Abstract
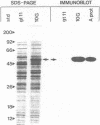
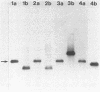
A gene bank of DNA from the fish pathogenic bacterium Aeromonas salmonicida was constructed in the bacteriophage lambda gt11. Phage lambda gt11/10G, a recombinant carrying a 4.0-kilobase fragment of A. salmonicida DNA, was found to express the surface array protein (A protein) in Escherichia coli. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis showed that the protein expressed from the cloned gene had a subunit molecular weight of 49,000, which was identical to that of subunits in the native assembled A layer. Genomic Southern analysis showed that the gene coding for this predominant cellular protein was in a single copy on the chromosome and was conserved among a wide range of A. salmonicida strains with different phenotypic characteristics and isolated from diverse geographic locations, fish species, and means of pathogenesis. Results of genomic blotting experiments also showed that loss of expression of the A layer resulting from growth at 30 degrees C was accompanied by genetic rearrangement in which N-terminal sequences of the gene for A protein were lost by deletion.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. M., Freitag C. S., Clements J. R., Eisenstein B. I. An invertible element of DNA controls phase variation of type 1 fimbriae of Escherichia coli. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5724–5727. doi: 10.1073/pnas.82.17.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belland R. J., Trust T. J. Synthesis, export, and assembly of Aeromonas salmonicida A-layer analyzed by transposon mutagenesis. J Bacteriol. 1985 Sep;163(3):877–881. doi: 10.1128/jb.163.3.877-881.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström S., Robbins K., Koomey J. M., Swanson J. Piliation control mechanisms in Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3890–3894. doi: 10.1073/pnas.83.11.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J. Ultrastructure, chemistry, and function of the bacterial wall. Int Rev Cytol. 1981;72:229–317. doi: 10.1016/s0074-7696(08)61198-5. [DOI] [PubMed] [Google Scholar]
- Borst P., Greaves D. R. Programmed gene rearrangements altering gene expression. Science. 1987 Feb 6;235(4789):658–667. doi: 10.1126/science.3544215. [DOI] [PubMed] [Google Scholar]
- Chart H., Shaw D. H., Ishiguro E. E., Trust T. J. Structural and immunochemical homogeneity of Aeromonas salmonicida lipopolysaccharide. J Bacteriol. 1984 Apr;158(1):16–22. doi: 10.1128/jb.158.1.16-22.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley J. S., Lallier R., Trust T. J. Surface antigens of virulent strains of Aeromonas hydrophila. Vet Immunol Immunopathol. 1986 Jun;12(1-4):339–344. doi: 10.1016/0165-2427(86)90138-8. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I. Operon fusion of the phase variation switch. A virulence factor in Escherichia coli. Infection. 1982;10(2):112–115. doi: 10.1007/BF01816739. [DOI] [PubMed] [Google Scholar]
- Glauert A. M., Thornley M. J. The topography of the bacterial cell wall. Annu Rev Microbiol. 1969;23:159–198. doi: 10.1146/annurev.mi.23.100169.001111. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Ishiguro E. E., Ainsworth T., Trust T. J., Kay W. W. Congo red agar, a differential medium for Aeromonas salmonicida, detects the presence of the cell surface protein array involved in virulence. J Bacteriol. 1985 Dec;164(3):1233–1237. doi: 10.1128/jb.164.3.1233-1237.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro E. E., Kay W. W., Ainsworth T., Chamberlain J. B., Austen R. A., Buckley J. T., Trust T. J. Loss of virulence during culture of Aeromonas salmonicida at high temperature. J Bacteriol. 1981 Oct;148(1):333–340. doi: 10.1128/jb.148.1.333-340.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay W. W., Phipps B. M., Ishiguro E. E., Olafson R. W., Trust T. J. Surface layer virulence A-proteins from Aeromonas salmonicida strains. Can J Biochem Cell Biol. 1984 Nov;62(11):1064–1071. doi: 10.1139/o84-137. [DOI] [PubMed] [Google Scholar]
- Kay W. W., Phipps B. M., Ishiguro E. E., Trust T. J. Porphyrin binding by the surface array virulence protein of Aeromonas salmonicida. J Bacteriol. 1985 Dec;164(3):1332–1336. doi: 10.1128/jb.164.3.1332-1336.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mayer H. Optimization of the EcoRI-activity of EcoRI endonuclease. FEBS Lett. 1978 Jun 15;90(2):341–344. doi: 10.1016/0014-5793(78)80400-1. [DOI] [PubMed] [Google Scholar]
- Mescher M. F., Strominger J. L. Structural (shape-maintaining) role of the cell surface glycoprotein of Halobacterium salinarium. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2687–2691. doi: 10.1073/pnas.73.8.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn C. B., Ishiguro E. E., Kay W. W., Trust T. J. Role of surface components in serum resistance of virulent Aeromonas salmonicida. Infect Immun. 1982 Jun;36(3):1069–1075. doi: 10.1128/iai.36.3.1069-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps B. M., Trust T. J., Ishiguro E. E., Kay W. W. Purification and characterization of the cell surface virulent A protein from Aeromonas salmonicida. Biochemistry. 1983 Jun 7;22(12):2934–2939. doi: 10.1021/bi00281a023. [DOI] [PubMed] [Google Scholar]
- Polisky B., Greene P., Garfin D. E., McCarthy B. J., Goodman H. M., Boyer H. W. Specificity of substrate recognition by the EcoRI restriction endonuclease. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3310–3314. doi: 10.1073/pnas.72.9.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers on bacteria. Annu Rev Microbiol. 1983;37:311–339. doi: 10.1146/annurev.mi.37.100183.001523. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B. Regular arrays of macromolecules on bacterial cell walls: structure, chemistry, assembly, and function. Int Rev Cytol. 1978;53:1–62. doi: 10.1016/s0074-7696(08)62240-8. [DOI] [PubMed] [Google Scholar]
- Smit J., Agabian N. Cloning of the major protein of the Caulobacter crescentus periodic surface layer: detection and characterization of the cloned peptide by protein expression assays. J Bacteriol. 1984 Dec;160(3):1137–1145. doi: 10.1128/jb.160.3.1137-1145.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A., Nickel P., Meyer T. F., So M. Opacity determinants of Neisseria gonorrhoeae: gene expression and chromosomal linkage to the gonococcal pilus gene. Cell. 1984 Jun;37(2):447–456. doi: 10.1016/0092-8674(84)90375-1. [DOI] [PubMed] [Google Scholar]
- Stewart M., Beveridge T. J., Trust T. J. Two patterns in the Aeromonas salmonicida A-layer may reflect a structural transformation that alters permeability. J Bacteriol. 1986 Apr;166(1):120–127. doi: 10.1128/jb.166.1.120-127.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoenner H. G., Dodd T., Larsen C. Antigenic variation of Borrelia hermsii. J Exp Med. 1982 Nov 1;156(5):1297–1311. doi: 10.1084/jem.156.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker N. G., Fairweather N. F., Spratt B. G. Versatile low-copy-number plasmid vectors for cloning in Escherichia coli. Gene. 1982 Jun;18(3):335–341. doi: 10.1016/0378-1119(82)90172-x. [DOI] [PubMed] [Google Scholar]
- Swanson J., Bergström S., Barrera O., Robbins K., Corwin D. Pilus- gonococcal variants. Evidence for multiple forms of piliation control. J Exp Med. 1985 Aug 1;162(2):729–744. doi: 10.1084/jem.162.2.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]