Abstract
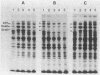
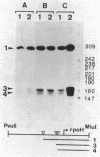
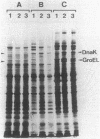
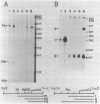
Temperature-resistant pseudorevertants were isolated from rpoH (htpR) mutants of Escherichia coli K-12 that cannot grow at a high temperature owing to a deficiency in sigma 32 required for the induction of heat shock proteins. Among them was a class of revertants carrying a suppressor mutation, designated suhA, that suppressed all the nonsense and missense rpoH mutations tested. suhA is located at 77 min, about 1 min away from rpoH, on the genetic map. In contrast to the rpoH mutants, the suhA revertants that contained both rpoH (nonsense) and suhA mutations were fully or partially proficient in the induction of heat shock proteins upon exposure to a high temperature. Under these conditions, transcription from two heat shock promoters as determined by operon fusion was transiently activated. In one of the rpoH(Am) suhA revertants studied in detail, an increase in temperature caused the synthesis of significant amounts of sigma 32, accompanied by increased stability and accumulation of rpoH mRNAs. On the other hand, the same mutation (suhA6) only weakly suppressed the rpoH deletion mutant; however, two of the major heat shock genes, dnaK and groE, were apparently induced in the absence of sigma 32. Thus, suhA6 seems to bring about the induction of heat shock genes by at least two mechanisms, one increasing the level of sigma 32 synthesis, and the other activating some transcription factor other than sigma 32.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Cowing D. W., Bardwell J. C., Craig E. A., Woolford C., Hendrix R. W., Gross C. A. Consensus sequence for Escherichia coli heat shock gene promoters. Proc Natl Acad Sci U S A. 1985 May;82(9):2679–2683. doi: 10.1073/pnas.82.9.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. A., Goldberg A. L. Production of abnormal proteins in E. coli stimulates transcription of lon and other heat shock genes. Cell. 1985 Jun;41(2):587–595. doi: 10.1016/s0092-8674(85)80031-3. [DOI] [PubMed] [Google Scholar]
- Grossman A. D., Erickson J. W., Gross C. A. The htpR gene product of E. coli is a sigma factor for heat-shock promoters. Cell. 1984 Sep;38(2):383–390. doi: 10.1016/0092-8674(84)90493-8. [DOI] [PubMed] [Google Scholar]
- Grossman A. D., Taylor W. E., Burton Z. F., Burgess R. R., Gross C. A. Stringent response in Escherichia coli induces expression of heat shock proteins. J Mol Biol. 1985 Nov 20;186(2):357–365. doi: 10.1016/0022-2836(85)90110-x. [DOI] [PubMed] [Google Scholar]
- Kleckner N., Roth J., Botstein D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977 Oct 15;116(1):125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- Landick R., Vaughn V., Lau E. T., VanBogelen R. A., Erickson J. W., Neidhardt F. C. Nucleotide sequence of the heat shock regulatory gene of E. coli suggests its protein product may be a transcription factor. Cell. 1984 Aug;38(1):175–182. doi: 10.1016/0092-8674(84)90538-5. [DOI] [PubMed] [Google Scholar]
- Lemaux P. G., Herendeen S. L., Bloch P. L., Neidhardt F. C. Transient rates of synthesis of individual polypeptides in E. coli following temperature shifts. Cell. 1978 Mar;13(3):427–434. doi: 10.1016/0092-8674(78)90317-3. [DOI] [PubMed] [Google Scholar]
- Mori K., Aiba H. Evidence for negative control of cya transcription by cAMP and cAMP receptor protein in intact Escherichia coli cells. J Biol Chem. 1985 Nov 25;260(27):14838–14843. [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A. Positive regulatory gene for temperature-controlled proteins in Escherichia coli. Biochem Biophys Res Commun. 1981 May 29;100(2):894–900. doi: 10.1016/s0006-291x(81)80257-4. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Vaughn V. The genetics and regulation of heat-shock proteins. Annu Rev Genet. 1984;18:295–329. doi: 10.1146/annurev.ge.18.120184.001455. [DOI] [PubMed] [Google Scholar]
- Osawa T., Yura T. Effects of reduced amount of RNA polymerase sigma factor on gene expression and growth of Escherichia coli: studies of the rpoD450 (amber) mutation. Mol Gen Genet. 1981;184(2):166–173. doi: 10.1007/BF00272900. [DOI] [PubMed] [Google Scholar]
- Shiba K., Ito K., Yura T. Suppressors of the secY24 mutation: identification and characterization of additional ssy genes in Escherichia coli. J Bacteriol. 1986 Jun;166(3):849–856. doi: 10.1128/jb.166.3.849-856.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W. E., Straus D. B., Grossman A. D., Burton Z. F., Gross C. A., Burgess R. R. Transcription from a heat-inducible promoter causes heat shock regulation of the sigma subunit of E. coli RNA polymerase. Cell. 1984 Sep;38(2):371–381. doi: 10.1016/0092-8674(84)90492-6. [DOI] [PubMed] [Google Scholar]
- Tilly K., Erickson J., Sharma S., Georgopoulos C. Heat shock regulatory gene rpoH mRNA level increases after heat shock in Escherichia coli. J Bacteriol. 1986 Dec;168(3):1155–1158. doi: 10.1128/jb.168.3.1155-1158.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K., McKittrick N., Zylicz M., Georgopoulos C. The dnaK protein modulates the heat-shock response of Escherichia coli. Cell. 1983 Sep;34(2):641–646. doi: 10.1016/0092-8674(83)90396-3. [DOI] [PubMed] [Google Scholar]
- Tobe T., Ito K., Yura T. Isolation and physical mapping of temperature-sensitive mutants defective in heat-shock induction of proteins in Escherichia coli. Mol Gen Genet. 1984;195(1-2):10–16. doi: 10.1007/BF00332716. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Yamamori T., Ito K., Nakamura Y., Yura T. Transient regulation of protein synthesis in Escherichia coli upon shift-up of growth temperature. J Bacteriol. 1978 Jun;134(3):1133–1140. doi: 10.1128/jb.134.3.1133-1140.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamori T., Yura T. Genetic control of heat-shock protein synthesis and its bearing on growth and thermal resistance in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1982 Feb;79(3):860–864. doi: 10.1073/pnas.79.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamori T., Yura T. Temperature-induced synthesis of specific proteins in Escherichia coli: evidence for transcriptional control. J Bacteriol. 1980 Jun;142(3):843–851. doi: 10.1128/jb.142.3.843-851.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano R., Imai M., Yura T. The use of operon fusions in studies of the heat-shock response: effects of altered sigma 32 on heat-shock promoter function in Escherichia coli. Mol Gen Genet. 1987 Apr;207(1):24–28. doi: 10.1007/BF00331486. [DOI] [PubMed] [Google Scholar]
- Yura T., Tobe T., Ito K., Osawa T. Heat shock regulatory gene (htpR) of Escherichia coli is required for growth at high temperature but is dispensable at low temperature. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6803–6807. doi: 10.1073/pnas.81.21.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]