Abstract
To determine whether junctional communication between pancreatic acinar cells contributes to their secretory function in vivo, we have compared wild-type mice, which express the gap junctional proteins connexin32 (Cx32) and connexin26, to mice deficient for the Cx32 gene. Pancreatic acinar cells from Cx32 (−/−) mice failed to express Cx32 as evidenced by reverse transcription–PCR and immunolabeling and showed a marked reduction (4.8- and 25-fold, respectively) in the number and size of gap junctions. Dye transfer studies showed that the extent of intercellular communication was inhibited in Cx32 (−/−) acini. However, electrical coupling was detected by dual patch clamp recording in Cx32 (−/−) acinar cell pairs. Although wild-type and Cx32 (−/−) acini were similarly stimulated to release amylase by carbamylcholine, Cx32 (−/−) acini showed a twofold increase of their basal secretion. This effect was caused by an increase in the proportion of secreting acini, as detected with a reverse hemolytic plaque assay. Blood measurements further revealed that Cx32 (−/−) mice had elevated basal levels of circulating amylase. The results, which demonstrate an inverse relationship between the extent of acinar cell coupling and basal amylase secretion in vivo, support the view that the physiological recruitment of secretory acinar cells is regulated by gap junction mediated intercellular communication.
Pancreatic acinar cells are a valuable model to study the signal transduction mechanisms that regulate secretion of ions and digestive enzymes. It is well established that many hormones and neurotransmitters, such as acetylcholine, cholecystokinine, and bombesin, bind to Gq-linked membrane receptors, resulting in the production of diacylglycerol and inositol 1,4,5-trisphosphate (IP3). The activation of protein kinase C by diacylglycerol and the release of Ca2+ from intracellular stores by IP3 are key events in the regulation of pancreatic secretion (Nishizuka, 1986; Yule and Williams, 1994; Berridge, 1997), which is produced by clusters of acinar cells that individually show morphological and biochemical differences (Hellman et al., 1962; Bendayan, 1985). This heterogeneity and the observation that different stimuli evoke the release of various amounts of pancreatic enzymes (Rothman, 1976; Beaudoin et al., 1983), suggest that secretion of the exocrine pancreas may not be similarly contributed by each subunit of the gland. In keeping with this idea, a substantial heterogeneity has been reported in the response to secretagogues of individual pancreatic acini and acinar cells (Bosco et al., 1988, 1994; Willems et al., 1993).
Previous studies have also documented that dispersion of pancreatic acinar cells results in loss of secretion (Amsterdam and Jamieson, 1974; Gardner and Jackson, 1977). The finding that re-establishment of cell–cell contacts promotes basal and stimulated secretion indicates that some mechanism involving cell–cell interactions participates in the regulation of the exocrine function of the pancreas (Bosco et al., 1994). In this respect, direct cell-to-cell communication via gap junctions has received most attention. Gap junctions are assemblies of transmembrane channels that provide a selective pathway for the transfer of ions and signaling molecules between cells in contact. These channels are formed by a multigene family of related proteins, referred to as connexins (Cx).1 Acinar cells are extensively coupled through Cx32- and Cx26-formed gap junction channels (Meda et al., 1988). It is assumed that gap junctional communication is important for the integration of signaling among cells. Consistent with this view, in vitro studies have reported that diffusion through gap junction channels of Ca2+ or Ca2+-mobilizing molecules may coordinate the oscillations of the second messenger between cells of individual acini, thereby promoting the recruitment of acinar cells to secrete (Nathanson et al., 1992; Ngezahayo and Kolb, 1993; Loessberg Stauffer et al., 1993; Bosco et al., 1994; Yule et al., 1996). In apparent contradiction with these reports, cell-to-cell communication has also been repeatedly shown to decrease during maximal stimulation of acinar cells by Ca2+-mobilizing secretagogues (Petersen and Ueda, 1976; Iwatsuki and Petersen, 1978; Petersen and Iwatsuki, 1979; Chanson et al., 1991). That acute closure of gap junction channels modulates enzyme secretion was further strengthened by the observation that acinar cell uncouplers increase the basal release of amylase without affecting the maximally stimulated secretion of dispersed acini (Meda et al., 1986, 1987; Chanson et al., 1989; Loessberg Stauffer et al., 1993). It has not been clarified, however, whether a chronic alteration in the extent of cell-to-cell communication could affect the secretory activity of acinar cells in vivo. Moreover, the mechanism whereby cell uncoupling affects secretion also remains undetermined.
To address these questions, we have used the homozygous Cx32 deficient mice (Cx32 [−/−]) that have been recently generated by gene targeting (Nelles et al., 1996). We have first characterized gap junctions and cell-to-cell communication in wild-type (C57BL/6 as well as C57BL/6 × 129 SV-F1) and Cx32-deficient pancreatic acinar cells. Second, we have studied the release of amylase under basal conditions and after stimulation by carbamylcholine (CCh). Third, we have measured circulating levels of amylase. The results indicate that lack of Cx32 protein alters the in vivo function of the exocrine pancreas and suggest that the extensive coupling normally observed between acinar cells mediates an inhibitory effect that downregulates the number of acinar cells recruited for amylase secretion. Once this inhibition is relieved by partial loss of gap junctional communication, the recruitment of secreting acinar cells is enhanced.
Materials and Methods
Animals
Mice deficient in Cx32 have been generated by targeted homologous recombination (Nelles et al., 1996). Due to the breeding protocol chosen, these animals exhibit a genetic background mixing features of the C57BL/6 and 129 SV strains (Temme et al., 1997). Mice heterozygous for the Cx32 nil transgene were crossed with C57BL/6 mice to expand the population of Cx32-deficient animals. Thus, the Cx32 (−/−) mice used in this study have a background closer to that of C57BL/6 mice than to the original C57BL/6 × 129 SV-F1 animals. To assess whatever these differences in genetic background impact on the coupling and secretion characteristics, we have used as appropriate wild-type controls C57BL/6 mice as well as the F1 generation of C57BL/6 × 129 SV mice. In some experiments, female Cx32-deficient animals were also used as controls. Due to the phenomenon of X chromosome inactivation, and the localization of Cx32 on this chromosome, these mice express a Cx32 (+/−) genotype that would be present in all wild-type animals as well. Accordingly, they showed coupling and secretory characteristics similar to those of wild-type mice.
Acini and Cell Preparations
Dispersed acini were prepared by collagenase digestion of pancreata from Cx32 (−/−) and wild-type mice, according to a method previously described (Bruzzone et al., 1985). Cell pairs were prepared by resuspending the intact acini in a Ca2+- and Mg2+-free Ringer-bicarbonate medium, buffered to pH 7.4 with 12.5 mM Hepes, and containing 3 mM EGTA. The resulting cell suspension was passed repeatedly through a 18-gauge needle and centrifuged 3 min at 100 g in a Ringer-bicarbonate containing Ca2+ and Mg2+, and supplemented with 4% BSA (KRB-BSA). Samples of acini and of cell clusters were rinsed in KRB-BSA by centrifugation and plated in 10 × 35-mm plastic dishes that had been previously coated with 0.5 mg/ml poly-l-lysine (mol wt 150,000–300,000; Sigma Chemical Co., St. Louis, MO) in distilled water. Acini and cells were allowed to attach for 20–30 min at room temperature before the experiments.
RNA Isolation and Reverse Transcription–PCR Amplification
Total cellular RNA was isolated from pancreas of wild-type and Cx32 (−/−) mice that had been frozen in liquid nitrogen, using the guanidinium isothiocyanate/acid phenol extraction method (Vozzi et al., 1995). Liver RNA to be used as positive control was similarly isolated from C57BL/6 mice. To enrich for the mRNA fraction, total RNA was passed through an oligodT column (Quiagen AG, Basel, Switzerland, or Pharmacia Biotech, Dübendorf, Switzerland), according to the manufacturer's instructions. Reverse transcription (RT) was carried out using random hexamers and the resulting cDNA was amplified by PCR using primer pairs specific for Cx26 (sense 5′-GTCCACTGAGCGCAGCCTCCA-3′ and antisense 5′-CTGCAGAGCCCAGAGCCGGAT-3′; the predicted size of the amplified fragments was 435 bp) and for Cx32 (sense 5′-AGTGCCAGGGAGGTGTGAAT-3′ or 5′-GGACAGGTCTATACACCTTG-3′ and antisense 5′-GGAACACCACACTGATGACA-3′; the predicted sizes of the amplified fragments were 452 or 416 bp, respectively). After a 5-min start at 94°C, amplification was carried out for 31 cycles, each composed of 1 min at 94°C, 1 min at 65°C, and 1 min at 72°C, using an UNOII PCR cycler (Biometra GmBH, Göttingen, Germany). After the last cycle, an elongation step of 5 min was performed at 72°C. Amplified DNA fragments were separated in a 2% agarose gel and visualized after ethidium bromide staining. No products were amplified in the absence of reverse transcriptase (not shown).
Immunofluorescence
For immunofluorescence labeling, small pancreatic fragments from wild-type and Cx32 (−/−) mice were rapidly frozen by immersion in 2-methylbutane cooled with liquid nitrogen. Sections were then cut on a cryostat (Leica Instruments, Nussloch, Germany), collected on silane-coated slides and fixed 3 min in cold (−20°C) acetone. Immunofluorescence analysis was performed according to standard protocols (Meda et al., 1993). Briefly, sections were incubated 2 h at room temperature with affinity- purified rabbit or rat sera against liver Cx32 (Dermiezel et al., 1984; Stevensen et al., 1986) and Cx26 (Traub et al., 1989). Antibodies were used at dilutions ranging from 1:400–1:1,000 with the exception of the R5-21C mAb against liver Cx32 that was used undiluted from culture supernatants. The second incubation was carried out for 1 h using fluoresceinated anti–rabbit or anti–rat antibodies, which ever applicable, diluted 1:400. After rinsing, sections were covered with 0.025% paraphenylene-diamine in PBS-glycerol (1:2, vol/vol) and photographed on an Axiophot microscope (Carl Zeiss, Inc., Oberkochen, Germany) equipped with filters for fluorescein detection. Negative and positive controls of the immunolabeling were carried out on tissues of wild-type animals, as previously described (Meda et al., 1993).
Electron Microscopy
Wild-type and Cx32 (−/−) pancreata were fixed 60 min at room temperature in 2.5% glutaraldehyde prepared in a 0.1 M phosphate buffer (pH 7.4). For freeze-fracture, samples were infiltrated for 60 min in 30% phosphate-buffered glycerol and frozen in Freon 22 that had been cooled with liquid nitrogen. The pellets were fractured and shadowed in a Balzers BAF 301 apparatus (High Vacuum Corp., Balzers, Lichtenstein). The replicas were washed in a sodium hypochlorite solution, rinsed in distilled water, mounted on Formvar and carbon-coated copper grids and examined in a Philips EM 301 (Philips Electron Optics, Mahwah, NJ) electron microscope. For conventional electron microscopy, the glutaraldehyde-fixed samples were postfixed in 2% phosphate-buffered osmium tetroxide, dehydrated in graded ethanols, and then embedded in Epon. Semithin sections were examined under phase-contrast illumination using an Axiophot microscope (Carl Zeiss, Inc.). Thin sections were examined in a Philips EM 301 electron microscope.
Gap Junction Analysis
The area and numerical density of gap junctions was evaluated in the membranes of acinar cells of Cx32 (−/−) and wild-type mice. To this end, randomly selected gap junctions were photographed on freeze-fracture replicas at the fixed magnification of ×23,000, and the area of each junction was measured on positive prints enlarged at the final magnification of ×69,000, using a graphic tablet (Tektronix, Inc., Beaverton, OR) connected to a personal computer. The numerical density of gap junctions was evaluated by counting the junctional plaques on fractured membranes (E and P faces), whose areas were measured as outlined above. All data were expressed as mean ± SEM and compared using an unpaired t test.
Junctional Communication
For dye transfer studies, dishes with attached acini were transferred to the stage of an inverted ICM405 Zeiss microscope and kept at 37°C. To assess coupling, one cell was impaled within each acinus with a high resistance microelectrode filled with a 4% Lucifer yellow CH (Sigma Chemical Co.) solution in 150 mM LiCl, buffered to pH 7.2 with 10 mM Hepes. The tracer was iontophoretically injected for 3 min by passing 0.1 nA negative square pulses of 900 ms duration and 0.5-Hz frequency, according to Meda et al. (1986). At the end of the injection period, acini were photographed under fluorescence and phase-contrast illuminations. To quantitate the extent of dye coupling, color slides of the microinjected acini were projected on a graphic tablet connected to a personal computer to measure the area of the acinus profile and that of the Lucifer yellow–labeled cells. The extent of cell coupling was evaluated by the area labeled by Lucifer yellow as percentage of the acinus area. Dye injections were performed both in a control KRB-BSA medium and after a 5–30-min exposure of the acini to 3.5 mM heptanol or to concentrations of CCh ranging from 10 nM to 10 mM. All data were expressed as mean ± SEM and compared using an unpaired t test.
For electrical coupling studies, dishes with attached acinar cell pairs were transferred to the stage of an inverted microscope (TMD300; Nikon AG, Küsnacht, Switzerland). Throughout the experiments, cells were continuously superfused with a solution containing 136 mM NaCl, 4 mM KCl, 1 mM CaCl2, 1 mM MgCl2, and 2.5 mM glucose, which was buffered to pH 7.4 with 10 mM Hepes. In some cases, this solution was supplemented with 2 mM halothane as indicated in the text. Gap junctional coupling was evaluated using patch electrodes and a dual whole cell voltage-clamp method (Neyton and Trautmann, 1985). Patch electrodes were filled with 139 mM KCl, 1 mM NaCl, 2 mM MgCl2, 0.5 mM EGTA, and 10 mM Hepes, pH 7.2, and had resistances of 3–8 MΩ. The two cells of each pair were voltage clamped at a common holding potential of 0 mV. To measure gap junctional currents (Ij), transjunctional potential differences (Vj) were elicited by changing the holding potential of one of the cells of a pair. Ij was defined as the current recorded in the cell kept at a 0 mV. Gap junctional conductance (gj) was then calculated according to the equation gj = Ij/Vj. In pairs in which gj was reduced by halothane (Burt and Spray, 1991), gating of unitary gap junction channels could be detected. Current flowing through these channels were discriminated as step-like changes of opposite polarities but identical amplitudes that were recorded simultaneously in the current traces of the two cells. All current and voltage signals were acquired at 2 kHz sampling rate using the Pulse software (Heka Elektronik, Lambrecht, Germany), and stored on the hard disk of a Power Macintosh computer (Apple Computer Co., Cupertino, CA). Digitized current traces were filtered at 0.1–2 kHz for analysis and display of Ij traces was made using the IGOR software (WaveMetrics Inc., Lake Oswego, OR).
Amylase Secretion
Secretion of amylase was evaluated using both a biochemical assay and a reverse hemolytic plaque assay. For the biochemical assay, 100 mg (wet weight) of dispersed acini were preincubated in 10 ml of KRB-BSA at room temperature. After 15 min, the acini were allowed to sediment, the supernatant was removed and replaced with fresh KRB-BSA for another 15 min. Aliquots of this acinar suspension were placed in glass vials and incubated 30 min at 37°C with the appropriate test medium, as indicated in the text. Samples were then transferred to Eppendorf tubes and centrifuged 5 min at 500 g. Amylase activity was measured using the Phadebas amylase test (Pharmacia Diagnostics, Zürich, Switzerland) in both pellets and supernatants and the amount of amylase released was expressed as percentage of the total amylase present in acini before the agonist stimulation (Willems et al., 1993). Pancreatic amylase activities were normalized to total protein content, as determined by a Bio-Rad protein assay (BIO RAD Laboratories GmBH, München, Germany). All data were expressed as mean ± SEM and compared using an unpaired t test.
For the reverse hemolytic plaque assay, dispersed acini were mixed with 4% (vol/vol) packed sheep red blood cells that had been coated with protein A and placed into Cunningham's chambers coated with poly- l-lysine, as previously described (Bosco et al., 1988). After a 45-min incubation period to allow for cell attachment, the chambers were rinsed and incubated three times for 5 min at 37°C before starting the secretion test. To this end, acini were incubated 30 min at 37°C in KRB-BSA supplemented with an amylase antiserum (Sigma Chemical Co.) diluted 1:2. The chambers were then rinsed and incubated 60 min at 37°C with guinea pig complement (Behring Institut, Marburg, Germany). At the end of this incubation period, the chambers were further incubated 5 min with 0.02% trypan blue, rinsed, and then fixed in a 1% solution of glutaraldehyde in 0.1 M phosphate buffer. The chambers were first screened to determine the proportion of plaque-forming acini. To this end, about 100 acini were evaluated per condition in each experiment. The amount of amylase secreted by the acini was then evaluated by measuring the area of the hemolytic plaque formed around each acinus. The amylase output of an entire acinus population was given by total plaque development, which was calculated by multiplying the proportion of secreting acini by the average area of their hemolytic plaques (Bosco et al., 1988, 1994). Data from three separate experiments were compared using a paired t test.
Circulating Amylase Levels
For in vivo measurement of amylase activity, blood samples from wild-type and Cx32 (−/−) mice that were weight and age matched were collected in heparinized tubes and centrifuged at 4°C to prepare plasma. Amylase activity was determined in each plasma sample using the Phadebas test and normalized to the total protein concentration, as described above. All data were expressed as mean ± SEM and compared using an unpaired t test.
Results
Pancreatic Connexins and Gap Junctions
The pancreas of Cx32 (−/−) mice appeared organized as that of wild-type animals (Fig. 1). The density of islets of Langerhans (representing 1.14 ± 0.4% of pancreatic tissue) and of secretory ducts (0.39 ± 0.07%) measured in three Cx32 (−/−) pancreata was not different from that observed in wild type. However, acini composed of larger acinar cells were detected in some Cx32 (−/−) pancreata (Fig. 1), in which they represented 0.71–12.4% of the pancreatic tissue. These cells were 1.5 times larger (388 ± 6.3 mm2; n = 614) than normal acinar cells (253.3 ± 4.2 mm2; n = 726) and were characterized by the presence of numerous zymogen granules.
Figure 1.
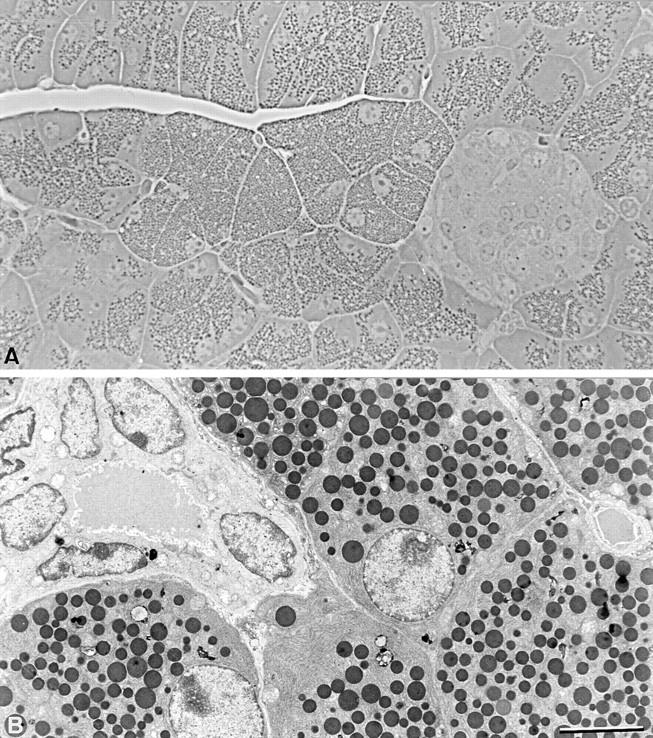
Organization of Cx32 (−/−) pancreas. (A) Semithin sections of Cx32 (−/−) pancreas. Cx32 (−/−) pancreas shows a typical organization of exocrine and endocrine tissue. Note that large acinar cells containing numerous zymogen granules are observed. (B) Electron microscope view of Cx32 (−/−) pancreas showing the characteristic ultrastructure of fully differentiated acinar and duct cells. Bar: (A) 40 μm; (B) 8 μm.
As compared with what was observed in wild-type mice, Cx32 mRNA and proteins were not detected in the pancreas of Cx32 (−/−) mice (Fig. 2, A, B, and C). The expression of Cx26 protein was also strongly decreased in Cx32 (−/−) pancreas (Fig. 2, D and E), although Cx26 mRNA was detected by RT-PCR (Fig. 2 A).
Figure 2.
Expression of Cx32 in wild-type and Cx32 (−/−) pancreatic acinar cells. (A) RT-PCR of mRNA isolated from mouse liver and pancreas using Cx32 primer pairs (lanes 1–3) or Cx26 primer pairs (lane 4–6). Amplification products of the expected sizes were detected for Cx32 and Cx26 mRNAs in liver (lanes 1 and 4) and pancreas of C57BL/6 mice (lanes 3 and 6). mRNA for Cx26 (lane 5), but not for Cx32 (lane 2), was detected in pancreas of Cx32 (−/−) mice. Size markers are shown on each side of the gel. (B–E) Indirect immunofluorescence of pancreas cryostat sections. Specific punctate labeling for Cx32 and Cx26 was detected in wild-type pancreas (B and D, respectively). In contrast, no immune reactivity was observed for Cx32 (C) and attenuated labeling was seen for Cx26 (E) in Cx32 (−/−) pancreas. Bar, 10 μm.
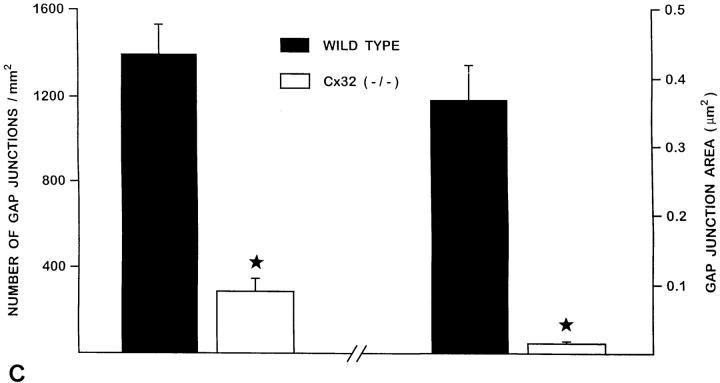
Freeze-fracture analysis of acinar cell membranes showed that the number of gap junctions was decreased 4.8-fold in Cx32 (−/−) exocrine pancreas. The area of the gap junctions located at basolateral membranes was also decreased 25-fold (Fig. 3, A and B).
Figure 3.
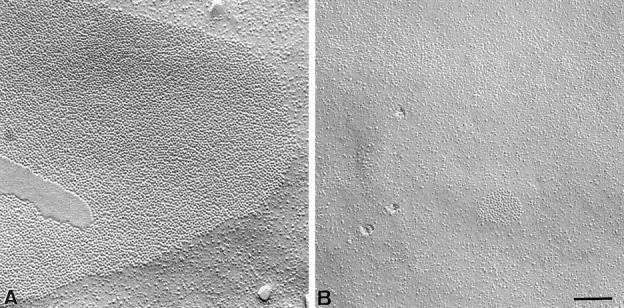
Freeze-fracture analysis of gap junctions in pancreatic acinar cells. In contrast to gap junctions observed in wild-type animals (A), pancreatic acinar cells from Cx32 (−/−) mice showed less frequent gap junctions plaques of smaller areas (B). (C) Quantitative evaluation of gap junctions in wild-type and Cx32 (−/−) pancreatic acinar cells. The number of gap junction plaques was reduced five fold in the pancreas of Cx32 (−/−) mice (open columns) as compared with that evaluated in wild-types (solid columns). The size of gap junction plaques was also reduced twenty fold in Cx32 (−/−) exocrine pancreas. Stars, differences at a P < 0.001 level. Bar, 130 nm.
Intercellular Communication
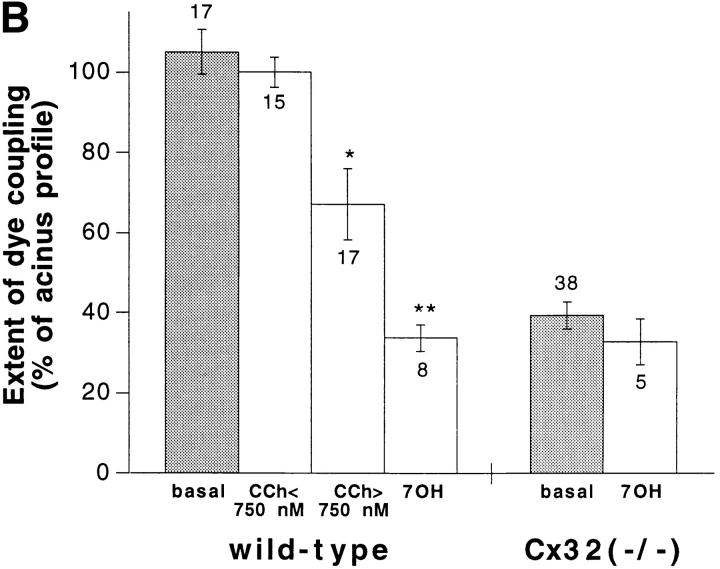
Extent of intercellular communication was first studied by injecting Lucifer yellow. The fluorescent dye rapidly diffused from the injected cell into all its neighbors in wild-type C57BL/6 acini, the surface labeled by the tracer representing on average 105.1 ± 5.6% (n = 17) of the acinus profile (Fig. 4, A and B). A similar extent of communication (96 ± 4%; n = 7, and 111.6 ± 5.4%; n = 6) was observed in acini of C57BL/6 × 129 SV-F1 and heterozygote Cx32 (+/−) mice. Addition of 3.5 mM heptanol to the wild-type acini markedly decreased (P < 0.001) acinar cell coupling. The extent of dye coupling was also reduced in the presence of concentrations of CCh >750 nM (Fig. 4 B). In contrast, acini isolated from Cx32 (−/−) pancreata showed a restricted diffusion of Lucifer yellow that on the average (39.3 ± 3.4% of the acinus profile; n = 38) was similar to that observed in wild-type mice treated with heptanol (Fig. 4, A and B). Addition of heptanol to Cx32 (−/−) acini did not further decrease the extent of dye coupling (Fig. 4 B).
Figure 4.
Dye coupling in acini dispersed from wild-type and Cx32 (−/−) pancreata. (A) Fluorescence views of wild-type and Cx32 (−/−) acini injected 3 min with Lucifer yellow. All the cells of the wild-type acinus were found coupled to each other, whereas in the Cx32 (−/−) acinus, the diffusion of the fluorescent dye was restricted to the injected cell. (B) Quantitative evaluation of dye coupling in wild-type and Cx32 (−/−) acini. Under resting conditions (basal) and in the presence of <750 nM CCh, dye transfer was extensive between wild-type acinar cells. Dye transfer was significantly decreased by >750 nM CCh and by 3.5 mM heptanol (7OH), indicating uncoupling of acinar cells. In Cx32 (−/−) acini, dye transfer was markedly reduced under resting conditions and was not further decreased in the presence of 3.5 mM heptanol. Stars, differences at P < 0.005 and P < 0.001 levels, respectively. Bar, 30 μm.
To determine if the decrease in dye transfer reflected a complete closure of gap junction channels, junctional conductance (gj) was monitored in pairs of acinar cells using a dual patch clamp approach. Cell pairs of Cx32 (−/−) mice showed gj values averaging 17 ± 3.5 nSiemens (n = 7). As shown in Fig. 5 A, gj had the characteristics expected for a junctional conductance, including its fully reversible blockade by halothane. To characterize the properties of gap junctional currents (Ij) in Cx32 (−/−) mice, we first evaluated the Ij sensitivity to transjunctional potentials (Vj). To this end, long (8 s) Vj pulses were applied and Ij was measured at the onset and end of the voltage protocol. As shown in Fig. 5 B, almost no difference was observed between instantaneous and steady-state values over the Vj range studied (±80 mV), indicating a weak dependence on transjunctional voltage of the gap junction channels present in Cx32 (−/−) acinar cells. Second, we studied the gating of individual gap junction channels in pairs in which gj had been reduced by halothane (Fig. 5 C). Large transitions of opposite polarities but similar amplitudes were recorded simultaneously in both current traces, exhibiting typical junctional channels with an average amplitude of 120–145 pS (n = 160).
Figure 5.
Electrical coupling between Cx32 (−/−) acinar cell pairs. (A) When evaluated as a function of time, junctional conductance was found to be rapidly abolished by halothane in a reversible manner. The dashed line indicates the zero conductance value. (B) Current-voltage relationships of gap junctional currents measured at the onset (○) and end (•) of an 8-s voltage pulse protocol. This relationship was almost linear for both measurements, indicating a modest sensitivity of the gap junctions to transjunctional voltage. Similar observations were made in two other cell pairs. (C) Example of unitary gap junction channel activity recorded in a Cx32 (−/−) acinar cell pair that was monitored in the presence of halothane. Current transitions evoked during a 40 mV transjunctional potential correspond to unitary conductances of about 125–140 pS.
Amylase Secretion
Amylase secretion was studied in acini dispersed from wild-type and Cx32 (−/−) pancreata following a 30-min incubation in the presence of increasing concentrations of CCh (Fig. 6). This secretagogue stimulated the release of amylase in wild-type C57BL/6 acini in a dose-dependent manner from a basal level to a plateau. The CCh-stimulated secretion of CX32 (−/−) acini was not different. However, the basal release of amylase was increased (P < 0.05) about two- to threefold in Cx32 (−/−) acini as compared with that of acini from wild-type mice. Both C57BL/6 and C57BL/6 × 129 SV-F1 mice showed a comparable basal amylase output (Fig. 6). No difference in total amylase content was observed between Cx32 (−/−) and wild-type pancreata (2.2 ± 0.3 U/mg total protein, n = 4; and 2.16 ± 0.13 U/mg, n = 4, respectively).
Figure 6.
Effects of CCh on amylase secretion of pancreatic acini dispersed from wild-type and Cx32 (−/−) pancreata. CCh evoked a S-shaped stimulation of amylase release in wild-type C57BL/6 (○) and Cx32 (−/−) acini (•). However, Cx32 (−/−) acini showed an increase in basal release, as compared with controls. Stars indicate differences at a P < 0.05 level. Values are mean ± SEM of 9 and 12 independent experiments on preparations of wild-type C57BL/6 and Cx32 (−/−) acini, respectively. The ▵ indicates the average basal amylase secretion of C57BL/6 × 129 SV-F1 acini, as evaluated in six independent experiments.
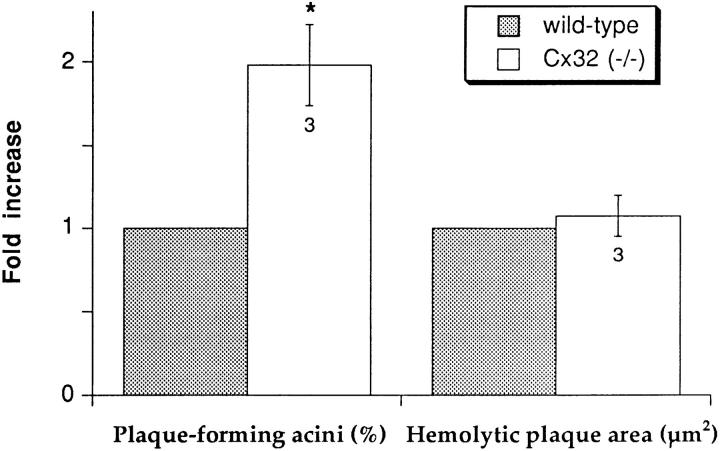
To further analyze basal secretion of wild-type (C57BL/6) and Cx32 (−/−) acini, we used an immunological assay for amylase. In three separate experiments, acini from Cx32 (−/−) mice formed hemolytic plaques more frequently (P < 0.04) than acini from wild-type mice studied simultaneously under identical conditions (Fig. 7). In contrast, Cx32 (−/−) acini did not differ from wild-type acini in terms of average areas of hemolytic plaque that formed as a result of amylase release (Fig. 7). This change resulted in an almost twofold increase in total plaque development of Cx32 (−/−) acini (4,811 ± 1,171 μm2) as compared with wild-type acini (2,506 ± 1,343 μm2).
Figure 7.
Primary parameters evaluating basal secretion of wild-type (C57BL/6) and Cx32 (−/−) pancreatic acini. Although the area of hemolytic plaques was not different, acini from Cx32 (−/−) mice showed higher proportion of secreting acini as compared with wild-type mice. For each experiment, the proportion of plaque-forming acini from Cx32 (−/−) mice and the area of their hemolytic plaques were normalized to values obtained for wild-type acini and averaged. Star, differences at a P < 0.04 level.
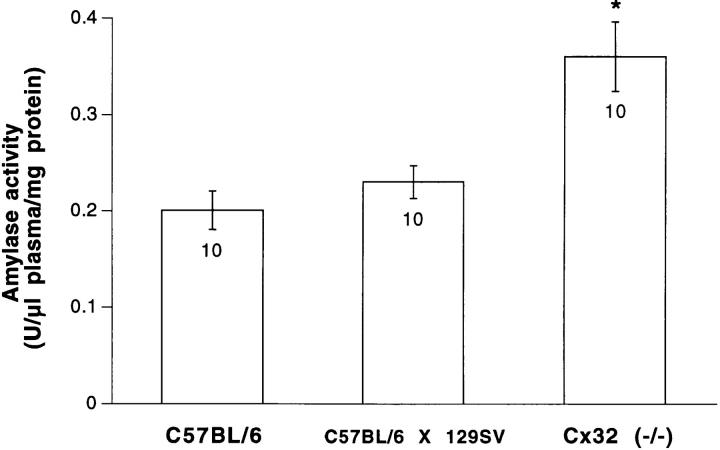
Plasmatic amylase levels were measured to determine if the enhanced secretory activity of Cx32 (−/−) acini were also detectable in the living animals. As shown in Fig. 8, the amount of plasmatic amylase was significantly (P < 0.01) increased in Cx32 (−/−) mice as compared with that of both C57BL/6 and C57BL/6 × 129 SV-F1 animals, that had similar levels of circulating amylase.
Figure 8.
Plasmatic levels of amylase in C57BL/6, C57BL/6 × 129 SV-F1 and Cx32 (−/−) mice. Amylase activity was twofold higher in Cx32 (−/−) than in the two lines of wild-type mice screened. Number within columns indicate the number of mice studied (one measurement per mouse). Star, differences at a P < 0.01 level.
Discussion
Direct cell–cell interactions via gap junctional communication contribute to the homeostasis of differentiated cells. The physiological role of gap junctional communication in the function of nonexcitable cells remains, however, a matter of debate. Previous in vitro studies have documented a possible link between this communication and the secretion of pancreatic acinar cells (Meda et al., 1986, 1987; Bruzzone et al., 1987; Chanson et al., 1989, 1991; Loessberg Stauffer et al., 1993; Bosco et al., 1994). The recent generation of viable mice deficient for the Cx32 gene (Nelles et al., 1996), provides a model to directly investigate the participation of gap junction channels in the function of tissues expressing Cx32 (Nelles et al., 1996; Anzini et al., 1997), including the exocrine pancreas (Chanson et al., 1991; Meda et al., 1993).
Pancreatic acinar cells of Cx32 (−/−) mice lacked Cx32, as determined by RT-PCR and immunolabeling. The absence of this connexin was associated with much smaller and less abundant gap junction plaques, as revealed by freeze-fracture analysis of acinar cell membranes. In agreement with these morphological data, the extent of acinar cell-to-cell communication was dramatically altered in Cx32 (−/−) mice. Hence, the diffusion of iontophoretically injected Lucifer yellow, which labeled within a minute every cell in acini of wild-type mice of both the C57BL/6 and the C57BL/6 × 129 SV-F1 strains as well as those of Cx32 (+/−) mice, was markedly decreased in Cx32 (−/−) animals in which it was comparable to that of wild-type cells exposed to the gap junction blocker heptanol (Meda et al., 1986; Chanson et al., 1989). In spite of this change, electrical coupling was still detected using the dual patch clamp approach between the acinar cells of Cx32 (−/−) mice. The intercellular currents recorded in Cx32 (−/−) acinar cell pairs could be reversibly blocked with uncouplers, showed moderate sensitivity to transjunctional voltage within a ±80-mV range and were mediated by gap junction channels with a unitary conductance of 120–145 pS. These properties, which are consistent with those attributed to Cx26 in exogenous expression systems (Barrio et al., 1991; Bukauskas et al., 1995; Kwak et al., 1995), suggest that the electrical coupling observed between acinar cells of Cx32 (−/−) mice is maintained by gap junction channels made of Cx26. This protein was still detected in pancreas of Cx32 (−/−) mice, albeit at a reduced level compared with that observed in wild-type mice. These results are analogous to those recently reported in hepatocytes (Nelles et al., 1996), in which Cx26 and Cx32 colocalize within the same gap junction plaques (Nicholson et al., 1987). Whether stable incorporation of Cx26 in gap junctions requires the presence of Cx32 remains to be investigated. Alternatively, the lower expression of Cx26 may reflect an altered assembly and/or degradation of connexins at gap junctional membranes (Musil and Goodenough, 1991; Falk et al., 1994; Lampe, 1994; Laing and Beyer, 1995).
Pancreatic acinar cells of Cx32 (−/−) mice did not show apparent alterations in spatial organization and ultrastructure. In agreement with this observation, Cx32 (−/−) acinar cells appeared fully operational since they were stimulated to release amylase in a dose-dependent way by CCh. Although the dose-response relationship was similar in wild-type and Cx32 (−/−) acini, the basal secretion of the latter acini was twice that of controls in spite of a comparable content of amylase, the predominant enzyme of rodent pancreas. The amount of amylase released by individual acini was also similar for wild-type and Cx32 (−/−) pancreata, as evaluated by reverse hemolytic plaque assay (Bosco et al., 1988). However, this assay showed that the proportion of acini secreting under basal conditions was larger in preparations of Cx32 (−/−) pancreata. This change is of interest with regard to the previous observation that the number of secreting acini, but not the amount of amylase released, was increased in pancreatic acinar cells exposed to heptanol (Bosco et al., 1988; Chanson et al., 1989). Thus, reduction of acinar cell-to-cell communication is associated with the recruitment of individual acini to secrete. Apparently, this phenomenon does not require the complete closure of gap junction channels since large electrical coupling remained detectable between Cx32 (−/−) acinar cells. At any rate, the enhanced basal secretion of amylase was sufficient to markedly increase the levels of the enzyme that were found in the blood under resting conditions. The secretion of the exocrine pancreas is regulated by numerous factors, including hormones and neurotransmitters. The novel finding that a chronic decrease in cell coupling results in increased plasmatic levels of amylase, points to gap junctional communication as a relevant mechanism controlling enzyme secretion in vivo.
Increasing evidence indicates that open gap junction channels are required to fulfill the secretory function of acinar cells during stimulation by Ca2+-mobilizing secretagogues (Amsterdam and Jamieson, 1974; Gardner and Jackson, 1977; Nathanson et al., 1992; Loessberg Stauffer et al., 1993; Ngezahayo and Kolb, 1993; Bosco et al., 1994; Yule et al., 1996). Whereas this evidence may not appear immediately conciliable with the repeated observation that the same secretagogues decrease intercellular communication while maximally stimulating the secretory activity of acinar cells (Petersen and Ueda, 1976; Iwatsuki and Petersen, 1978; Petersen and Iwatsuki, 1979; Chanson et al., 1991; Bosco et al., 1994), this discrepancy is only apparent. Indeed, under conditions of gap junction blockade, the potency of several agonists in stimulating enzyme release was found to be reduced when the effect of cell uncoupling on basal secretion was taken into account (Meda et al., 1986; Loessberg Stauffer et al., 1993). Hence, the enhanced basal secretion observed during uncoupling of acinar cells may represents a mechanism to sustain exocytosis during maximal stimulation by Ca2+-mobilizing agonists. Among several other possibilities, it is conceivable that the intercellular diffusion of factor(s) that negatively control basal secretion is prevented upon downregulation of gap junctional communication, leading to enhanced recruitment of acinar cells for exocytosis. The persistence of electrical coupling in acinar cells of Cx32 (−/−) mice that showed this increased recruitment, further suggests that some cytoplasmic molecules play a key role in the tonic inhibitory influence exerted by gap junctional communication. Additional experiments aimed at studying the extent of communication between Cx32 (−/−) acini using dyes of variable size and charge is expected to help identifying these factors.
In conclusion, the finding that basal secretion is increased in pancreatic acinar cells of Cx32-deficient mice supports the view that gap junctional communication participates in regulating the function of the exocrine pancreas. The mechanisms by which Ca2+-mobilizing agonists induce gap junction closure and by which this closure leads to enhanced recruitment of secretory acinar cells remain to be elucidated. The modulation of acinar cell coupling by these secretagogues appears of physiological importance since closure of gap junction channels has been shown to occur in vivo during maximal stimulation of exocytosis (Chanson et al., 1991). We have now demonstrated that the chronic absence of at least one type of connexin channels disturbs the in vivo function of the exocrine pancreas.
Acknowledgments
We thank E. Sutter, A. Charollais, F. Cogne, T. Dudez, and I. Duperrut for excellent technical assistance. We also thank Dr. T. Ott for his help with the C57BL/6 × 129 SV-F1 mice.
This work was supported by grants from the Swiss National Science Foundation (32.34086.95 to P. Meda and 32-45845.95 to M. Chanson), the Société Académique de Genève, and the Association Française de Lutte Contre la Mucoviscidose (to M. Chanson), the Juvenile Diabetes Foundation International (197124 to P. Meda), the European Union (BMH4-CT96-1427 to P. Meda and K. Willecke), the German Research Association (SFB284, C1 to K. Willecke) and the Dr. Miltred Scheel Foundation for Cancer Research (to K. Willecke).
Abbreviations used in this paper
- CCH
carbamylcholine
- Cx
connexin
- RT
reverse transcription
Footnotes
Address all correspondence to Marc Chanson, Laboratory of Clinical Investigation 3, HUG, Department of Pediatrics, P.O. Box 14, 24 Micheli-du-Crest, 1211 Geneva 4, Switzerland. Tel.: (41 22) 37 24 609. Fax: (41 22) 37 24 088. E-mail: Marc.Chanson@hcuge.ch
References
- Amsterdam A, Jamieson JD. Studies on dispersed pancreatic exocrine cells. II. Functional characteristics of separated cells. J Cell Biol. 1974;63:1057–1073. doi: 10.1083/jcb.63.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzini P, Neuberg D-H, Schachner M, Nelles E, Willecke K, Zielasek J, Toyka KV, Suter U, Martini R. Structural abnormalities and deficient maintenance of peripheral nerve myelin in mice lacking the gap junction protein Cx32. J Neurosci. 1997;17:4545–4551. doi: 10.1523/JNEUROSCI.17-12-04545.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrio LC, Suchyna T, Bargiello T, Xu LX, Roginski RS, Bennett MVL, Nicholson BJ. Gap junctions formed by connexins 26 and 32 alone and in combination are differently affected by voltage. Proc Natl Acad Sci USA. 1991;88:8410–8414. doi: 10.1073/pnas.88.19.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin AR, Vachereau A, St-Jean P. Evidence that amylase is released from two distinct pools of secretory proteins in the pancreas. Biochim Biophys Acta. 1983;757:302–305. doi: 10.1016/0304-4165(83)90055-7. [DOI] [PubMed] [Google Scholar]
- Bendayan M. Morphometrical and immunocytchemical characterization of peri-insular and tele-insular acinar cells in rat pancreas. Eur J Cell Biol. 1985;36:263–268. [PubMed] [Google Scholar]
- Berridge MJ. Elementary and global aspect of calcium signaling. J Physiol. 1997;499:291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco D, Chanson M, Bruzzone R, Meda P. Visualization of amylase secretion from individual pancreatic acini. Am J Physiol. 1988;254:664–670. doi: 10.1152/ajpgi.1988.254.5.G664. [DOI] [PubMed] [Google Scholar]
- Bosco DJS, Soriano, Chanson M, Meda P. Heterogeneity and contact-dependent regulation of amylase release by individual acinar cells. J Cell Physiol. 1994;160:378–388. doi: 10.1002/jcp.1041600219. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Halban PA, Gjinovci A, Trimble ER. A new, rapid, method for preparation of dispersed pancreatic acini. Biochem J. 1985;226:621–624. doi: 10.1042/bj2260621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone R, Trimble ER, Gjinovci A, Traub O, Willecke K, Meda P. Regulation of pancreatic exocrine function: a role for cell-to-cell communication? . Pancreas. 1987;2:262–271. doi: 10.1097/00006676-198705000-00004. [DOI] [PubMed] [Google Scholar]
- Bukauskas FF, Elfgang C, Willecke K, Weingart R. Heterotypic gap junction channels (connexin26-connexin32) violate the paradigm of unitary conductance. Pfluegers Arch. 1995;429:870–872. doi: 10.1007/BF00374812. [DOI] [PubMed] [Google Scholar]
- Burt JM, Spray DC. Inotropic agents modulate gap junctional conductance between cardiac myocytes. Am J Physiol. 1991;254:1206–1210. doi: 10.1152/ajpheart.1988.254.6.H1206. [DOI] [PubMed] [Google Scholar]
- Chanson M, Bruzzone R, Bosco D, Meda P. Effects of n-alcohols on junctional coupling and amylase secretion of pancreatic acinar cells. J Cell Physiol. 1989;139:147–156. doi: 10.1002/jcp.1041390121. [DOI] [PubMed] [Google Scholar]
- Chanson M, Orci L, Meda P. Extent and modulation of junctional communication between pancreatic acinar cells in vivo. . Am J Physiol. 1991;261:28–36. doi: 10.1152/ajpgi.1991.261.1.G28. [DOI] [PubMed] [Google Scholar]
- Dermietzel R, Leibstein A, Frixen U, Janssen-Timmen U, Traub O, Willecke K. Gap junctions in several tissues share antigenic determinants with liver gap junctions. EMBO (Eur Mol Biol Organ) J. 1984;3:2261–2270. doi: 10.1002/j.1460-2075.1984.tb02124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk MM, Kumar NM, Gilula NG. Membrane insertion of gap junction connexins: Polytopic channel forming membrane proteins. J Cell Biol. 1994;127:343–355. doi: 10.1083/jcb.127.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JD, Jackson MJ. Regulation of amylase release from dispersed pancreatic acinar cells. J Physiol. 1977;270:439–454. doi: 10.1113/jphysiol.1977.sp011961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman B, Wallgren A, Petersson B. Cytosolic characteristics of the exocrine pancreatic cells with regards to their position in relation to the islets of Langherans. Acta Endocrinol. 1962;39:465–473. doi: 10.1530/acta.0.0390465. [DOI] [PubMed] [Google Scholar]
- Iwatsuki N, Petersen OH. Acetylcholine-evoked electrical uncoupling and its ionic dependency. J Physiol. 1978;274:81–96. doi: 10.1113/jphysiol.1978.sp012135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak BR, Hermans MMP, De Jonge HR, Lohmann SM, Jongsma HJ, Chanson M. Differential regulation of distinct types of gap junction channels by similar phosphorylating treatments. Mol Biol Cell. 1995;6:989–1002. doi: 10.1091/mbc.6.12.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing JG, Beyer EC. The gap junction protein connexin43 is degraded via the ubiquitin proteasome pathway. J Biol Chem. 1995;270:26399–26403. doi: 10.1074/jbc.270.44.26399. [DOI] [PubMed] [Google Scholar]
- Lampe P. Analyzing phorbol ester effect on gap junctional communication: a dramatic inhibition of assembly. J Cell Biol. 1994;127:1895–1905. doi: 10.1083/jcb.127.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loessberg Stauffer, P., H. Zhao, K. Luby-Phelps, R.L. Moss, R.A. Star, and S. Muallem. Gap junction communication modulates [Ca2+]ioscillations and enzyme secretion in pancreatic acini. J Biol Chem. 1993;268:19769–19775. [PubMed] [Google Scholar]
- Meda P, Bruzzone R, Knodel S, Orci L. Blockage of cell-to-cell communication within pancreatic acini is associated with increased basal release of amylase. J Cell Biol. 1986;103:475–483. doi: 10.1083/jcb.103.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda P, Bruzzone R, Chanson M, Bosco D, Orci L. Junctional coupling modulates secretion of exocrine pancreas. Proc Natl Acad Sci USA. 1987;84:4901–4904. doi: 10.1073/pnas.84.14.4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda P, Bruzzone R, Chanson M, Bosco D. Junctional coupling and secretion of pancreatic acinar cells. Mod Cell Biol. 1988;7:353–364. doi: 10.1002/jcp.1041390121. [DOI] [PubMed] [Google Scholar]
- Meda P, Pepper MS, Traub O, Willecke K, Gros D, Beyer EC, Nicholson BJ, Paul D, Orci L. Differential expression of gap junction connexins in endocrine and exocrine glands. Endocrinology. 1993;133:2371–2378. doi: 10.1210/endo.133.5.8404689. [DOI] [PubMed] [Google Scholar]
- Musil LS, Goodenough DA. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J Cell Biol. 1991;115:1357–1374. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson MH, Padfield PJ, O'Sullivan AJ, Burgstahler AD, Jamieson JD. Mechanism of Ca2+wave propagation in pancreatic acinar cells. J Biol Chem. 1992;267:18118–18121. [PubMed] [Google Scholar]
- Nelles E, Bützler C, Jung D, Temme A, Gabriel H-D, Dahl U, Traub O, Stümpel F, Jungermann K, Zielasek J, Toyka KV, Dermietzel R, Willecke K. Defective propagation of signals generated by sympathetic nerve stimulation in the liver of connexin32–deficient mice. Proc Natl Acad Sci USA. 1996;93:9565–9570. doi: 10.1073/pnas.93.18.9565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J, Trautmann A. Single-channel currents of an intercellular junction. Nature. 1985;317:331–335. doi: 10.1038/317331a0. [DOI] [PubMed] [Google Scholar]
- Ngezahayo A, Kolb H-A. Gap junctional conductance tunes phase difference of cholecystokinin evoked calcium oscillations in pairs of pancreatic acinar cells. Pfluegers Arch. 1993;422:413–415. doi: 10.1007/BF00374302. [DOI] [PubMed] [Google Scholar]
- Nicholson BJ, Dermietzel R, Teplow D, Traub O, Willecke K, Revel JP. Two homologous protein components of hepatic gap junctions. Nature. 1987;329:283–295. doi: 10.1038/329732a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986;233:305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Petersen, O.H, and N. Iwatsuki. 1979. Hormonal control of cell to cell coupling in the exocrine pancreas. In Hormone Receptors in Digestion and Nutrition. G. Rosselin, P. Fromageot, and S. Bonfils, editors. Elsevier/North Holland Biomedical Press, Amsterdam. 191–202.
- Petersen OH, Ueda N. Pancreatic acinar cells: the role of calcium in stimulus-secretion coupling. J Physiol. 1976;254:583–606. doi: 10.1113/jphysiol.1976.sp011248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman SS. Independent secretion of different digestive enzymes by the pancreas. Am J Physiol. 1976;231:1847–1851. doi: 10.1152/ajplegacy.1976.231.6.1847. [DOI] [PubMed] [Google Scholar]
- Stevensen BR, Siciliano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temme A, Buchmann A, Gabriel H-D, Nelles E, Schwarz M, Willecke K. High incidence of spontaneous and chemically induced liver tumors in mice deficient for Cx32. Curr Biol. 1997;7:713–716. doi: 10.1016/s0960-9822(06)00302-2. [DOI] [PubMed] [Google Scholar]
- Traub O, Look J, Dermietzel R, Brümmer F, Hülser D, Willecke K. Comparative characterization of the 21-kD and 26-kD gap junction proteins in murine liver and cultured hepatocytes. J Cell Biol. 1989;108:1039–1051. doi: 10.1083/jcb.108.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vozzi C, Ullrich S, Charollais A, Philippe J, Orci L, Meda P. Adequate connexin-mediated coupling is required for proper insulin production. J Cell Biol. 1995;131:1561–1572. doi: 10.1083/jcb.131.6.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems PHGM, Van Emst-De-Vries SE, Van Os CH, De Pont JJHHM. Dose-dependent recruitment of pancreatic acinar cells during receptor-mediated calcium mobilization. Cell Calcium. 1993;14:145–159. doi: 10.1016/0143-4160(93)90084-j. [DOI] [PubMed] [Google Scholar]
- Yule, D.I., and J.A. Williams. 1994. Stimulus-secretion coupling in the pancreatic acinus. In Physiology of the Gastrointestinal Tract (3rd ed.). L.R. Johnson, editor. Raven Press, New York. 1447–1472.
- Yule DI, Stuenkel E, Williams JA. Intercellular calcium waves in rat pancreatic acini: mechanism of transmission. Am J Physiol. 1996;271:1285–1294. doi: 10.1152/ajpcell.1996.271.4.C1285. [DOI] [PubMed] [Google Scholar]