PH, Pope CA, III, Schwartz J, Dockery DW, Lichtenfels AJ, Salge JM, et al. Air pollution and mortality in elderly people: a time-series study in Sao Paulo, Brazil. Arch Environ Health. 1995;50(2):159–163. doi: 10.1080/00039896.1995.9940893. [DOI] [PubMed] [Google Scholar]
- Samoli E, Touloumi G, Zanobetti A, Le Tertre A, Schindler C, Atkinson R, et al. Investigating the dose-response relation between air pollution and total mortality in the APHEA-2 multicity project. Occup Environ Med. 2003;60:977–982. doi: 10.1136/oem.60.12.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena R, Srivastava S, Trivedi D, Anand E, Joshi S, Gupta SK. Impact of environmental pollution on the eye. Acta Ophthalmol Scand. 2003;81(5):491–494. doi: 10.1034/j.1600-0420.2003.00119.x. [Online 26 September 2003] [DOI] [PubMed] [Google Scholar]
- Seltzer J, Scanlon PD, Drazen JM, Ingram RH, Jr, Reid L. Morphologic correlation of physiologic changes caused by SO2 in dogs. The role of inflammation. Am Rev Respir Dis. 1984;129(5):790–797. doi: 10.1164/arrd.1984.129.5.790. [DOI] [PubMed] [Google Scholar]
- Singh R, Joseph A, Umapathy T, Tint NL, Dua HS. Impression cytology of the ocular surface. Br J Ophthalmol. 2005;89:1655–1659. doi: 10.1136/bjo.2005.073916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza MB, Saldiva PH, Pope CA, 3rd, Capelozzi VL. Respiratory changes due to long-term exposure to urban levels of air pollution: a histopathologic study in humans. Chest. 1998;113(5):1312–1318. doi: 10.1378/chest.113.5.1312. [DOI] [PubMed] [Google Scholar]
- Versura P, Profazio V, Cellini M, Torreggiani A, Caramazza R. Eye discomfort and air pollution. Ophthalmologica. 1999;213(2):103–109. doi: 10.1159/000027401. [DOI] [PubMed] [Google Scholar]
- Wei ZG, Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells are preferentially located in fornical epithelium: implications on conjunctival epithelial homeostasis. Invest Ophtalmol Vis Sci. 1995;36(1):236–246. [PubMed] [Google Scholar]
- White R, Zoppi AL, Haroz RK, Broillet A. Sulfur dioxide induced bronchitis in rats. Arch Toxicol Suppl. 1986;9:431–435. doi: 10.1007/978-3-642-71248-7_88. [DOI] [PubMed] [Google Scholar]