Abstract
Desmoplakin (DP), plakoglobin (PG), and plakophilin 1 (PP1) are desmosomal components lacking a transmembrane domain, thus making them candidate linker proteins for connecting intermediate filaments and desmosomes. Using deletion and site-directed mutagenesis, we show that remarkably, removal of ∼1% of DP's sequence obliterates its ability to associate with desmosomes. Conversely, when linked to a foreign protein, as few as 86 NH2-terminal DP residues are sufficient to target to desmosomes efficiently. In in vitro overlay assays, the DP head specifically associates with itself and with desmocollin 1a (Dsc1a). In similar overlay assays, PP1 binds to DP and Dsc1a, and to a lesser extent, desmoglein 1 (Dsg1), while PG binds to Dsg1 and more weakly to Dsc1a and DP. Interestingly, like DP, PG and PP1 associate with epidermal keratins, although PG is considerably weaker in its ability to do so. As judged by overlay assays, the amino terminal head domain of type II keratins appears to have a special importance in establishing these connections. Taken together, our findings provide new insights into the complexities of the links between desmosomes and intermediate filaments (IFs). Our results suggest a model whereby at desmosome sites within dividing epidermal cells, DP and PG anchor to desmosomal cadherins and to each other, forming an ordered array of nontransmembrane proteins that then bind to keratin IFs. As epidermal cells differentiate, PP1 is added as a molecular reinforcement to the plaque, enhancing anchorage to IFs and accounting at least partially for the increase in numbers and stability of desmosomes in suprabasal cells.
Anumber of cell types display self-recognition by virtue of homophilic interactions with cell surface cadherins (for reviews see Schmidt et al., 1994; Green and Jones, 1996; Marrs and Nelson, 1996; Gumbiner, 1996). Cadherins form cell–cell junctions that are stabilized by connecting to the cytoskeletal network, either through actin microfilaments (Pasdar et al., 1991; Pasdar and Nelson, 1988) or through intermediate filament networks (Mueller and Franke, 1983; Stappenbeck and Green, 1992; Stappenbeck et al., 1993; Kouklis et al., 1994). IF-connecting cell–cell junctions, or desmosomes, are especially prominent in heart muscle cells and in stratified squamous epithelial cells (keratinocytes) where they form symmetrical plaques several microns in diameter and ∼100 nm thick. Each half of the desmosome is derived from an adjacent cell, and IFs from both cells appear to anchor through the cytoplasmic peripheries of the structure (Kelly, 1966).
Desmosomes contain two subtypes of the transmembrane glycoprotein superfamily of cadherins—desmogleins (Dsgs)1 and desmocollins (Dscs)—each encoded by at least three differentially expressed genes (Koch et al., 1992; Buxton et al., 1993; Koch and Franke, 1994; King et al., 1997). Dsg and Dsc are both essential for desmosomal cadherin clustering within the membrane, and for cell–cell adhesion (Chitaev and Troyanovsky, 1997). Typical of all cadherin-mediated junctions, desmosomes contain plakoglobin (PG), a member of the armadillo gene family (Cowin et al., 1986; for review see Barth et al., 1997). Desmosomal cadherins also associate with two additional nontransmembrane proteins not found in classical adherens junctions: plakophilins, cousins of PG (Hatzfeld et al., 1994; Heid et al., 1994), and desmoplakins (DPs), members of a small cytoskeletal linker family (Green et al., 1992). DPs contain a central α-helical rod domain, predicted to form an ∼130-nm coiled-coil homodimer, flanked by globular head (1014 amino acids [aa]) and tail (924 aa) domains (O'Keefe et al., 1989; Green et al., 1990; Virata et al., 1992). The major desmoplakin expressed in all cells that possess desmosomes is DPI, encoded by the same gene as DPII but displaying a longer rod domain (Green et al., 1988; Green et al., 1990).
Desmoplakins, plakoglobin, and plakophilins all reside in the desmosomal zone between the plasma membrane and IFs (Miller et al., 1987; Jones and Grelling, 1989; Moll et al., 1997). Whereas DP and PG are found in many cell types, PP1 expression is largely restricted to differentiating stratified and complex epithelia (Kapprell et al., 1988). Given their ubiquitous expression pattern, their abundance, and the absence of a homologue in actin-mediated cell–cell adherens junctions, DPI and DPII have always been the leading candidates for making molecular connections between desmosomes and IFs. Indeed, when NH2-terminally truncated DPI molecules are transiently expressed at very high levels in simple epithelial (COS) cells that have few desmosomes, or in mouse fibroblasts that have none, they colocalize with and result in disruption of endogenous IF networks (Stappenbeck and Green, 1992; Stappenbeck et al., 1993; Bornslaeger et al., 1996). The COOH-terminal tail segment of DP associates with IFs in cultured keratinocytes, although under these conditions a decoration rather than destabilization of the keratin network more typically occurs (Kouklis et al., 1994).
In vitro studies with recombinant DP tail reveal that it directly interacts with the head domain of type II epidermal keratins (Kouklis et al., 1994). This association has recently been confirmed by yeast two-hybrid analyses (Meng et al., 1997). The only other desmosomal protein reported to bind to IFs is PP1 (band 6), which interacted with type I and type II epidermal keratins in an overlay assay using radiolabeled keratins (Kapprell et al., 1988; Hatzfeld et al., 1994; Heid et al., 1994).
At the other end of the desmosome–IF connection are the proteins and/or domains involved in attaching the linker proteins to the desmosome. Troyanovsky et al. (1993) discovered that plakoglobin, desmoplakin, and the IF network were all recruited in vivo to the cytoplasmic tail segment of a chimeric Dsc1a linked to the transmembrane domain of a connexin. Deletion mutagenesis suggested that whether direct or indirect, sequences needed to recruit DP to Dsc-connexin differed from those necessary for PG, suggesting the existence of multiple modes of interactions by the nontransmembrane linker proteins (Troyanovsky et al., 1994). In similar studies with Dsg1, PG was found to be necessary for recruiting DP to artificial desmosome-like plaques generated by an Ecadherin-Dsg1 transgene chimera (Kowalczyk et al., 1997; Roh and Stanley, 1995). These findings suggested a linear sequence of interactions among Dsg1, PG, and DP. It is presently unclear whether the differences observed in these studies result from inherent differences between Dscs and Dsgs, differences among the various culture systems used, or whether these differences reflect artifacts introduced by the use of chimeric proteins.
At present, only a few studies have revealed direct interactions between the nontransmembrane linker proteins and the desmosomal cadherins. The most extensively documented is an interaction between PG and Dsgs, which occurs more strongly than that between PG and Dscs (Mathur et al., 1994; Troyanovsky et al., 1994; Troyanovsky et al., 1996; Chitaev et al., 1996; Wahl et al., 1996; Witcher et al., 1996; Kowalczyk et al., 1997). Whether PP1 associates with desmosomal cadherins is less clear, although in one study (Mathur et al., 1994) an overlay interaction was detected between radiolabeled Dsg1 and a desmosomal band of 75 kD likely to be PP1. This said, plakophilins seem to be localized more distantly from the midline of the desmosome than is plakoglobin, raising the possibility that their modes of interaction may differ in vivo (Mertens et al., 1996). Such differences could be important in light of the fact that desmosomes increase in number and stability as epidermal cells terminally differentiate and concomitantly increase plakophilin expression.
An interaction between the desmosomal cadherins and desmoplakin has yet to be identified, despite the prediction of its existence (Troyanovsky et al., 1994). Stappenbeck et al. (1993) demonstrated that the 1014–amino acid residue NH2-terminal head domain associates with desmosomal proteins in simple epithelial cells and fibroblasts in vivo, and Kowalczyk et al. (1997) showed that 584 residues of the DP head can form a triprotein complex with plakoglobin and Dsg1 in fibroblasts. However, examining these associations in desmosome-abundant cells was hampered by the fact that the desmoplakin (DPH) protein was proteolytically cleaved in A431 human epithelioid carcinoma cells, resulting in removal of the epitope tag and making it impossible to distinguish the transgene product from endogenous desmoplakin (Bornslaeger et al., 1996). Moreover, in vitro associations between desmoplakin and desmosomal cadherins have thus far gone undetected, although an interaction between the DP head segment and PG was observed in a yeast two-hybrid system (Kowalczyk et al., 1997). This has led to the postulate that DP may interact only indirectly with the desmosomal cadherins.
We now describe experiments aimed at delineating the sequences involved in the association between the DP head segment and the desmosome, and at understanding in greater detail how the desmosome associates with IFs. We have identified an NH2-terminal segment representing <3% of the DP head domain that can confer desmosomal association to foreign proteins in cultured human epidermal keratinocytes. Deleting only a portion of this segment is sufficient to abrogate this association in vivo. We provide in vitro studies that demonstrate direct associations between DP and PP1, PG, DP itself, and Dsc1a. We also show that PP1 binds preferentially to Dsc1a over Dsg1, while PG binds more strongly to Dsg1. Finally, we demonstrate that PP1 associates with keratins directly, and it does so more strongly than PG. Uncovering these novel associations has important new implications for our understanding of cytoarchitecture and desmosomal adherens junctions in the epidermal keratinocyte.
Materials and Methods
Plasmid Constructs
The junctions of all ligated DNAs and all PCR-generated fragments were confirmed by DNA sequence analysis. Any manipulations to alter restriction enzyme sites were engineered in a manner such that the wild-type amino acid sequence was retained. The mammalian expression vector used for all DP constructs is PECE-FLAG, obtained from Dr. Xiao-Kun Zang (Burnham Institute, La Jolla, CA). It contains an SV40 major early promoter and enhancer to drive expression.
Full-length DPI DNA was made by combining two DP fragments at a common XbaI site (nucleotide 3620): (a) keratinocyte cDNA beginning at 127 bp 5′ from the translation initiation start site and extending past the XbaI site; and (b) phDP1rt (Kouklis et al., 1994), a human genomic clone encompassing nucleotide 3450 to +500 bp 3′ from the translation stop codon of DP. To facilitate cloning, the XbaI site at nucleotide 5858 was removed by site-directed mutagenesis. The FLAG epitope tag was added by conventional DNA recombinant technology using a synthetic oligonucleotide containing the end of the DP coding sequence, followed by a sequence encoding the FLAG epitope (MDYKDDDDK), followed by a stop codon. The hybrid gene cDNA encoding DPI FLAG and DPII FLAG was then subcloned into the PECE backbone vector to produce pDPFLAG.
Deletion Constructs
To create the DPH deletion constructs, the PECE-FLAG vector was modified such that the multiple cloning site was positioned 5′ of the sequences encoding the FLAG tag, referred to here as PECE FLAG 3′. pDPH 1222 was created by insertion of an EcoRV DPI cDNA fragment into PECE FLAG 3′. COOH-terminal deletions of this DPH sequence were made in pDPH1222 at the following sites: Bsu 361 (pDPH968), MscI (pDPH 824), BsaAI (pDPH493), and AccI (pDPH 387). In all cases, strategies were used to ensure that the FLAG and DPH sequences were in frame; all were verified by sequence analysis. NH2-terminal deletions of 9 and 16 amino acids were made by PCR deletion mutagenesis, retaining the Kozak sequence and translation start codon of the DPH. The NH2-terminal 29-aa deletion was made by digesting pDPH 824 with BstE II and NcoI, and by treating with Klenow fragment and religation where NcoI had been inserted by site-directed mutagenesis at the DPI translation initiation codon. To generate point mutants, we used synthetic oligonucleotides and PCR-based cloning. Again, all PCR fragments and junctions were sequenced to confirm their identity.
DP Green Fluorescent Protein(GFP) Fusion Proteins
GFP clone pJK19-1 was kindly provided by Dr. Pamela Silver (Dept. of Biological Chemistry and Molecular Pharmacology, Harvard University). It contains two mutations—S65T and V164A—to enhance immunofluorescence detection and solubility, respectively. The full GFP cDNA was engineered to contain a molecular cloning site 3′ of the GFP initiator Met, which was then used along with an endogenous EcoRV site in the 3′ untranslated region to excise the cDNA. Construction of all fusion proteins was then made by using natural restriction endonuclease sites within the DPH cDNA sequence to isolate the appropriate fragments, and by using DNA recombinant technology to place them in frame upstream from the GFP sequence.
Recombinant Proteins
The pGex-2TK vector (Pharmacia Biotech., Inc., Piscataway, NJ) was used to generate GST fusion proteins. The pET8c bacterial expression vector (Studier and Moffat, 1986) was used to generate all other recombinant proteins. To facilitate cloning procedures for DP head constructs, an NcoI site was engineered at the initiator ATG of DPH824 by site-directed mutagenesis; this site also appears in the initiator ATG of pET8c. pET8c-DPH824 was then generated by inserting the NcoI–MscI fragment of pDPH824 into pET8c. pET8c-DPH NΔ29 and pET8c pDPH180 GFP were then engineered starting from pET8cDPH824.
Human plakoglobin cDNA and bovine Dsc1a cDNA (BDClr) were generous gifts from Dr. Werner Franke (German Cancer Center, Heidelberg, Germany). To generate pET8c-PG, an NcoI site was engineered at the ATG initiator codon using a G:C mutation at nucleotide 119 of the clone. The NcoI–BamHI fragment from this clone was then inserted into the NcoI/BamHI site of pET8c. pBDClr was subcloned as an EcoRI fragment into pGex2TK (Pharmacia Biotech., Inc.) to generate pGex2TK-Dsc. PCR was then used to engineer a DraI site at the transmembrane– cytoplasmic junction such that the sequence was changed from GTT AAG to TTT AAA. The sequences encompassing the 5′ EcoRI site to the DraI site of Dsc1a were then removed from pGex2TK-Dsc to generate pGex2TK-Dsc tail.
To engineer recombinant desmoglein-tail, we used clone pDGK3C (generous gift of Dr. W.W. Franke) containing bovine Dsg1 cDNA. The Sca1–BsaA1 fragment, containing nucleotide residues 1592–3326, were subcloned into the NcoI–BamHI sites of bacterial expression vector Pet15b (Novagen, Inc., Madison, WI). This construct encoded 50 amino acids of extracellular and the entire transmembrane and cytoplasmic domains of Dsg1.
A full-length plakophilin 1 cDNA was obtained from a mouse skin cDNA library, subcloned into Superscript™ (Promega Corp.), and was fully sequenced.
Antibodies
Polyclonal anti-DP head antiserum (αDPH) was prepared by injecting rabbits with an initial 400 μg of purified recombinant human DPH followed by two 200-μg boosts separated at 4-wk intervals. 3 mo later, two more boosts of 150 μg each were given at 3-wk intervals. Western blot analyses using affinity-purified antibody revealed that the pattern of detection was identical to that of known desmoplakin antibodies, revealing exclusively the expected doublet at >200 kD.
Antibodies used for immunofluorescence were as follows: guinea pig αK5 (1:100; Lersch et al., 1989), mouse monoclonal anti-FLAG M2 (αFg, 10 μg/ml; IBI Kodak, New Haven, CT) or 1 μg/ml rabbit anti-FLAG (αFg, D8) polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA), affinity-purified αDPH (1:10), αDP recognizing the rod/tail of DP (5 μg/ml DP2.15; ICN Biomedicals, Costa Mesa, CA), rabbit anti-GFP (10 μg/ml αGFP; CLONTECH Laboratories, Inc., Palo Alto, CA), mouse monoclonal anti-Dsg 3.10 (5 μg/ml αDsg; Koch et al., 1990), and rabbit anti-plakoglobin (1:150 PG; a generous gift from Dr. Jackie Papkoff, Megabios Corp., Burlingame, CA). Antibodies used for Western blotting were as follows: anti-FLAG (αFg, D8) at 1:7,000; GFP (1 μg/ml), αDPH (1:100), αDsg (5 μg/ml), mouse anti-DscII 7G6 (1:500, αDsc; a generous gift from Dr. Margaret Wheelock, University of Toledo, OH), mouse anti-plakoglobin 5.1 (1:2500, PG; ARP, Belmont, MA), and mouse anti-plakophilin PP102D6.1.4 (αPP, 1:100; a generous gift from Dr. Werner Franke). All secondary antibodies, conjugated to FITC, Texas Red, Alexa 488, or Alexa 594 (Molecular Probes, Inc., Eugene, OR; immunofluorescence), or to HRP (Western blots) were obtained from Jackson ImmunoResearch Laboratories, Inc., (West Grove, PA).
Overlay Binding Assays
Most protein probes used in the overlay assays were prepared by in vitro transcription/translation (TNT Coupled Reticulocyte Lysate system™; Promega Corp.) in the presence of Pro-mix l-[35S] in vitro cell labeling mix (Amersham Corp., Arlington Heights, IL). In one case, recombinant plakoglobin was biotinylated as in Kouklis et al. (1994). After labeling, probes were diluted 10× in overlay buffer and subjected to centrifugation at 3000 g for 90 min in Centricon 10™ units (Amicon W.R. Grace & Co., Beverly, MA). This was repeated 3× in order to remove the majority of the free [35S]-labeled amino acids. Quantitation and purity of protein probes were then evaluated by SDS-PAGE and phosphorImager analysis using the Image Quant Program phosphorImager analysis using the Image Quant Program (Molecular Dynamics, Sunnyvale, CA).
The overlay assay was performed essentially as described (Merdes et al., 1991). 1-mm–thick SDS-polyacrylamide gels were made using ultra pure electrophoresis reagents (Bio-Rad Laboratories, Hercules, CA). After electrophoresis of samples in quadruplicate, one gel was stained with Coomassie Blue to visualize the proteins, and the other three were transferred to nitrocellulose membranes for the test overlay and for control overlays with either radiolabeled GFP or reticulocyte reaction omitting the plasmid DNA. Probe was diluted in blocking solution such that each probe had the same count/vol. Blots were probed overnight at 4°C, and were then washed for 30 min at room temperature (4×) and either exposed to x-ray film or analyzed with a phosphorImager.
Recombinant Proteins Used in Overlays
Bacterially expressed proteins were isolated from inclusion bodies. DPH proteins were further purified by passage through a Hi Load 16/60 Superdex 200 size exclusion column (Pharmacia Biotech, Inc., Piscataway, NJ) in 6 M urea, 10 mM Tris, pH 7.0, 200 mM NaCl, and 0.1% β-mercaptoethanol. Plakoglobin was passaged through a Mono Q HR 10/10 anion exchange column (Pharmacia Biotech, Inc.) using two buffers (Buffer A: 6 M urea, 20 mM Tris, pH 7.0; and Buffer B: 6 M urea, 20 mM Tris, pH 7.0, 1 M NaCl). Recombinant GST-Dsc and GST were soluble, and were purified using an anti-GST affinity column (Pharmacia, Piscataway, NJ). Recombinant human K5 proteins were purified as described (Wilson et al., 1992). BSA Fraction V is from Sigma Chemical Co. (St. Louis, MO), and size standards are from Bio-Rad Laboratories.
Results
Specific Association of 387 Amino Acid Residues of the DPI Head Domain With Desmosomes in Cultured Human Epidermal Keratinocytes
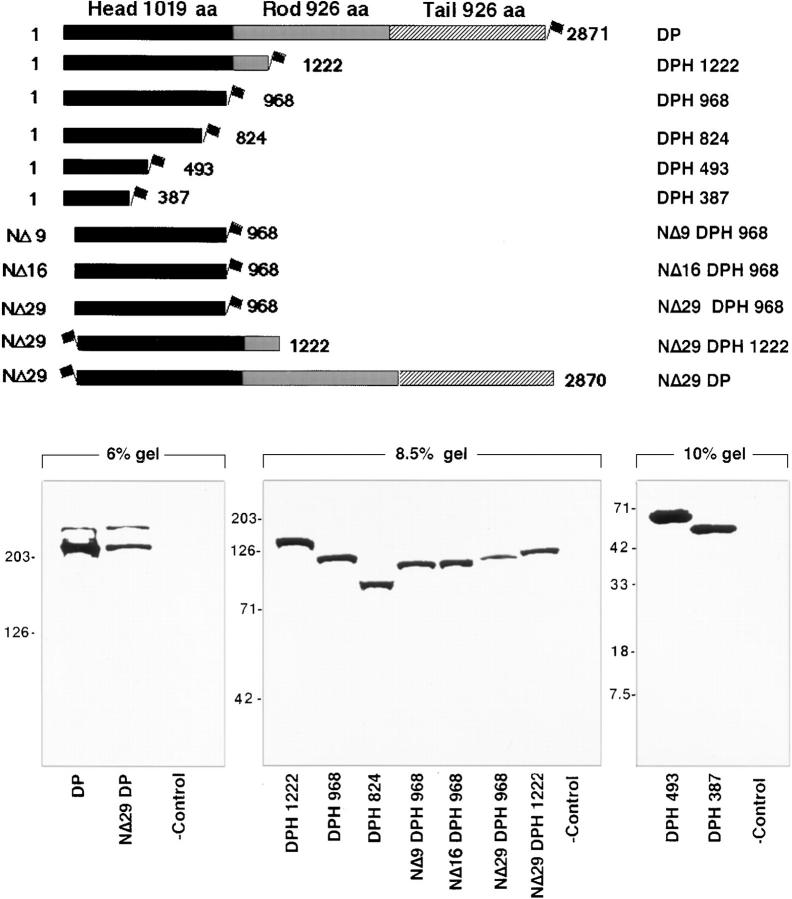
We first verified that the ATG translation initiation codon is as reported in GenBank (accession No. M77830). As judged by secondary structure predictions, the amino terminal head domain of desmoplakin (DPH) is now predicted to be 1014 amino acid residues. To explore the association between DPH and desmosomes, and to further delineate the nature of the desmosomal recognition site, we began by preparing mammalian expression vectors encoding either the full-length DPI or various deletion mutants of DPI, most tagged with the FLAG epitope at their COOH terminus (Fig. 1). Whenever NH2-terminal mutations were engineered, we preserved the first ATG translation initiation codon and surrounding Kozak sequences (Kozak, 1997) to ensure efficient expression. Western blot analyses confirmed that the clones were expressed comparably, and as single species of the expected size in transiently transfected COS epithelial cells (Fig. 1). The two bands in the full-length DP and NΔ29 DP lanes most likely represent DPI and DPII, since the expression construct contains the DP introns that are differentially spliced to produce these products.
Figure 1.
Desmoplakin deletion mutants and their expression in cultured epithelial cells. (Top) Shown are diagrams of truncated mutant desmoplakin proteins whose expression was genetically engineered to be under the control of the SV40 major early promoter and enhancer. In all cases, the FLAG epitope tag was placed in-frame (depicted by the flag). The full-length DP transgene was engineered from part DP cDNA and part DP gene, such that both DPI and DPII would be expressed. The coiled coil rod and flanking globular head and tail segments are demarcated according to the Coils Version 2.1 secondary structure prediction program (www site, ISREC, Switzerland). Numbers shown are in amino acid residues; nomenclature is at right. (Bottom) Western blot analyses of protein extracts from COS epithelial cells transfected with the constructs indicated above and harvested 48 h after transfection. Proteins were resolved by electrophoresis through SDS polyacrylamide gels (percentages noted), transferred to nitrocellulose paper by electroblotting, and then visualized by binding to anti-FLAG. Antibody binding was developed using biotinylated secondary antibodies as outlined by Kouklis et al. (1994). (-control) Extracts from mock-transfected cells. Migration of size standards are indicated at left, in kD. Since some transfections were conducted at different times and since variations existed in plasmid preparations and transfection efficiencies, loadings were done to reflect similar transgene protein levels, rather than total protein levels.
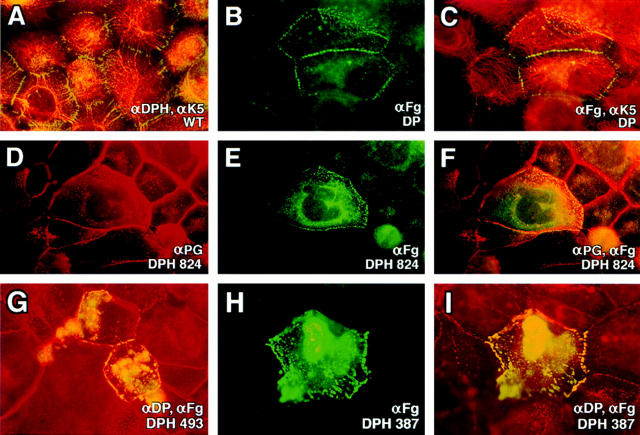
Human epidermal keratinocytes display an extensive array of desmosomes that anchor the keratin IFs at the cell periphery. These membrane plaques exhibited the expected punctate pattern when stained with a polyclonal antiserum that we prepared against recombinant DPH (Fig. 2 A). When transiently expressed in keratinocytes, FLAG-tagged full-length recombinant DP localized to these junctions (Fig. 2, B and C). No anti-FLAG staining was observed in the desmosomes of surrounding untransfected cells, although some transfected cells showed a mixture of cytoplasmic and desmosomal αFg staining. The ratio of cytoplasmic to desmosomal staining was consistently higher in more brightly stained cells. This finding suggested that the cytoplasmic staining was a consequence of transgene overexpression, resulting in saturation of all the desmosomal binding sites and cytoplasmic accumulation of the excess protein.
Figure 2.
Localization of full-length and COOH-terminally truncated desmoplakins to desmosomes. Human epidermal keratinocytes (SCC-13) were transfected with expression vectors encoding proteins denoted at the lower right of each frame. After transfection, cells were fixed and labeled with antibodies indicated, followed by the appropriate fluorescently conjugated secondary antibodies. Representative cells shown were visualized through a Zeiss Axiophot immunofluorescence microscope with a 100×or 63× objective. Punctate staining at cell borders is typical of desmosomal staining; filamentous staining is typical of keratin network staining. WT, wild-type.
Desmosomal localization was also observed with transgenes encoding smaller portions of the NH2 terminus of DP (Fig. 2, D–I). Costaining with αFg and antibodies specific for various desmosomal components (some of which are shown here) revealed that recruiting DPH segments did not interfere with localization of the other desmosomal components. More weakly αFg-staining cells exhibited largely desmosomal localization, and ∼50–85% of all transfected cells exhibited unequivocal desmosomal targeting. We narrowed the desmosomal recognition domain to within the 387 NH2-terminal residues of the DP head. As we began to delete further into the carboxy terminus, these severely truncated proteins were often unstable and more difficult to analyze. We revisited this issue later using fusion proteins (see below).
Collectively, our observations were in good agreement with and extended previous results obtained with other cell types and with larger head portions of DP (Stappenbeck et al., 1993). We further observed that similar to the larger DP head mutants (Bornslaeger et al., 1996), the smaller DP head mutants also disrupted desmosomes and keratin networks when expressed in long-term transfected keratinocytes selected with a drug-resistance marker (E. Smith and E. Fuchs, unpublished results). This finding suggested that our DP head mutants not only bound to components within the epidermal desmosomes, but also prevented and/or perturbed some of the protein interactions necessary for structure stabilization.
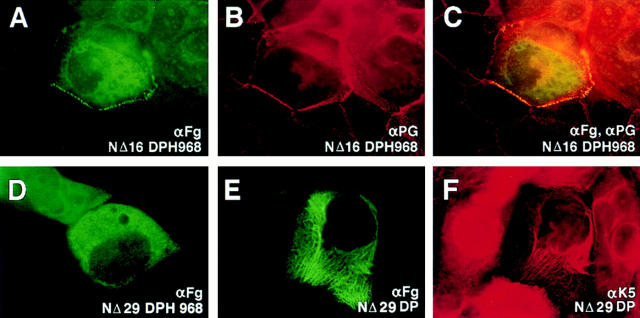
Deletion of 29 Residues From the NH2 Terminus of DP Abrogates Its Association with Desmosomes
We next turned our attention towards engineering NH2-terminal deletions of the DP head segment (see Fig. 1 for constructs and protein expression analyses). Surprisingly, only a few amino acids could be removed from the NH2 terminus before desmosomal recognition was significantly weakened. Thus, although deletion of the first 16 amino acid residues still allowed appreciable localization of the DP head to desmosomes (Fig. 3, A–C), deleting an additional 13 residues resulted in a loss of association in the majority of transfected keratinocytes (Fig. 3 D). Furthermore, the full-length DP with a FLAG tag in the place of the first 29 amino acid residues, was also unable to associate with desmosomes, although it retained its ability to colocalize with keratin filaments (Fig. 3, E and F). Therefore, we concluded that removing these additional 13 residues either removed all or a part of the desmosomal recognition site, or alternatively, created an alteration in DP head conformation such that the desmosomal recognition site(s) was masked.
Figure 3.
Localization of NH2-terminally truncated desmoplakins to desmosomes. Human epidermal keratinocytes were transfected with expression vectors encoding FLAG epitope–tagged desmoplakin proteins. After transfection, cells were fixed and stained with the indicated antibodies. Representative cells were photographed using a 100× objective. Note loss of punctate staining and appearance of diffuse cytoplasmic staining in transfected cells in D; note loss of punctate staining and appearance of filamentous staining in transfected cell in E and F.
Is the N16-N29 Segment Sufficient for DP Head–Desmosome Recognition?
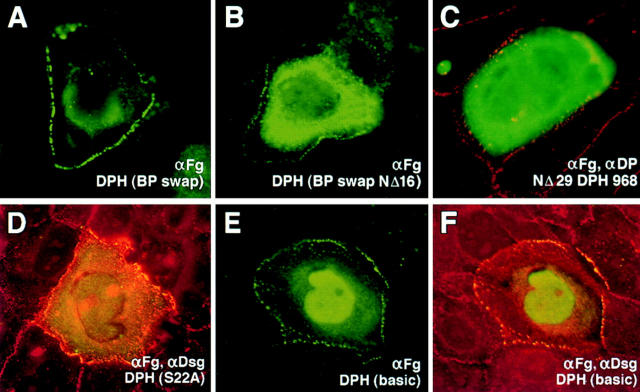
To further investigate the role of the N16-N29 segment of the DP head in desmosome recognition, we used site-directed mutagenesis to substitute a number of the residues within this sequence (Fig. 4). Some of our substitutions within these 13 residues were made in NΔ16 DPH, while others were made in the complete DPH. We also swapped the N16-N29 residues with sequences that might be equivalent in the epidermal form of the bullous pemphigoid antigen 1 protein (BPAG1e). This protein, referred to as DPH (BPswap), is interesting in that BPAG1e is a cousin of DP that associates with hemidesmosomes rather than desmosomes (Schmidt et al., 1994; Green and Jones, 1996). Mutant cDNAs were sequenced for verification, and were then stably expressed in cultured cells. The transgene products were analyzed by PAGE and immunoblot analyses to confirm that the proteins were of the expected size (Fig. 4).
Figure 4.
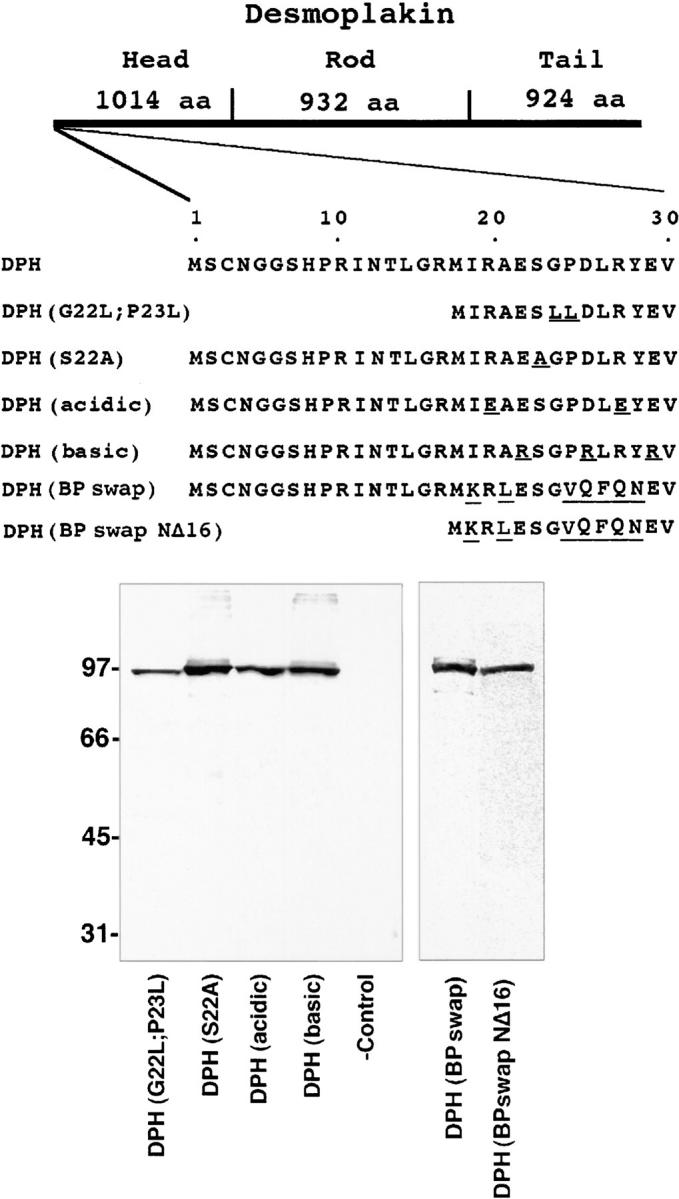
Construction and expression of mutants within N16-N29 of the desmoplakin head domain. (Top) Diagram depicting the first 29 amino acid residues of the DPH and the various constructs engineered in which residues were mutated within this sequence. The most dramatic change was the swapping of the N16-N29 residues within the equivalent sequence of BPAG1e to create DPH (BPswap), which was engineered in the context of the full-length head domain, and in the context of DPH NΔ16. (Bottom)Immunoblot anlaysis of extacts isolated from cells 48 h after transfection with vectors encoding these proteins. (-control) Extract from mock-transfected cells. Molecular mass standards in kD are indicated at left.
When transiently transfected into keratinocytes, most mutant N16-N29 DPH proteins gave desmosomal localization as judged by anti-FLAG antibodies. This included the DPH (BPswap) protein, harboring seven amino acid substitutions (Fig. 5 A). One mutation that significantly affected localization was the DPH (BPswapNΔ16) (Fig. 5 B). The ability of this double mutant to localize to desmosomes was significantly weaker than either of the single mutants, i.e., NΔ16DPH or DPH (BPswap), but it was not as severe as deletion of the first 29 amino acid residues altogether (Fig. 5 C). Overall, this finding revealed the importance of the first 16 residues not previously detected within the context of an otherwise wild-type DPH domain. Furthermore, the fact that the double mutant still showed some, albeit weak, ability to localize to desmosomes, was intriguing, and suggested that the binding domain(s) for desmosomal recognition is likely to encompass additional residues extending beyond these 29. Further evidence for this possibility is provided below.
Figure 5.
Changes in the localization of the desmoplakin head domain upon mutation within the first 29 NH2-terminal residues of DPH. Keratinocytes were transfected with the vectors encoding mutant DPH proteins. After transfection, cells were fixed and stained with antibodies as indicated. Note progressive loss of cell border staining going from DPH (BPswap) in A to DPH(BPswapNΔ16) in B to NΔ29DPH in C; note addition of nuclear staining in E and F.
Desmosomal localization was observed with most of the other mutants, including (a) a G22L/P23L double point mutant, which made the segment more compatible with an α-helical conformation; (b) an S22A mutant, which removed the only serine residue in this stretch, eliminating any possibility for its phosphorylation (Fig. 5 D); and (c) DPH (acidic), a mutant generated by converting the two positively charged arginine residues to negatively charged glutamate residues, thereby removing all positive charge from this segment. The only other mutation that affected localization considerably was the DPH(basic) protein, containing an N16-N29 segment with the three negatively charged residues mutated to arginines. This mutant partitioned between desmosomes and the nucleus, presumably because the positively charged segment now acted as a nuclear localization signal sequence (Fig. 5, E and F). Nuclear localization of DPH(basic) suggested that the amino terminus of the DP head is exposed on the surface of the protein.
Evidence for a Desmosomal Binding Domain Within the NH2 Terminus of the DP Head
All of our studies performed thus far suggested that somewhere within the DP head, there resided a domain that conferred desmosomal association. We were unsuccessful in demonstrating desmosomal targeting of purified biotinylated N16-N29 DP peptide microinjected into the cytoplasm of cultured keratinocytes (data not shown). We were also unsuccessful at expressing very small NH2-terminal segments of the DP head in transfected keratinocytes (not shown). We therefore turned our attention towards engineering fusion proteins and testing whether small DP head segments could confer the ability upon foreign proteins to associate with desmosomes (Fig. 6).
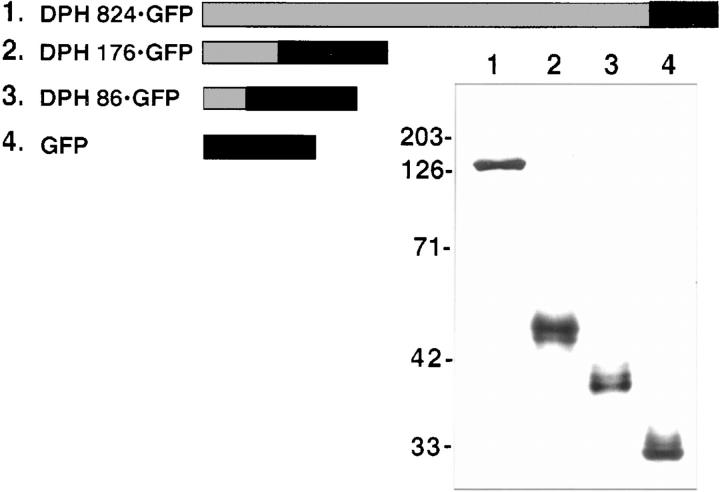
Figure 6.
Construction and expression of DPH fusion proteins. (Top) Diagram indicating fusion proteins generated from portions of DPH (grey) linked to GFP (black bars). (Bottom) Cells were transfected with expression vectors encoding the DPH-GFP fusion proteins. After transfection, extracts were isolated and subjected to electrophoresis through 10% SDS polyacrylamide gels, electroblotted onto nitrocellulose paper, probed with anti-GFP and subjected to chemiluminescence. Molecular mass standards in kD at left.
We first tested the ability of 824 amino terminal residues of the DP head domain to confer desmosomal recognition to GFP, a cytoplasmic protein with completely different sequence and structure from DP. As judged by αGFP immunofluorescence of transfected keratinocytes, the DPH 824 GFP transgene product targeted efficiently to desmosomes without perturbations (not shown). This control demonstrated that adding GFP to the COOH terminus of a major portion of the DPH head domain did not affect its desmosomal localization. Next we discovered that either 176 or 86 amino terminal residues were sufficient to confer efficient desmosomal recognition to GFP (Fig. 7, A–D). In DPH 176 GFP transfected cells, 68 ± 7% of the cells showed colocalization between αGFP and antibodies against a variety of desmosomal proteins, including endogenous DP (shown; COOH-terminal antibody), and desmocollins. In DPH 86 GFP-transfected cells, 48 ± 8% of the cells showed targeting to desmosomes. In contrast, when expressed on its own, GFP partitioned between the cytoplasm and the nucleus, displaying no cell border staining (Fig. 7, E–F). Only a few (6 ± 2%) of these GFP-transfected cells showed some membrane staining. The reduction in efficiency of desmosomal targeting between DPH 176 GFP and DPG 86 GFP was consistent with the notion that smaller DPH segments do not possess the entire desmosomal targeting sequence(s). Similar results were obtained when we repeated these experiments with hybrid proteins engineered with DP and BPAG1e head domains, FLAG-tagged at the COOH terminus (data not shown).
Figure 7.
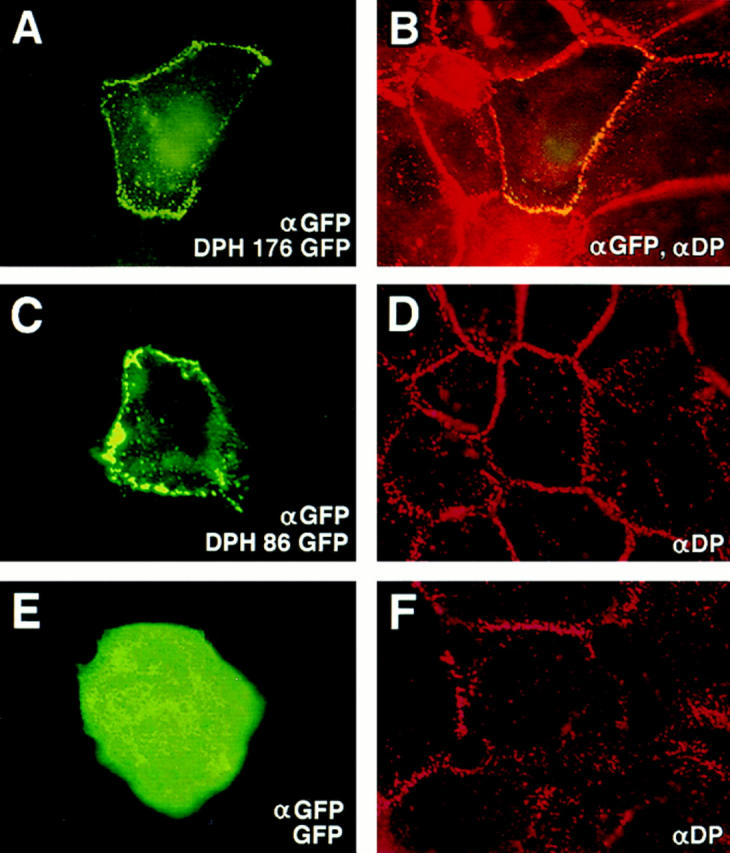
Localization of desmoplakin fusion proteins to desmosomes. Keratinocytes were transfected with expression vectors encoding desmoplakin fusion proteins. After transfection, cells were fixed and stained with the indicated antibodies (lower right). The αDP used was to the tail segment of DP, not present in the fusion protein. Representative cells for each construct were photographed under an 100× objective, and frames are displayed here in pairs, with transgene product staining at left and double-staining at right. Note that cells expressing DPH 176 GFP or DPH 86 GFP displayed predominantly punctate αGFP/αDP staining at the periphery, typical of desmosomal localization; in contrast, cells expressing GFP exhibited cytoplasmic staining with little or no membrane staining.
Identifying the Desmosomal Proteins that Directly Interact with the Amino Terminus of the DP Head
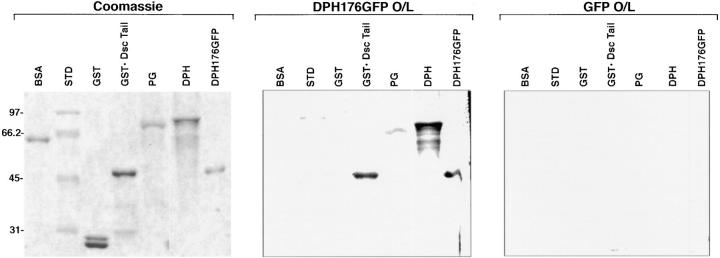
For the remainder of our studies, we focused on analyzing the 176-aa NH2-terminal segment of DP, since this domain contained an efficient desmosomal recognition sequence. To address whether this segment can directly associate with desmosomal components, and to delineate further the nature of these interactions, we conducted overlay assays with [35S]-labeled recombinant DPH 176 GFP, produced by in vitro transcription and translation in a reticulocyte lysate system. Electrophoretically resolved proteins were either fixed and stained with Coomassie Blue, or were transferred to nitrocellulose and hybridized with radiolabeled protein probe. The DPH segment bound to at least two desmosomal proteins: the Dsc1a cytoplasmic tail segment and the DP head itself, under conditions where GFP alone showed no binding (Fig. 8). Self-association of the DPH segment with DP was detectable in whole skin extracts, but the levels of endogenous Dsc1a were too low to be detected clearly in overlay assays using crude extracts. Representative examples of these points are provided below. While the insolubility of bacterially expressed recombinant DPH 176 GFP precluded our ability to conduct immunoprecipitations as a complementary method of examining binding (data not shown), the associations that we observed by overlay assays were specific: BSA, five molecular mass standards, and glutathione S transferase (GST) showed no binding (Fig. 8), nor did we detect binding of total proteins isolated from fibroblast extracts (data not shown). The direct interaction between the DP head and the Dsc1a tail provides in vitro evidence to explain the in vivo results of Troyanovsky et al. (1994), who identified a deletion mutant of connexin-Dsc1a that failed to bind plakoglobin, but still recruited desmoplakin and IFs to the membranes of cultured fibroblasts.
Figure 8.
Evidence for direct interaction between 176 residues of DPH and the cytoplasmic domain of Dsc1a and the DPH itself. Recombinant proteins were engineered as described in Materials and Methods, and resolved in triplicate by SDS PAGE. One gel was stained with Coomassie Blue to visualize the proteins, and the others were transferred to nitrocellulose membrane and then subjected to overlay assays using equal amounts of [S35]methionine-labeled DPH176GFP (test) and GFP (control), respectively. Note binding of DPH176GFP to the Dsc1a tail, DPH, and DPH176GFP, under conditions where radiolabeled GFP showed no binding. Molecular mass standards in kilodaltons at left.
Associations Between Desmosomes and Plakophilin 1 and Plakoglobin
We next focused on the remaining two major desmosomal proteins—plakoglobin and plakophilin 1—that lack a transmembrane domain. While plakoglobin localizes to both E-cadherin and desmosomal cadherin cell–cell junctions, plakophilin 1 seems to be specific for desmosomes (Kapprell et al., 1988). This result suggested that the association between these two armadillo family members and desmosomal components may differ. To test this hypothesis, we used overlay assays to compare the relative behaviors of radiolabeled recombinant PP1 and PG.
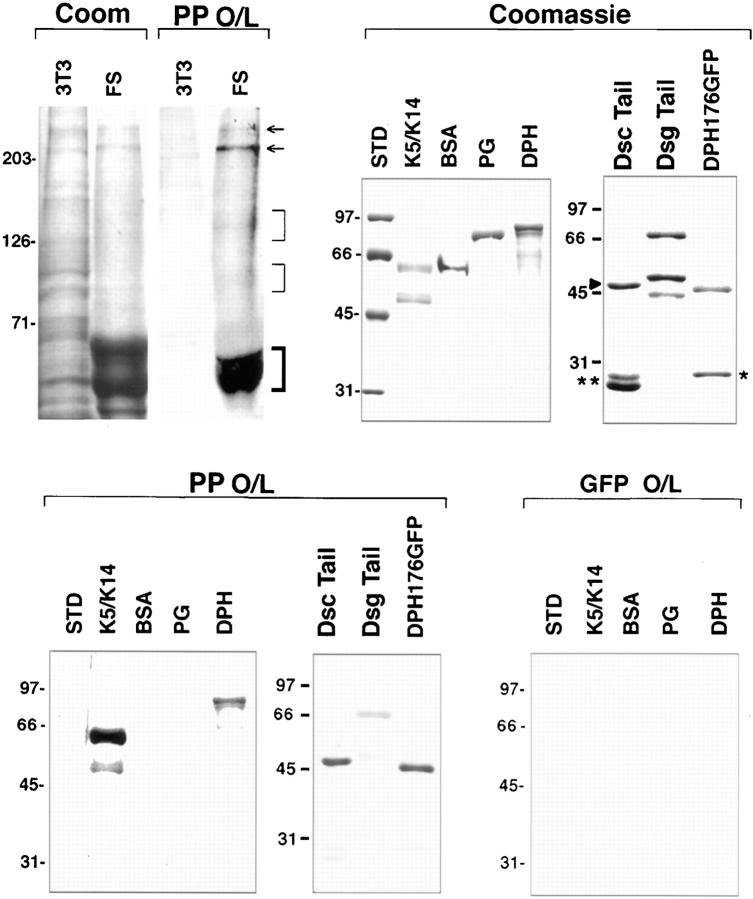
In vitro–translated, [S35]-labeled PP1 interacted specifically with several epidermal proteins under conditions where associations with fibroblast proteins were not observed (Fig. 9). In skin protein extracts, PP1 in electrophoretic mobilities bound prominently to bands corresponding to those of DPI/DPII and keratins. The identity of these interacting proteins was confirmed by overlay assays using recombinant proteins. The use of recombinant proteins allowed us to demonstrate further that within the DP protein, the head domain was a target for PP1 binding (Fig. 9).
Figure 9.
Evidence for direct interaction between plakophilin 1 and desmosomal proteins. (Top, from left to right) Proteins from the Triton X-100 insoluble fraction of mouse 3T3 fibroblasts or human foreskin were resolved by electrophoresis through a 6% SDS polyacrylamide gel, and were either stained with Coomassie Blue to visualize proteins (Coom) or subjected to overlay assays (O/L) using S35-methionine–labeled plakophilin 1 (PP). Note binding of PP to bands of the mobility of desmoplakins I and II (arrows), and to keratins (bold bracket). Note also faint binding to two additional groups of skin bands (thin brackets), which were of the mobility of the desmogleins and desmocollins. Interactions between PP1 and desmosomal components were verified using recombinant proteins. Coomassie Blue–stained gels of bacterially expressed and purified recombinant proteins are shown, along with overlay assays using equal amounts of S35-labeled PP1 (test) and GFP (control). Double asterisk and single asterisk denote GST and GFP, respectively, added to the lanes containing DscTail and DPH176GFP as internal controls. Dsctail, GST-Dsc1a-tail fusion protein; Dsgtail, Dsg1-tail; Std, molecular mass standards (sizes denoted in kD at left).
In skin extract overlays, two groups of very faint bands were detected in the vicinity of the sizes expected for the desmogleins (130–165 kD) and desmocollins (100–110 kD), respectively. To explore this possibility further, we engineered recombinant GST-Dsc1a and Dsg1 proteins containing the cytoplasmic tail segments of the cadherins. GST protein was added to the GST-Dsc1a tail sample as an internal control (see Coomassie Blue–stained gels: arrowhead, GST-Dsc1a tail; double asterisk, GST). An appreciable band of the size expected for intact tail segment was observed for each cadherin sample (46 kD and 66 kD, respectively), although a few specific proteolytic cleavage products were generated in the Dsg1 tail sample. Intriguingly, PP1 appeared to bind more strongly to recombinant Dsc1a tail segment than to the Dsg1 tail (Fig. 9). In contrast, neither GFP (asterisk; coadded to the lane containing DPH 176 GFP) nor the degradation products of the Dsg1 tail showed appreciable binding under these conditions. As judged by immunoblot analysis, these smaller Dsg1 tail fragments lacked the COOH terminus.
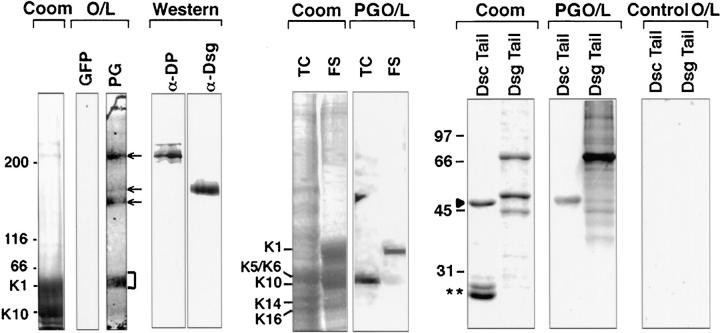
We next conducted similar studies with radiolabeled PG. PG bound to several bands in whole-skin extracts corresponding to the expected sizes of DPI and DPII, desmogleins, and keratins (Fig. 10). αDP and αDsg1 antibodies confirmed that DPI, DPII, and Dsg1 (165 kD) migrated at these positions. The interaction between PG and desmoplakin had been detected, although it was weak, in the reverse assay using [S35]-labeled DPH 176 GFP and recombinant PG (Fig. 8); PG-DP interactions have also previously been demonstrated by yeast two-hybrid assays (Kowalczyk et al., 1997).
Figure 10.
Evidence for direct interaction between plakoglobin and a number of desmosomal proteins. (Top, from left to right) Proteins from the Triton X-100–insoluble fraction of human foreskin were resolved by electrophoresis through a 6% SDS polyacrylamide gel and either stained with Coomassie Blue to visualize proteins (Coom) or subjected to overlay assays (O/L) using equivalent amounts of [S35]methionine- labeled GFP (control) or plakoglobin (PG, test). Note clear binding of PG to bands of the mobility of desmoplakins (top arrow), desmogleins (bottom two arrows), and to keratins (bracket). Western blot analysis confirmed the presence of desmoplakins and desmogleins in this extract. Binding of PG to the keratins was confirmed using extracts from human epidermal cultures (TC) and from foreskin (FS), depicting predominantly K5/6 interactions in TC extracts and K1 interactions in FS extracts. Interactions between PG and the desmosomal cadherins were further examined using recombinant proteins. Coomassie Blue–stained gels of bacterially expressed and purified GST-Dsc1a-tail (Dsc Tail) fusion protein and recombinant Dsg1-tail (Dsg Tail) are shown, along with overlay assays using equal amounts of S35-labeled PG. Double asterisk denotes GST, added to the lane containing DscTail as an internal control. Molecular mass standards are shown in kD at left.
The interaction between PG and Dsg1 was confirmed using recombinant Dsg1 tail protein (PG O/L). The interaction could be readily detected using 35S-labeled PG, and was significantly stronger in both whole cell extracts and in recombinant protein overlays than that seen with PP1. PG–Dsg1 interactions have been observed previously using a variety of different methods (Mathur et al., 1994; Roh and Stanley, 1995; Chitaev et al., 1996; Chitaev et al., 1997; Witcher et al., 1996). Interestingly, under conditions where PG and Dsg1 interacted strongly, significantly weaker interaction was seen with Dsc1 (Fig. 10). While PG's preference for Dsg1 vs. Dsc1a has been reported previously (Mathur et al., 1994; Chitaev et al., 1996), the result was significantly more meaningful in the context of parallel studies with PP1, since PG's and PP1's preference for these desmosomal cadherins was exactly the opposite (compare Figs. 9 and 10). The comparison allowed us to rule out trivial explanations for this preference, e.g., that only one of the two desmosomal cadherin fusion proteins was fully intact and/or fully resembled its native conformation in the overlay assay. Moreover, these findings revealed a distinct difference in the preference of PP1 and PG for the two desmosomal cadherins.
Associations Between Keratins and Plakophilin 1/Plakoglobin
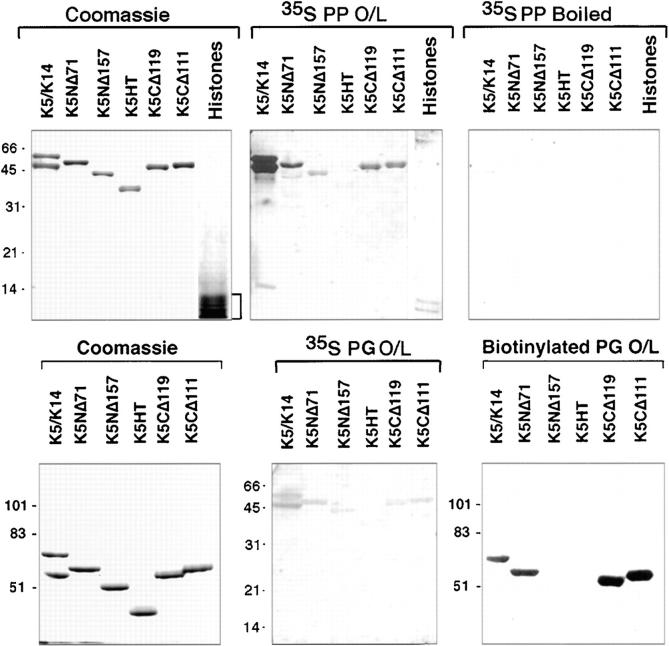
With a 275-mM NaCl wash buffer, both PP1 and PG displayed affinity for epidermal keratins as judged by overlay assays of skin extracts (Figs. 9 and 10, respectively). This interaction was strongest with bands corresponding to the mobilities of type II keratins, although PP1 also shared appreciable association with the type I keratins. To explore these interactions in more detail, we repeated the overlay assays, this time using mutants of K5, the type II keratin most abundantly expressed in the dividing layer of the epidermis (Wilson et al., 1992; Kouklis et al., 1994).
When using equivalent amounts of in vitro–translated S35-labeled PP1 and PG, PP1 showed markedly stronger binding to both type I and type II keratins than did PG, a feature that was also seen in our previous overlay assays using whole skin extracts. To detect associations between PG and recombinant keratins effectively, we had to purify PG using fplc chromatography, and then biotinylate the protein. The biotinylated PG probe enabled us to use higher concentrations of protein, thereby favoring the equilibrium of binding, and it also enabled us to enhance the signal by chemiluminescence. Under these conditions, associations between PG and mutant type II keratins could be examined (Fig. 11).
Figure 11.
Comparison of the interactions among PP1, PG, and keratins. Interactions between keratins and plakophilin/plakoglobin were examined in greater detail using recombinant keratins and overlay assays with equal amounts of in vitro–translated S35-labeled PP1 and S35-labeled PG proteins that we determined to have comparable specific activities. Overlays were also performed with biotinylated recombinant PG. As a control for nonspecific charge interactions, we included a mixture of bovine histones (Sigma Chemical Co., St. Louis, MO) and a control, whereby the probe was boiled before hybridization. Recombinant K5 mutant proteins were as described previously (Wilson et al., 1992; Kouklis et al., 1994). Note that under equivalent conditions, the interactions with keratins were significantly stronger for PP1 than PG, and that the more sensitive biotinylated PG probe was needed to analyze PG-IF interactions. Note also that only those K5 proteins with an intact amino terminal domain hybridized with the armadillo probes.
Both PP1 and PG associated with mutant versions of K5 that contained an intact amino terminal head domain. Whereas S35-labeled PP1 showed some ability to associate with the K5 missing its NH2-terminal 157 residues, PG showed no interaction with this mutant, even when biotinylated. Neither of the two armadillo proteins showed appreciable association with the K5 mutant missing the entire nonhelical K5 head and tail segments.
The K5 head domain is basic (pKi = 12.24), raising the question as to whether PP1 and PG might bind nonspecifically to any highly basic protein. Charge alone did not appear to be sufficient, as judged by the fact that (a) a mixture of histones showed no appreciable interactions on the overlay assays (Fig. 11; PP O/L), and (b) boiled probe obliterated the majority of hybridization (Fig. 11; PP O/L boiled). This said, given the nature of the K5 head segment, it seems likely that charge interactions will be important mechanistically in the interaction.
Discussion
Elucidating the Desmosomal Recognition Site in the Desmoplakin Head Domain
We have confirmed and extended earlier in vivo studies reporting the existence of a desmosomal recognition site within the desmoplakin head domain (Stappenbeck et al., 1993; Bornslaeger et al., 1996; Kowalczyk et al., 1997). We have narrowed the molecular definition of this recognition site to somewhere within the first 86 to 176 amino acids of the 1014 residue DP head, and have demonstrated that it functions in desmosome-rich cells, namely epidermal keratinocytes. While deletion of 29 NH2-terminal residues abrogated the ability of either DP or the DP head domain to target to desmosomes, we could not produce compelling evidence that these 29 residues alone were sufficient for efficient desmosomal recognition.
We have not ruled out the possibility that there might be other desmosomal recognition sites within the >300 kD desmoplakin protein. However, since removing 29 NH2-terminal amino acids from DP abrogates desmosomal recognition, this small deletion must elicit a change sufficient to perturb all the recognition sites within the DP protein. This argument aside, our overlay assays indicate that within this head segment, multiple desmosomal proteins can bind, underscoring its importance for desmosome architecture.
In Vitro, Desmoplakin Can Interact Directly With Desmoplakin (Self-recognition), Desmocollin 1a, and Plakoglobin: Implications for Desmosome Organization
Recently, Chitaev and Troyanovsky (1997) showed convincingly that desmocollins and desmogleins are both essential for desmosomal cadherin clustering and for cell–cell adhesion. The prevailing model for desmosomal structure is that plakoglobins and desmoplakins then sequentially assemble in a linear array onto the cadherin scaffold, forming dangling chain-like structures extending perpendicularly from the plasma membrane and linking to IFs through DP (Kowalczyk et al., 1997). Indeed, our overlay assays (Kouklis et al., 1994; present report) provide evidence in support of each of the interactions postulated in this linear model. This said, our studies reveal additional interactions hitherto unappreciated, including direct binding of DP to Dsc1a, an interaction first postulated from in vivo studies (Troyanovsky et al., 1994). When this complexity is taken into consideration, an alternative model emerges whereby DP and PG form a layer(s) of laterally interconnected proteins anchored beneath the desmosome membrane through linkage to desmosomal cadherins.
The differential preference of PG and DP for desmogleins and desmocollins, respectively, renders to them the capacity to bind noncompetetively to a developing plaque. If plakoglobin's ability to bind to Dsg1 and Dsc1a is not mutually exclusive, PG might serve at least in part as a cadherin-linking protein, stabilizing the tails of the clustered transmembrane proteins within the plaque. A function of this sort for plakoglobin would explain why desmosomes are greatly diminished in plakoglobin-null tissues that do not express plakophilins (Ruiz et al., 1996; Bierkamp et al., 1996). Epidermal desmosomes in PG null mice are significantly less perturbed than desmosomes in other tissues, which might be an indication that plakophilin 1 can compensate for the absence of plakoglobin (Ruiz et al., 1996; Bierkamp et al., 1996). If so, then the differential preferences that we have detected between the desmosomal cadherins and PP1 and PG are not likely to be essential features of desmosome structure and/or regulation.
In contrast to plakoglobin, desmoplakin can self-associate through head–head interactions. Furthermore, desmoplakin is likely to dimerize through coiled-coil interactions within the rod domain (O'Keefe et al., 1989; Green et al., 1992), rendering to each DP dimer the capacity to interact laterally through its two-head domains to form an interconnected sheet of DP beneath the desmosomal membrane. When coupled with the additional finding that DP and PG can interact, a versatile selection of interactions becomes possible, enabling DP and PG to interact laterally to create a molecular filling sandwiched between the membrane and the IF network.
A lateral array of interconnected PG and DP molecules beneath the desmosomal cadherins might provide greater stability to the desmosome than a linear array. Additionally, the lateral model would explain why desmoplakin and IFs preferentially associate with desmosomes, while plakoglobin associates with most if not all cadherins. Both linear and parallel arrangements for DP and PG within the desmosomes are consistent with the studies of Kowalczyk et al. (1997), who found that a chimeric Ecadherin-Dsg1 requires both plakoglobin and desmoplakin to produce desmosome-like structures at the membrane. Indeed, both linear and lateral associations may occur within the plaque.
Differential Behavior of Plakophilin and Plakoglobin: Implications for Desmosome–IF Interactions and for Terminal Differentiation
When taken together with previous data, our in vitro studies suggest myriad molecular routes by which desmosomes can potentially link to IFs. Since PP1 is most abundant in terminally differentiating epidermal cells, these routes are likely to change significantly as epidermal cells withdraw from the cell cycle and commit to differentiate terminally. Based on relative preferences in overlay assays, we surmise that in the basal layer, the links between desmosomes and IFs are strongest through desmoglein–plakoglobin– desmoplakin–IF and desmocollin–desmoplakin–IF interactions. As differentiating cells increase expression of PP1, additional links through desmocollin–plakophilin–desmoplakin–IF and desmocollin–plakophilin–IF could occur.
The differential preference of plakoglobin and plakophilin for desmosomal cadherins might allow newly synthesized plakophilin to associate with the desmosomal plaque without displacing plakoglobin. In this way, plakophilin might act as a molecular cement to solidify links to IFs. These changes may explain at least in part why desmosomes increase in number and stability in the differentiating spinous layers, which receive their name from this notable morphological change. As keratinocytes differentiate, enhancing desmosome–IF interactions may create a less dynamic and more stable structure, a feature that may help keratinocytes to maintain their adhesive properties and to lay down the framework for subsequent cornified envelope formation, as suggested recently by Candi et al. (1998).
An Increased Importance of the Type II Keratin Head Domain
As judged by overlay assays, plakophilin 1, and to a lesser extent plakoglobin, interacted directly with keratin. Intriguingly, these interactions were most potent with the type II keratins, and in particular, the nonhelical head segment of the type II keratin. This domain of keratin had already been found to associate with the cytoplasmic tail segment of desmoplakin both in overlay assays (Kouklis et al., 1994) and in yeast two-hybrid assays (Meng et al., 1997). This domain is also the target for K5 mutations in the rare human genetic skin disorder epidermolysis bullosa simplex with mottled pigmentation, in which the keratin filaments seem deficient in their ability to anchor to desmosomes and to organize melanosomes (Uttam et al., 1996; Irvine et al., 1997). Similarly, a mutation in this domain of K1 has been identified in patients with nonepidermolytic palmoplantar keratoderma, displaying suprabasal cell fragility (Kimonis et al., 1994). Taken together, these findings strengthen the notion that the head domain of the type II epidermal keratins possesses a special importance in interactions with desmosomal components.
Do the associations that we have uncovered between IFs and plakoglobin/plakophilin occur in vivo? A priori, one might argue against this notion, given that plakoglobin has been found in E-cadherin–mediated junctions that associate with actin filaments rather than IFs. However, it is possible that the interactions between plakoglobin and E-cadherin junctions mask the ability of plakoglobin to associate with IFs in a way that does not occur in desmosomal junctions. Moreover, in contrast to plakoglobin, plakophilin 1 is less promiscuous, associating exclusively with desmosomes. Our finding that plakophilin 1 binds better than plakoglobin to keratin IFs is consistent with this behavior.
Perhaps the most compelling evidence in support of an in vivo interaction between plakophilin 1 and keratin IFs is the recent revelation that a form of congenital ectodermal dysplasia has been associated with null mutations in both alleles of plakophilin 1 (McGrath et al., 1997). This disorder is typified by a dramatic loss of cell–cell adhesion and a reduction in desmosomes within the terminally differentiating cells of the epidermis (Fitzpatrick et al., 1993). McGrath et al. (1997) noted that in the skin of these patients, the association between keratin IFs and desmosomes was perturbed in the upper layers, leaving the keratin IFs compacted in a perinuclear distribution. Even more intriguing is the finding that plakoglobin, desmogleins, and desmocollins were still localized to the cell–cell borders in the suprabasal keratinocytes, but desmoplakin localization was partially perturbed. When taken together, our in vitro overlay assays, showing direct interaction between plakophilin and keratin IFs, provide a molecular understanding for the defects in the keratin cytoskeleton and desmosomal connections observed in the terminally differentiating epidermal cells of congenital ectodermal dysplasia patients.
Our data provide a number of missing links in our understanding of the associations between IFs and desmosomes. By using radiolabeled proteins produced by in vitro transcription/translation, and by engineering desmoplakin fusion proteins, we have circumvented the problems of protein insolubility that we and others have encountered previously with a number of the desmosomal components. We do not yet know whether all of the direct interactions that we have uncovered between desmosomal components occur within the desmosome, or alternatively whether some may reflect intermediate interactions in the assembly process. However, our findings indicate that desmosome assembly is complex, and is not a simple linear sequence of events. The in vitro interactions that we observed were specific, and were consistent with previous in vivo studies exploring a subset of the associations that we have described here. While crystallography will be necessary to provide a precise solution to the nature of these various interactions, identifying the interacting domains and producing soluble binding segments for some of these components now begins to pave the way for this approach in the future.
Acknowledgments
We thank Dr. Mary Beth McCormick (Department of Pharmacology, University of Wisconsin, Madison, WI) and Dr. Panos Kouklis for their initial screening and partial characterization of human desmoplakin cDNA clones while in this laboratory. A special thank you goes to Dr. Panos Kouklis for his generous and expert advice in the various biochemical aspects of this work, and to Dr. Elizabeth Allen and Elizabeth M. Hutton for their suggestions and discussions. We also thank Paul Gardner for his technical assistance in the automated sequencing. Finally, we thank Chuck Wellek for his kind help in preparing the computer-assisted color artwork for this manuscript.
Abbreviations used in this paper
- aa
amino acids
- BPAGIe
epidermal bullous pemphigoid antigen 1 protein
- DPH
desmoplakin
- DP
desmoplakin
- Dscs
desmocollins
- Dsgs
desmogleins
- IFs
intermediate filaments
- PG
plakoglobin
- PP1
plakophilin 1
Footnotes
E.F. is an Investigator of the Howard Hughes Medical Institute.
References
- Barth AI, Nathke IS, Nelson WJ. Cadherins, catenins and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr Opin Cell Biol. 1997;9:683–690. doi: 10.1016/s0955-0674(97)80122-6. [DOI] [PubMed] [Google Scholar]
- Bierkamp C, McLaughlin KJ, Schwarz H, Huber O, Kemler R. Embryonic heart and skin defects in mice lacking plakoglobin. Dev Biol. 1996;180:780–785. doi: 10.1006/dbio.1996.0346. [DOI] [PubMed] [Google Scholar]
- Bornslaeger EA, Corcoran CM, Stappenbeck TS, Green KJ. Breaking the connection: displacement of the desmosomal plaque protein desmoplakin from cell-cell interfaces disrupts anchorage of intermediate filament bundles and alters intercellular junction assembly. J Cell Biol. 1996;134:985–1001. doi: 10.1083/jcb.134.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton RS, Cowin P, Franke WW, Garrod DR, Green KJ, King IA, Koch PJ, Magee AI, Rees DA, Stanley JR, Steinberg MS. Nomenclature of the desmosomal cadherins. J Cell Biol. 1993;121:481–483. doi: 10.1083/jcb.121.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candi E, Tarcsa E, Digiovanna JJ, Compton JG, Elias PM, Marekov LN, Steinert PM. A highly conserved lysine residue on the head domain of type II keratins is essential for the attachment of keratin intermediate filaments to the cornified cell envelope through isopeptide crosslinking by transglutaminases. Proc Natl Acad Sci USA. 1998;95:2067–2072. doi: 10.1073/pnas.95.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitaev NA, Leube RE, Troyanovsky RB, Eshkind LG, Franke WW, Troyanovsky SM. The binding of plakoglobin to desmosomal cadherins: patterns of binding sites and topogenic potential. J Cell Biol. 1996;133:359–369. doi: 10.1083/jcb.133.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitaev NA, Troyanovsky SM. Direct Ca2+-dependent heterophilic interaction between desmosomal cadherins, desmoglein and desmocollin, contributes to cell-cell adhesion. J Cell Biol. 1997;138:193–201. doi: 10.1083/jcb.138.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowin, P., H.-P. Kapprell, W.W. Franke, J. Tamkun, and R.O. Hynes. 1986. Plakoglobin: a protein common to different kinds of intercellular adhering junctions. Cell. 1063–1073. [DOI] [PubMed]
- Fitzpatrick, T.B., A.Z. Eisen, K. Wolff, I.M. Freedberg, and K.F. Austen. 1993. Dermatology in general medicine. 4th ed. Vol. I and II. McGraw-Hill, Inc., New York.
- Green KJ, Goldman RD, Chisholm RL. Isolation of cDNAs encoding desmosomal plaque proteins: evidence that bovine desmoplakins I and II are derived from two mRNAs and a single gene. Proc Natl Acad SciUSA. 1988;85:2613–2617. doi: 10.1073/pnas.85.8.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KJ, Parry DAD, Steinert PM, Virata MLA, Wagner RM, Angst BD, Nilles LA. Structure of the human desmoplakins: implications for function in the desmosomal plaque. J Biol Chem. 1990;265:2603–2612. [PubMed] [Google Scholar]
- Green KJ, Virata ML, Elgart GW, Stanley JR, Parry DA. Comparative structural analysis of desmoplakin, bullous pemphigoid antigen and plectin: members of a new gene family involved in organization of intermediate filaments. Int J Biol Macromol. 1992;14:145–153. doi: 10.1016/s0141-8130(05)80004-2. [DOI] [PubMed] [Google Scholar]
- Green KJ, Jones JC. Desmosomes and hemidesmosomes: structure and function of molecular components. FASEB J. 1996;10:871–881. doi: 10.1096/fasebj.10.8.8666164. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Hatzfeld M, Kristjansson GI, Plessman U, Weber K. Band 6 protein, a major constituent of desmosomes from stratified epithelia, is a novel member of the armadillo multigene family. J Cell Sci. 1994;107:2259–2270. doi: 10.1242/jcs.107.8.2259. [DOI] [PubMed] [Google Scholar]
- Heid HW, Schmidt A, Zimbelmann R, Schafer S, Winter-Simanowski S, Stumpp S, Keith M, Figge U, Schnolzer M, Franke WW. Cell type-specific desmosomal plaque proteins of the plakoglobin family:plakophilin 1 (band 6 protein) Differentiation. 1994;58:113–131. doi: 10.1046/j.1432-0436.1995.5820113.x. [DOI] [PubMed] [Google Scholar]
- Irvine AD, McKenna KE, Jenkinson H, Hughes AE. A mutation in the V1 domain of keratin 5 causes epidermolysis bullosa simplex with mottled pigmentation. J Investig Dermatol. 1997;108:809–810. doi: 10.1111/1523-1747.ep12292263. [DOI] [PubMed] [Google Scholar]
- Jones JC, Grelling KA. Distribution of desmoplakin in normal cultured human keratinocytes and in basal cell carcinoma cells. Cell Motil Cytoskeleton. 1989;13:181–194. doi: 10.1002/cm.970130306. [DOI] [PubMed] [Google Scholar]
- Kapprell HP, Owaribe K, Franke WW. Identification of a basic protein of Mr 75,000 as an accessory desmosomal plaque protein in stratified and complex epithelia. J Cell Biol. 1988;106:1679–1691. doi: 10.1083/jcb.106.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DE. Fine structure of desmosomes, hemidesmosomes, and an epidermal globular layer in developing newt epidermis. J Cell Biol. 1966;28:51–72. doi: 10.1083/jcb.28.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimonis V, DiGiovanna JJ, Yang JM, Doyle SZ, Bale SJ, Compton JG. A mutation in the V1 end domain of keratin 1 in non-epidermolytic palmar-plantar keratoderma. J Investig Dermatol. 1994;103:764–769. doi: 10.1111/1523-1747.ep12412771. [DOI] [PubMed] [Google Scholar]
- King IA, Angst BD, Hunt DM, Kruger M, Arnemann J, Buxton RS. Hierarchical expression of desmosomal cadherins during stratified epithelial morphogenesis in the mouse. Differentiation. 1997;62:83–96. doi: 10.1046/j.1432-0436.1997.6220083.x. [DOI] [PubMed] [Google Scholar]
- Koch PJ, Walsh MJ, Schmelz M, Goldschmidt MD, Zimbelmann R, Franke WW. Identification of desmoglein, a constitutive desmosomal glycoprotein, as a member of the cadherin family of cell adhesion molecules. Eur J Cell Biol. 1990;53:1–12. [PubMed] [Google Scholar]
- Koch PJ, Goldschmidt MD, Zimblemann R, Troyanovsky R, Franke WW. Complexity of expression patterns of the desmosomal cadherins. Proc Natl Acad Sci USA. 1992;89:353–357. doi: 10.1073/pnas.89.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch PJ, Franke WW. Desmosomal cadherins: another growing multigene family of adhesion molecules. Curr Opin Cell Biol. 1994;6:682–687. doi: 10.1016/0955-0674(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Kouklis P, Hutton E, Fuchs E. Making the connection: keratin intermediate filaments and desmosomes proteins. J Cell Biol. 1994;127:1049–1060. doi: 10.1083/jcb.127.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk AP, Bornslaeger EA, Borgwardt JE, Palka HL, Dhaliwal AS, Corcoran CM, Denning MF, Green KJ. The amino-terminal domain of desmoplakin binds to plakoglobin and clusters desmosomal cadherin-plakoglobin complexes. J Cell Biol. 1997;139:773–784. doi: 10.1083/jcb.139.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Recognition of AUG and alternative initiator codons is augmented by G in position +4 but is not generally affected by the nucelotides in position +5 and +6. EMBO (Eur Mol Biol Organ) J. 1997;16:2482–2492. doi: 10.1093/emboj/16.9.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lersch R, Stellmach V, Stocks C, Giudice G, Fuchs E. Isolation, sequence and expression of a human keratin K5 gene: transcriptional regulation of keratins and insights into pair-wise control. Mol Cell Biol. 1989;9:3155–3168. doi: 10.1128/mcb.9.9.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs JA, Nelson WJ. Cadherin cell adhesion molecules in differentiation and embryogenesis. Int Rev Cytol. 1996;165:159–205. doi: 10.1016/s0074-7696(08)62222-6. [DOI] [PubMed] [Google Scholar]
- Mathur M, Goodwin L, Cowin P. Interactions of the cytoplasmic domain of the desmosomal cadherin Dsg1 with plakoglobin. J Biol Chem. 1994;269:14075–14080. [PubMed] [Google Scholar]
- McGrath JA, McMillan JR, Shemanko CS, Runswick SK, Leigh IM, Lane EB, Garrod DR, Eady RAJ. Mutations in the plakophilin 1 gene result in ectodermal dysplasia/skin fragility syndrome. Nat Genet. 1997;17:240–244. doi: 10.1038/ng1097-240. [DOI] [PubMed] [Google Scholar]
- Meng JJ, Bornslaeger EA, Green KJ, Steinert PM, Ip W. Two-hybrid analysis reveals fundamental differences in direct interactions between desmoplakin and cell type-specific intermediate filaments. J Biol Chem. 1997;272:21495–21503. doi: 10.1074/jbc.272.34.21495. [DOI] [PubMed] [Google Scholar]
- Merdes A, Brunkener M, Horstmann H, Geortgatos SD. Filensin: a new vimentin-binding, polymerization-competent, and membrane-associated protein of the lens fiber cell. J Cell Biol. 1991;115:397–410. doi: 10.1083/jcb.115.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens C, Kuhn C, Franke WW. Plakophilin 2a and 2b: constitutive proteins of dual location in the karyoplasm and the desmosomal plaque. J Cell Biol. 1996;135:1009–1025. doi: 10.1083/jcb.135.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K, Mattey D, Measures H, Hopkins C, Garrod D. Localisation of the protein and glycoprotein components of bovine nasal epithelial desmosomes by immunoelectron microscopy. EMBO (Eur Mol Biol Organ) J. 1987;6:885–889. doi: 10.1002/j.1460-2075.1987.tb04834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll I, Kurzen H, Langbein L, Franke WW. The distribution of the desmosomal protein, plakophilin 1, in human skin and skin tumors. J Investig Dermatol. 1997;108:139–146. doi: 10.1111/1523-1747.ep12332388. [DOI] [PubMed] [Google Scholar]
- Mueller H, Franke WW. Biochemical and immunological characterization of desmoplakins I and II, the major polypeptides of the desmosomal plaque. J Mol Biol. 1983;163:647–671. doi: 10.1016/0022-2836(83)90116-x. [DOI] [PubMed] [Google Scholar]
- O'Keefe EJ, Erickson HP, Bennett V. Desmoplakin I and desmoplakin II: purification and characterization. J Biol Chem. 1989;264:8310–8318. [PubMed] [Google Scholar]
- Pasdar M, Krzeminski KA, Nelson WJ. Regulation of desmosome assembly in MDCK epithelial cells: coordination of membrane core and cytoplasmic plaque domain assembly at the plasma membrane. J Cell Biol. 1991;113:645–655. doi: 10.1083/jcb.113.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasdar M, Nelson WJ. Kinetics of desmosome assembly in Madin-Darby canine kidney epithelial cells: temporal and spatial regulation of desmoplakin organization and stabilization upon cell-cell contact. II. Morphological analysis. J Cell Biol. 1988;106:687–695. doi: 10.1083/jcb.106.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh JY, Stanley JR. Plakoglobin binding by human Dsg3 (pemphigus vulgaris antigen) in keratinocytes requires the cadherin-like intracytoplasmic segment. J Investig Dermatol. 1995;104:720–724. doi: 10.1111/1523-1747.ep12606963. [DOI] [PubMed] [Google Scholar]
- Ruiz P, Brinkmann V, Ledermann B, Behrend M, Grund C, Thalhammer C, Vogel F, Birchmeier C, Gunthert U, Franke WW, Birchmeier W. Targeted mutation of plakoglobin in mice reveals essential functions of desmosomes in the embryonic heart. J Cell Biol. 1996;135:215–225. doi: 10.1083/jcb.135.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Heid HW, Schafer S, Nuber UA, Zimbelmann R, Franke WW. Desmosomes and cytoskeletal architecture in epithelial differentiation: cell type-specific plaque components and intermediate filament anchorage. Eur J Cell Biol. 1994;65:229–245. [PubMed] [Google Scholar]
- Stappenbeck TS, Bornslaeger EA, Corcoran CM, Luu HH, Virata ML, Green KJ. Functional analysis of desmoplakin domains: specification of the interaction with keratin versus vimentin intermediate filament networks. J Cell Biol. 1993;123:691–705. doi: 10.1083/jcb.123.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stappenbeck TS, Green KJ. The desmoplakin carboxyl terminus coaligns with and specifically disrupts intermediate filament networks when expressed in cultured cells. J Cell Biol. 1992;116:1197–1209. doi: 10.1083/jcb.116.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier FW, Moffatt BA. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Troyanovsky SM, Eshkind LG, Troyanovsky RB, Leube RE, Franke WW. Contributions of cytoplasmic domains of desmosomal cadherins to desmosome assembly and intermediate filament anchorage. Cell. 1993;72:561–574. doi: 10.1016/0092-8674(93)90075-2. [DOI] [PubMed] [Google Scholar]
- Troyanovsky SM, Troyanovsky RB, Eshkind LG, Leube RE, Franke WW. Identification of amino acid sequence motifs in desmocollin, a desmosomal glycoprotein, that are required for plakoglobin binding and plaque formation. Proc Natl Acad Sci USA. 1994;91:10790–10794. doi: 10.1073/pnas.91.23.10790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyanovsky SM, Troyanovsky RB, Eshkind LG, Krutovskikh VA, Leube RE, Franke WW. Identification of the plakoglobin-binding domain in desmoglein and its role in plaque assembly and intermediate filament anchorage. J Cell Biol. 1994;127:151–160. doi: 10.1083/jcb.127.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyanovsky RB, Chitaev NA, Troyanovsky SM. Cadherin binding sites of plakoglobin:localization, specificity and role in targeting to adhering junctions. J Cell Sci. 1996;109:3069–3078. doi: 10.1242/jcs.109.13.3069. [DOI] [PubMed] [Google Scholar]
- Uttam J, Hutton E, Coulombe P, Anton-Lamprecht I, Yu Q-C, Gedde-Dahl T, Fine J-D, Fuchs E. The genetic basis of epidermolysis bullosa simplex with mottled pigmentation. Proc Natl Acad Sci USA. 1996;93:9079–9084. doi: 10.1073/pnas.93.17.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virata ML, Wagner RM, Parry DA, Green KJ. Molecular structure of the human desmoplakin I and II amino terminus. Proc Natl Acad Sci USA. 1992;89:544–548. doi: 10.1073/pnas.89.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl JK, Sacco PA, McGranahan-Sadler TM, Sauppe LM, Wheelock MJ, Johnson KR. Plakoglobin domains that define its association with the desmosomal cadherins and the classical cadherins:identification of unique and shared domains. J Cell Sci. 1996;109:1143–1154. doi: 10.1242/jcs.109.5.1143. [DOI] [PubMed] [Google Scholar]
- Wilson AK, Coulombe PA, Fuchs E. The roles of the head, tail and R/KLLEGE domains in keratin filament assembly in vitro. J Cell Biol. 1992;119:401–414. doi: 10.1083/jcb.119.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witcher LL, Collins R, Puttagunta S, Mechanic SE, Munson M, Gumbiner B, Cowin P. Desmosomal cadherin binding domains of plakoglobin. J Biol Chem. 1996;271:10904–10909. doi: 10.1074/jbc.271.18.10904. [DOI] [PubMed] [Google Scholar]