Abstract
Recognition of the exposure of phosphatidylserine (PS) on the outer surface of plasma membrane has been implicated in the phagocytosis of aged/apoptotic cells. Because oxidized low-density lipoprotein (OxLDL) has been reported to block the phagocytosis, here we examined whether lectin-like OxLDL receptor 1 (LOX-1), the OxLDL receptor in endothelial cells, mediates phagocytosis of aged/apoptotic cells by endothelial cells. Cultured bovine aortic endothelial cells (BAE) and Chinese hamster ovary (CHO) cells expressing bovine LOX-1 (BLOX-1-CHO), but not wild-type CHO-K1 cells, bound aged red blood cells (RBC) and apoptotic cells, which were further phagocytosed. The binding of aged RBC and the phagocytosis of apoptotic cells were inhibited by OxLDL, acetyl LDL, and other LOX-1 ligands in both BAE and BLOX-1-CHO. mAb against LOX-1 blocked the binding of aged RBC to BAE, suggesting a role for LOX-1 in the recognition of aged cells. The recombinant soluble LOX-1 inhibited the interactions of aged/apoptotic cells with both BLOX-1-CHO and BAE and distinguished aged RBC from native RBC and apoptotic cells from native cells. PS liposome inhibited these LOX-1-mediated interactions with aged/apoptotic cells, suggesting LOX-1 recognizes PS of the apoptotic cells. PS exposed on the surface of apoptotic cells is known to be procoagulant. Accordingly, increased OxLDL may be one of the reasons for enhanced coagulation in atherosclerosis, inhibiting the removal of apoptotic cells.
Phagocytosis of aged and apoptotic cells is an essential process to protect normal healthy cells from the harmful contents and debris of dying cells (1). Various changes on the surface of apoptotic/aged cells have been proposed for the mechanisms of the recognition by phagocytosing cells. Among them, translocation of phosphatidylserine (PS) from the inner leaflet of the plasma membrane to the outer leaflet has been known to be one of the earliest signals in the apoptotic process (2). Therefore, the recognition of PS exposed on the surface of the cells may be the most important mechanism to remove the apoptotic/aged cells. In macrophages, several receptors have been proposed to mediate the binding aged/apoptotic cells, including CD36 and class A scavenger receptors (3, 4). CD36 is involved in the recognition of apoptotic photoreceptor outer segments by retial epithelial cells (5), and that of thymocytes and neutrophils by monocyte-derived macrophages in cooperation with αvβ3 integrin and thrombospondin (3). In thymic macrophages, treatment of the cells with an anticlass A scavenger receptor antibody or disruption of the gene for class A scavenger receptor reduced the phagocytosis of apoptotic thymocytes (4). Although the macrophages are specialized in phagocytosis of cell aberrant materials and pathogenic organisms, nonmacrophage cells, mostly neighboring cells, also take part in the phagocytosis of apoptotic cells (1). Although the role of PS in phagocytosis by the above molecules is still unclear, it was reported that PS and oxidized low-density lipoprotein (OxLDL) competed with the phagocytosis of aged and apoptotic cells (6). This finding prompted us to examine whether the endothelial receptor for OxLDL, lectin-like OxLDL receptor 1 (LOX-1) (7), was involved in the phagocytosing system.
LOX-1 is the major receptor for OxLDL expressed in vascular endothelial cells, presumably mediating the effects of OxLDL on endothelial cells. Sequencing showed that LOX-1 is a type II membrane protein that consists of 273 amino acids and belongs to the C-type lectin family. The expression of LOX-1 was not restricted to cultured endothelial cells, but also was observed in atheromatous intima of the intact aorta and the human carotid artery in vivo. The expression of LOX-1 is induced by certain kinds of cytokines in vitro and is enhanced in hypertensive rats in vivo, indicating that LOX-1 expression is highly regulated by stimuli related to atherosclerosis (8, 9).
In the present study, we showed that LOX-1 mediated phagocytosis of aged/apoptotic cells in endothelial cells. Facing blood cells in vivo, LOX-1 on endothelial cells may physiologically remove aged/apoptotic cells from blood circulation. Because these cells express PS on their surface, which promotes blood coagulation, it appears that removal of aged/apoptotic cells is important for the procoagulant property of the cardiovascular system (10, 11).
MATERIALS AND METHODS
Cells.
Red blood cells (RBC) from fresh human blood were washed three times with PBS and resuspended at 20% hematocrit in PBS containing 0.1% glucose, then used as native RBC. Aged RBC were prepared by incubating cells in PBS (20% hematocrit) at 37°C for 4 days, except for the time-course experiment. Bovine aortic endothelial cells (BAE) were maintained as described previously (7). Jurkat and HL-60 cells were maintained in RPMI medium 1640 with 10% fetal bovine serum, 100 units⋅ml−1 of penicillin, 0.1 mg⋅ml−1 of streptomycin, and 250 ng⋅ml−1 of Fungizone (GIBCO). Chinese hamster ovary (CHO) cells stably expressing bovine LOX-1 (BLOX-1-CHO) and wild-type CHO-K1 cells were maintained as described previously (7).
Apoptosis Induction.
Apoptosis of HL-60 cells was induced by incubating cells with 50 μM cycloheximide at 37°C for 3 hr at a density of 1 × 106 cells⋅ml−1. Apoptosis of Jurkat cells was induced by incubating cells with 200 ng⋅ml−1 of anti-Fas IgM (Clone CH-11, Medical and Biological Laboratories, Nagoya, Japan) at 37°C for 6 hr at a density of 1 × 106 cells⋅ml−1. Apoptosis of BAE was induced by incubating cells with 50 ng/ml of recombinant human tumor necrosis factor α (R & D Systems) at 37°C for 6 hr.
RBC Binding.
RBC (hematocrit 1%) were incubated with BAE, CHO-K1 cells, or BLOX-1-CHO cells in the culture media at 37°C for 30 min. Unbound RBC were removed by extensive washing with the media, and the cells were fixed with PBS containing 4% formaldehyde and 0.2% glutaraldehyde. The number of bound RBC were enumerated (200–400 CHO cells or BAE were counted each time).
Apoptotic Cell Binding.
Apoptotic cells (1 × 106 cells⋅ml−1) were incubated with BAE, CHO-K1 cells, or BLOX-1-CHO cells in the culture media at 37°C for 30 min. Unbound cells were removed by extensive washing with the medium, and the cells were fixed with PBS containing 4% formaldehyde and 0.2% glutaraldehyde. The cells were stained with May-Giemsa, and the percentage of cells binding or ingesting one or more apoptotic cells was calculated (100–200 CHO cells or BAE were counted each time).
Lipoproteins.
Human LDL (d = 1.019–1.063) was isolated from fresh plasma by sequential ultracentrifugation as described previously (7). Oxidative modification and acetylation of LDL was carried out as described (12).
Preparation of Soluble LOX-1, LOX-Fc.
cDNA coding the extracellular domain of bovine LOX-1 (180–810) tagged with BamHI site was subcloned into BamHI site of pCd5lneg1 (13). Stable transformant of LOX-Fc (BLOX-Fc-CHO) was developed as described (7). LOX-Fc was prepared from the conditioned media of BLOX-Fc-CHO with Protein A-agarose (Bio-Rad) column chromatography.
Binding of Soluble LOX-1 to RBC.
RBC were fixed with 4% formaldehyde and 0.2% glutaraldehyde, and washed three times with PBS. RBC (1% hematocrit) then were incubated with 50 μg⋅ml−1 of LOX-Fc or normal human IgG in Ham’s F-12 containing 10% fetal bovine serum for 90 min at room temperature. After washing three times with medium, RBC were further incubated with biotinylated anti-human IgG (Fc) for 30 min at room temperature, stained by using the tyramide signal amplification procedure (TSA-direct green, DuPont/NEN) according to the manufacturer’s instructions and subjected to fluorescence microscopy.
Binding of Soluble LOX-1 to Apoptotic Cells.
Native or apoptotic Jurkat cells (1 × 106 cells⋅ml−1) were incubated with 50 μg⋅ml−1 of LOX-Fc or normal human IgG in the culture medium at 37°C for 60 min. Biotinylated anti-human IgG (1:250 dilution) was added, and the mixture was further incubated for 30 min at 37°C. The cells were fixed with 4% formaldehyde and 0.2% glutaraldehyde, washed three times with PBS, and stained by using tyramide signal amplification procedure (TSA-direct green, DuPont/NEN). Then, the cells were permeabilized with 0.1% Triton X-100, stained with 2.5 μg⋅ml−1 of propidium iodide, and examined by using a confocal microscopy system (Bio-Rad). Without permeabilization, staining of the cells with propidium iodide was not detectable.
PS Externalization on Aged RBCs and Apoptotic Cells.
Exposure of PS on the surface of aged RBC or apoptotic cells was detected by annexin V- fluorescein isothiocyanate using an ApoAlert Annexin V Apoptosis Kit (CLONTECH). Cells were analyzed by flow cytometry on a FACScalibur (Becton Dickinson).
Phospholipid Liposome Preparation.
Multilamellar liposomes were prepared containing the indicated phospholipid, phosphatidylcholine, and free cholesterol in a molar ratio of 1:1:1. The lipids were mixed in chloroform and dried under nitrogen gas. The dried lipids were resuspended in PBS at a final concentration of 10 mM. The mixture was mixed, sonicated for 10 min, and stored at 4°C until use.
RESULTS AND DISCUSSION
As shown in Fig. 1, we have found that BAE have a capacity to phagocytose aged and apoptotic cells. Because this phagocytotic activity was significantly inhibited by OxLDL, we examined whether LOX-1, the major OxLDL receptor in BAE (7), mediates it. The stable transformant, BLOX-1-CHO, bound and phagocytosed aged RBC and HL-60 cells undergoing apoptosis induced by cycloheximide treatment (Fig. 2 a–e). BLOX-1-CHO also bound and phagocytosed apoptotic Jurkat cells treated with anti-Fas antibody and apoptotic BAE treated with tumor necrosis factor åa (data not shown), suggesting that the engulfment of apoptotic cells by LOX-1 was not dependent on the kind of cells or the initiating signals. BLOX-1-CHO did not significantly bind native RBC or untreated HL-60 cells. Wild-type CHO cells did not bind aged RBC or apoptotic cells (data not shown). Thus, LOX-1 was found to mediate binding and phagocytosis of both aged RBC and apoptotic cells. The binding of aged RBC was dose-dependently inhibited by OxLDL and acetyl LDL but not by native LDL (Fig. 3a). The half-maximum inhibition by OxLDL was achieved at 0.1 μg⋅ml−1. In comparison with the reported concentrations at which OxLDL caused other biological responses (14–17), the effective concentration of OxLDL was very low in the present study.
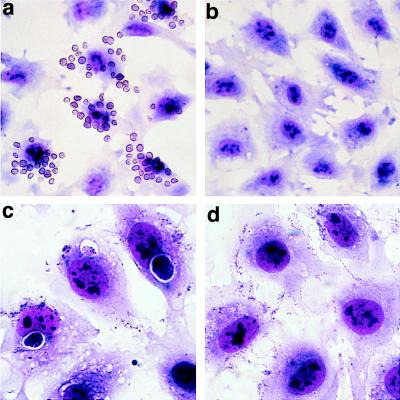
Figure 1.
Binding and phagocytosis of aged RBC and apoptotic cells by BAE. May-Giemsa stained preparations were observed under a light microscope. BAE were incubated with aged RBC (a) or apoptotic HL60 cells (c) as described in Materials and Methods. BAE did not bind native RBC (b) or untreated HL-60 cells (d). BAE also bound and phagocytosed apoptotic BAE induced by tumor necrosis factor α (data not shown).
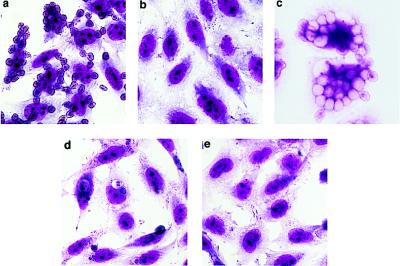
Figure 2.
Binding and phagocytosis of aged RBC and apoptotic cells by BLOX-1-CHO. BLOX-1-CHO bound aged RBC (a) and apoptotic HL-60 cells (d). BLOX-1-CHO did not bind native RBC (b) or untreated HL-60 cells (e). Aged RBC were engulfed by BLOX-1-CHO after 6-hr incubation (c). Wild-type CHO cells did not bind aged RBC or apoptotic cells (data not shown).
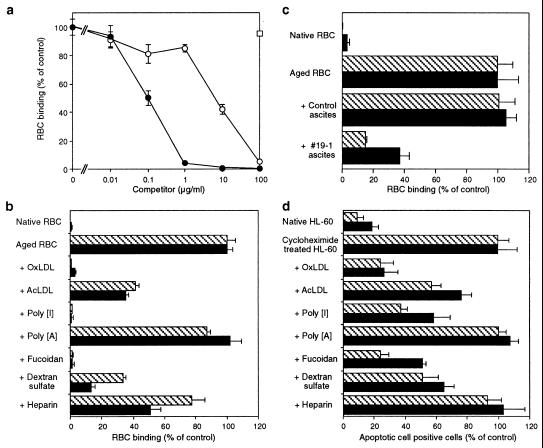
Figure 3.
(a) OxLDL (•) and acetyl LDL (AcLDL) (○) dose-dependently inhibited the binding of aged RBC binding to BLOX-1-CHO, but native LDL (□) did not. (b) Inhibition by various LOX-1 ligands of the binding of aged RBC to BLOX-1-CHO (cross-hatched bar) and BAE (solid bar). Inhibitors were used at a concentration of 50 μg⋅ml−1, except OxLDL and AcLDL, which were used at 10 μg⋅ml−1. (c) Inhibition by anti-BLOX-1 mAb (#19–1) of the binding of aged RBC to BLOX-1-CHO (cross-hatched bar) and BAE (solid bar). (d) Inhibition by various LOX-1 ligands of the binding of apoptotic HL-60 cells to BLOX-1-CHO (cross-hatched bar) and BAE (solid bar). All inhibitors were used at the concentration of 100 μg⋅ml−1. The values represent the mean ± SEM of triplicate determinations. Values of 100% control are the number of bound aged RBC or the number of CHO cells associated with cycloheximide-treated HL60 cells.
To examine whether LOX-1 works as the receptor for aged/apoptotic cells in BAE, we compared the inhibitory effects of various LOX-1 ligands between BAE and BLOX-1-CHO (Fig. 3b). The addition of OxLDL, fucoidan, and poly[I] eliminated most of the binding of aged RBC to BLOX-1-CHO. The weaker ligands of LOX-1, dextran sulfate (50 μg⋅ml−1), acetyl LDL (10 μg⋅ml−1), and heparin (50 μg⋅ml−1) also competed to a lesser extent. Poly(A), which is not a ligand of LOX-1, did not affect the binding, although poly[I], a ligand of LOX-1, competed well. The ligand specificity and rank order of the competition in BAE were the same as those in BLOX-1-CHO, suggesting that LOX-1 mediates the binding of aged RBC in BAE. Indeed, a mAb against LOX-1 (#19–1) blocked the binding of aged RBC to BAE as well as to BLOX-1-CHO (Fig. 3c).
The binding of apoptotic HL-60 to both BLOX-1-CHO and BAE competed with the compounds in the same rank order, suggesting LOX-1 also mediates the binding of apoptotic cells in BAE (Fig. 3d). The extent of inhibition was less than the case for aged RBC. This finding may be caused by the higher affinity of apoptotic cells to LOX-1 than the in vitro aged RBC.
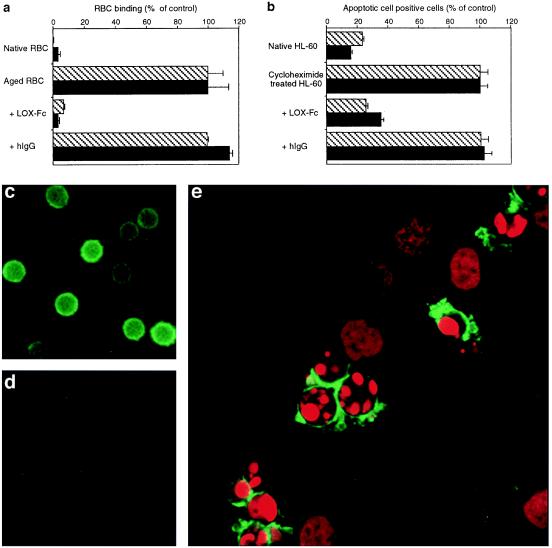
To confirm that LOX-1 directly recognized the subtle changes on the surface of aged and apoptotic cells, we used LOX-Fc, a fusion protein of the extracellular domain of LOX-1 and Fc region of human IgG. The use of LOX-Fc facilitates the detection of binding of LOX-1 to its ligand. Application of LOX-Fc to the culture medium effectively blocked the binding of both aged RBC (Fig. 4a) and apoptotic cells (Fig. 4b) to BLOX-1-CHO and BAE, whereas control human IgG did not affect the binding. Then, we tried staining aged cells and apoptotic cells with LOX-Fc. Staining with LOX-Fc visualized the putative LOX-1 ligand localized on the surface of aged RBC, but not on normal RBC (Fig. 4 c and d). Double staining with propidium iodide and LOX-Fc of anti-Fas antibody-treated Jurkat cells revealed that the putative LOX-1 ligand was selectively expressed on the surface of Jurkat cells with condensed chromatin, but not on the cells with uncondensed chromatin (Fig. 4e). This finding indicated that the expression of LOX-1 ligand on the surface of the cells occurred in the process of apoptosis.
Figure 4.
LOX-Fc inhibits the binding of aged RBC (a) and apoptotic HL-60 cells (b) to BLOX-1-CHO (cross-hatched bar) and BAE (solid bar). The extent of the binding of aged RBC and apoptotic HL60 cells was determined in the presence of LOX-Fc or normal hIgG (50 μg⋅ml−1). Data represent the mean ± SEM of triplicate determinations. Values of 100% control are the number of aged RBC or cycloheximide-treated HL-60 cells. Detection of LOX-1 ligand by LOX-Fc on the surface of aged RBC (c), but not native RBC (d). Binding of soluble LOX-1 to RBC was observed under a fluorescence microscope. (e) Confocal microscopy of Jurkat cells treated with anti-Fas IgM. The binding of soluble LOX-1 was visualized with tyramide- fluorescein isothiocyanate (green) and nuclei are stained with propidium iodide (red). Note that LOX-1 ligands were expressed only on the surface of apoptotic cells with condensed chromatin, and not on the cells with uncondensed chromatin. Staining with normal hIgG did not show any significant signals (not shown).
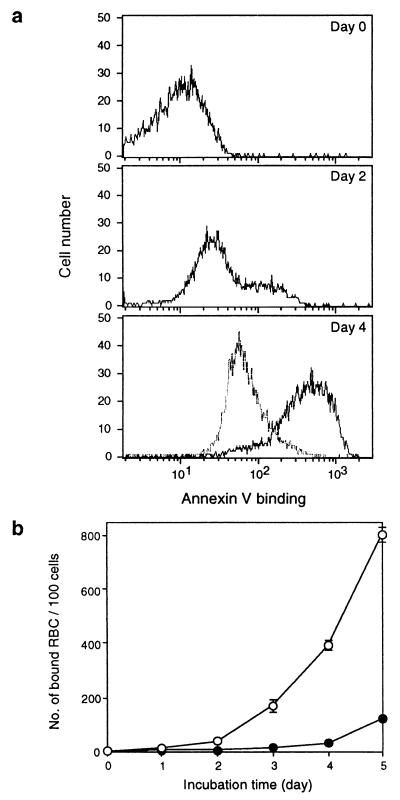
The anionic phospholipids of the plasma membrane in normal cells are asymmetrically distributed (18). Most of the phosphatidylinositol (PI) and virtually all of the PS resides in the inner leaflet of the plasma membrane. This asymmetry is maintained by the activity of the putative ATP-dependent translocase against “flip-flop” or “scrambling” movement of negative phospholipids (19, 20), and it is abolished under certain circumstances including apoptosis (21). This loss of asymmetry, PS externalization, has been regarded as a hallmark of the earliest process of apoptosis (2). Recently, a phagocytosing system, where apoptotic cells and aged cells compete with PS, has been reported (6, 22–24). This system appears to recognize the externalization of PS of the cells. In this context, we analyzed the relationship between the binding of aged/apoptotic cells to LOX-1 and PS. The exposure of PS, detected by the binding of annexin V, to the surface of aged RBC increased time-dependently as the case for apoptotic cells (Fig. 5a). These changes were accompanied by a time-dependent increase of the binding to BLOX-1-CHO (Fig. 5b). Physiological concentrations of glucose inhibited the exposure of PS to the surface of RBC (Fig. 5a), suggesting ATP depletion-dependent mechanisms are involved in PS externalization and also inhibited the increase in binding ability to BLOX-1-CHO during aging (Fig. 5b).
Figure 5.
Binding of aged RBC to LOX-1 is accompanied by PS externalization. (a) PS exposure on the surface of aged RBC. RBC (20% hematocrit) were incubated in PBS (solid line) or in PBS containing 0.1% glucose (dashed line) at 37°C for the indicated periods, and binding of annexin V-fluorescein isothiocyanate to RBC was analyzed by flow cytometry. A total of 5,000 cells were analyzed in each condition. (b) Binding of aged RBC to BLOX-1-CHO. RBC were incubated in PBS (○) or in PBS containing 0.1% glucose (•) as described above, and binding of RBC to BLOX-1-CHO was determined. Values represent the number of bound RBC per 100 BLOX-1-CHO cells and are expressed as the mean ± SEM of triplicate determinations.
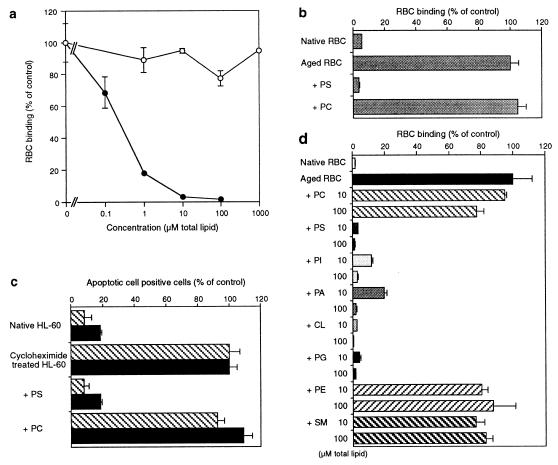
Furthermore, phospholipid liposomes containing PS inhibited the binding of aged RBC and apoptotic HL-60 to BLOX-1-CHO and BAE, whereas phosphatidylcholine (PC) liposome had no effect (Fig. 6 a–d). Inhibition by PS liposome was found to be concentration dependent, and complete inhibition was achieved at 1 μM of total phospholipid. Inhibition was not restricted to PS liposomes, anionic liposomes containing PI, phosphatidic acid, phosphatidylglycerol, and cardiolipin also inhibited the aged RBC binding, whereas no inhibition was observed by liposomes that consisted of neutral phospholipids such as phosphatidylethanolamine and sphingomyeline (Fig. 6e). The liposomes containing these negative phospholipids did not merely compete, but also were taken up by BLOX-1-CHO (data not shown). These results suggest that LOX-1 recognizes the exposure of PS or other anionic phospholipids on the plasma membrane induced by aging or apoptosis.
Figure 6.
Inhibitory effects of anionic phospholipid-liposomes on the binding of aged RBC and apoptotic HL-60 cells. (a) Binding of aged RBC to BLOX-1-CHO was inhibited by PS liposomes (•) dose-dependently, but not by PC liposomes (○). (b) Competition by PC and PS liposomes of the binding of aged RBC to BAE. The liposomes were used at 100 μM (total lipid). (c) Binding of apoptotic HL-60 cells to BLOX-1-CHO (cross-hatched bar) and BAE (solid bar) was inhibited by PS liposomes (100 μM total lipid), but not by the same amount of PC liposomes. (d) Inhibition by various phospholipid liposomes of the binding of aged RBC to BLOX-1-CHO. Binding of aged RBC was inhibited by anionic phospholipid liposomes, including PS, PI, phosphatidic acid (PA), cardiolipin (CL), and phosphatidylglycerol (PG), but not neutral phospholipid liposomes, PC, phosphatidylethanolamine (PE), and sphingomyeline (SM). Data represent the mean ± SEM of triplicate determinations. Values of 100% are the numbers of bound aged RBC or the numbers of BLOX-1-CHO associated with cycloheximide-treated HL-60 cells.
In the present study, the activity of phagocytosis was shown to be mostly mediated by LOX-1 (estimated as three-quarters from the efficiency of blocking antibody). In macrophages, several receptors, including CD36 (3) and class A scavenger receptors (4), have been reported to bind and/or phagocytose aged/apoptotic cells. Presumably they recognize different kinds of cells in different stages of apoptosis and aging. In contrast to macrophages, the lower molecular redundancy in endothelial cells suggests that LOX-1 is optimal for recognition of the change of apoptotic cells around them, e.g., blood cells, smooth muscle cells, and endothelial cells themselves.
Interestingly, all of the above receptors are known to bind OxLDL (25, 26). OxLDL may have the similar structure to that specifically expressed in apoptotic cells, such as PS. Although PS have been reported to be a competitive inhibitor of phagocytosis of apoptotic cells in peritoneal macrophages (6) and sertori cells (27), the inhibition by PS of the phagocytosis mediated by CD36 or class A scavenger receptors is unclear. There are conflicting reports regarding CD36. Ryeom et al. (5) demonstrated that the binding of apoptotic photoreceptor outer segment to CD36 in retinal pigment epithelium is inhibited by PS, whereas Fadok et al. (22) reported that the phagocytosis by monocyte-derived macrophages mediated by αvβ3 integrin/CD36 is not inhibited by PS. The latter phagocytosis depended rather on the RGDS sequence of thrombospondin (22). Macrosialin is thought to be a candidate molecule for the phagocytosis, because it binds both PS and OxLDL in macrophages. However, no direct evidence using an expression system has been provided so far (28). In the present study, we demonstrated that LOX-1-mediated phagocytosis was inhibited by both PS and OxLDL in both transfected cells and native endothelial cells. LOX-1 might be a part of the phagocytosing system in other kinds of cells, because LOX-1 also is expressed in several kinds of cells including macrophages (unpublished data).
It was reported that apoptotic cells are the focus of blood coagulation because of the procoagulant activity of PS on the surface of the cells (10, 11). Thus to maintain the anticoagulant state of blood, PS-exposing cells must be removed from circulation. This function may largely depend on the reticuloendothelial system, but the present results suggest the importance of a removal system in situ in vascular endothelial cells. LOX-1 may have developed in the process of evolution as one of the molecules that mediates the anticoagulant activity of endothelial cells. We suggest that the anticoagulant function of LOX-1 inhibited by OxLDL be considered in the promoted thrombosis in the pathophysiology of atherosclerosis. The circulation of aged/apoptotic cells, which should have been removed, also might affect other conditions in physiological homeostasis such as the immunological state (29), modulating the pathophysiology of atherosclerosis.
Lectin-like molecules have been speculated to be involved in phagocytosing systems for aged/apoptotic cells (30–32). LOX-1, with a lectin-like structure, also might be involved in the phagocytosis in these systems, although the binding specificity to carbohyrates remains to be determined.
In summary, we identified LOX-1 as the phagocytotic receptor in endothelial cells for aged/apoptotic cells, recognizing one of the earliest steps in the apoptotic process, the exposure of PS on the surface of the cells.
Acknowledgments
We gratefully acknowledge Dr. Tasuku Honjo for the critical reading of the manuscript and Dr. Brian Seed for pCd5lneg1. This work was supported, in part, by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan.
ABBREVIATIONS
- PS
phosphatidylserine
- OxLDL
oxidized low-density lipoprotein
- LOX-1
lectin-like OxLDL receptor 1
- BAE
bovine aortic endothelial cells
- CHO
Chinese hamster ovary
- BLOX-1-CHO
CHO cells expressing bovine LOX-1
- RBC
red blood cells
- PI
phosphatidylinositol
- PC
phosphatidylcholine
References
- 1.Savill J, Fadok V, Henson P, Haslett C. Immunol Today. 1993;14:131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- 2.Martin S J, Reutelingsperger C P, McGahon A J, Rader J A, van Schie R C, LaFace D M, Green D R. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savill J, Hogg N, Ren Y, Haslett C. J Clin Invest. 1989;90:1513–1522. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Platt N, Suzuki H, Kurihara Y, Kodama T, Gordon S. Proc Natl Acad Sci USA. 1996;93:12456–12460. doi: 10.1073/pnas.93.22.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryeom S W, Silverstein R L, Scotto A, Sparrow J R. J Biol Chem. 1996;271:20536–20539. doi: 10.1074/jbc.271.34.20536. [DOI] [PubMed] [Google Scholar]
- 6.Sambrano G R, Steinberg D. Proc Natl Acad Sci USA. 1995;92:1396–1400. doi: 10.1073/pnas.92.5.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T, Masaki T. Nature (London) 1997;385:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 8.Kume N, Sawamura T, Moriwaki T, Itokawa S, Hoshikawa H, Aoyama T, Nishi E, Ueno Y, Masaki T, Kita T. Circulation. 1997;96:I–104. [Google Scholar]
- 9.Nagase M, Hirose S, Sawamura T, Masaki T, Fujita T. Biochem Biophys Res Commun. 1997;237:496–498. doi: 10.1006/bbrc.1997.7176. [DOI] [PubMed] [Google Scholar]
- 10.Bevers E M, Comfurius P, Zwaal R F. Lupus. 1996;5:480–487. doi: 10.1177/096120339600500531. [DOI] [PubMed] [Google Scholar]
- 11.Casciola R L, Rosen A, Petri M, Schlissel M. Proc Natl Acad Sci USA. 1996;93:1624–1629. doi: 10.1073/pnas.93.4.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kume N, Arai H, Kawai C, Kita T. Biochim Biophys Acta. 1991;1091:63–67. doi: 10.1016/0167-4889(91)90223-k. [DOI] [PubMed] [Google Scholar]
- 13.Zettlmeissl G, Gregersen J-P, Duport J M, Mehdi S, Reiner G, Seed B. DNA Cell Biol. 1990;9:347–353. doi: 10.1089/dna.1990.9.347. [DOI] [PubMed] [Google Scholar]
- 14.Kugiyama K, Kerns S A, Morrisett J D, Roberts R, Henry P D. Nature (London) 1990;344:160–162. doi: 10.1038/344160a0. [DOI] [PubMed] [Google Scholar]
- 15.Murugesan G, Chisolm G M, Fox P L. J Cell Biol. 1993;120:1011–1019. doi: 10.1083/jcb.120.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon B C, Cunningham L D, Cohen R A. J Clin Invest. 1990;86:75–79. doi: 10.1172/JCI114718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan B V, Parthasarathy S S, Alexander R W, Medford R M. J Clin Invest. 1995;95:1262–1270. doi: 10.1172/JCI117776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zachowski A. Biochem J. 1993;294:1–14. doi: 10.1042/bj2940001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zachowski A, Devaux P F. Experientia. 1990;46:644–656. doi: 10.1007/BF01939703. [DOI] [PubMed] [Google Scholar]
- 20.Tang X, Halleck M S, Schlegel R A, Williamson P. Science. 1996;272:1495–1497. doi: 10.1126/science.272.5267.1495. [DOI] [PubMed] [Google Scholar]
- 21.Schlegel R A, Callahan M, Krahling S, Pradhan D, Williamson P. Adv Exp Med Biol. 1996;406:21–28. doi: 10.1007/978-1-4899-0274-0_3. [DOI] [PubMed] [Google Scholar]
- 22.Fadok V A, Savill J S, Haslett C, Bratton D L, Doherty D E, Campbell P A, Henson P M. J Immunol. 1992;149:4029–4035. [PubMed] [Google Scholar]
- 23.Fadok V A, Voelker D R, Campbell P A, Cohen J J, Bratton D L, Henson P M. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 24.Fadok V A, Laszlo D J, Noble P W, Weinstein L, Riches D W, Henson P M. J Immunol. 1993;151:4274–4285. [PubMed] [Google Scholar]
- 25.Endemann G, Stanton L W, Madden K S, Bryant C M, White R T, Protter A A. J Biol Chem. 1993;268:11811–11816. [PubMed] [Google Scholar]
- 26.Freeman M, Ekkel Y, Rohrer L, Penman M, Freedman N J, Chisolm G M, Krieger M. Proc Natl Acad Sci USA. 1991;88:4931–4935. doi: 10.1073/pnas.88.11.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiratsuchi A, Umeda M, Ohba Y, Nakanishi Y. J Biol Chem. 1997;272:2354–2358. doi: 10.1074/jbc.272.4.2354. [DOI] [PubMed] [Google Scholar]
- 28.Ramprasad M P, Fischer W, Witztum J L, Sambrano G R, Quehenberger O, Steinberg D. Proc Natl Acad Sci USA. 1995;92:9580–9584. doi: 10.1073/pnas.92.21.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kornbluth R S. Immunol Lett. 1994;43:125–132. doi: 10.1016/0165-2478(94)00149-9. [DOI] [PubMed] [Google Scholar]
- 30.Duvall E, Wyllie A H, Morris R G. Immunology. 1985;56:351–358. [PMC free article] [PubMed] [Google Scholar]
- 31.Dini L, Autuori F, Lentini A, Oliverio S, Piacentini M. FEBS Lett. 1992;296:174–178. doi: 10.1016/0014-5793(92)80373-o. [DOI] [PubMed] [Google Scholar]
- 32.Hall S E, Savill J S, Henson P M, Haslett C. J Immunol. 1994;153:3218–3227. [PubMed] [Google Scholar]