Abstract
The airway surface is an important host defense against pulmonary infection. Secretion of proteins with antimicrobial activity from epithelial cells onto the airway surface represents an important component of this innate immune system. Defensins are the best characterized epithelial-derived peptide antibiotics. A member of another family of peptide antibiotics called cathelicidins recently was identified from human bone marrow. We show in this paper that this human peptide named LL-37/hCAP-18 also may play a role in innate immunity of the human lung. In situ hybridization localized high levels of LL-37/hCAP-18 RNA to surface epithelial cells of the conducting airway as well as serous and mucous cells of the submucosal glands. LL-37/hCAP-18 peptide with antimicrobial activity was partially purified from airway surface fluid from human lung and a human bronchial xenograft model. The synthetic peptide LL-37 demonstrated antibiotic activity against a number of Gram-negative and Gram-positive organisms including Pseudomonas aeruginosa; bacterial killing of LL-37 was sensitive to NaCl and was synergistic with lactoferrin and lysozyme. In summary, we show that LL-37/hCAP-18 is a peptide with broad antimicrobial activity that is secreted onto the airway surface from epithelial cells of the human lung.
The innate immune system of the lung prevents the development of respiratory infections. A number of components contribute to this important host defense, including intact epithelia with mucociliary clearance, antimicrobial activity of the airway surface fluid, opsonization with surfactant protein, and bactericidal activity of the alveolar macrophages. Fluid lining the airway surface represents a complex mixture whose composition is critical for normal lung function. A number of molecules with antimicrobial activity are secreted onto the surface, including enzymes (e.g., lysozyme and secretory phospholipase A2), binding proteins (e.g., lactoferrin), cationic nuclear proteins (i.e., histones), and antimicrobial peptides (1, 2). The relative contribution each molecule plays toward the establishment of effective host defense is unclear.
One of the best characterized families of antimicrobial peptides is defensins, which are small cationic, cystine-rich peptides of broad antimicrobial activity (3, 4). Classic defensins have been isolated primarily from myeloid-derived cells, such as neutrophils and macrophages, where they reside in cytoplasmic granules and contribute to nonoxidative microbial killing. The first epithelial-derived defensin, identified and cloned from cow trachea, was tracheal antimicrobial peptide (TAP) (5). A homologous peptide called lingual antimicrobial peptide (LAP) was recovered from cow tongue (6). A human homolog of these β-defensins, called human β-defensin 1 (hBD-1), initially was purified from hemodialysate (7) and subsequently shown to be expressed in the surface epithelia and submucosal glands of the human airways (8, 9). hBD-1 has been implicated in pathogenesis of cystic fibrosis (CF) where it appears to be inactivated by the high-salt milieu of the CF surface fluid (8).
Another family of peptide antibiotics that is receiving increased attention is the cathelicidins (10–12). These peptides contain a highly conserved signal sequence and pro-region (“cathelin”) but show substantial heterogeneity in the C-terminal domain that encodes the mature peptide, which can range in size from 12 to 80 or more amino acids (13–15). The first human cathelicidin, LL-37/hCAP-18, was cloned from cDNA isolated from human bone marrow (16–18), and its gene was mapped (19–20). Subsequent studies have shown that LL-37/hCAP-18 is primarily expressed in myeloid cells where it resides in granules (21); expression of this gene also has been demonstrated in testis (16) and inflamed skin (22).
We demonstrate in this study that LL-37/hCAP-18 is expressed in humans diffusely throughout epithelia in many organs, including surface epithelia of the airways and serous and mucous cells of the proximal airway submucosal glands. Peptide with broad antimicrobial activity against Gram-positive and Gram-negative organisms is secreted into the airway surface fluid.
MATERIALS AND METHODS
General Methodology.
The cDNA of LL-37/hCAP-18 (16), cloned into pBluescript II KS−, and the polyclonal antibody against LL-37/hCAP-18 raised by injection of LL-37 into rabbits (20) were obtained from G. H. Gudmundsson (Karolinska Institute, Stockholm, Sweden).
RNA Extraction, Reverse Transcription–PCR (RT-PCR), and Characterization of the Products.
Poly(A)+ RNA was isolated from human large airways of healthy lungs by using Trizol (GIBCO/BRL) and oligo(dT) column chromatography. The lungs were explanted organs for which no suitable recipient was found. Additionally, total RNA was isolated from human primary respiratory epithelial cells (Clonetics, San Diego), grown in serum-free medium. Three primers specific to the LL-37/hCAP-18 cDNA were designed from the published LL-37/hCAP-18 cDNA sequence (GenBank accession no. Z38026) for RT-PCR as follows: GAA TTC CGG CCA TGA AGA CCC (fall 1, nucleotide 1–21), CAG AGC CCA GAA GCC TGA GC (fall 2, nucleotide 541–560), GAC TCT GTC CTG GGT ACA AGA TTC (fall 8, nucleotide 497–520). PCR was performed with the primer combinations fall 1/fall 2 and fall 1/fall 8. The PCR products were analyzed in a 1.5% agarose gel and blotted onto nylon membrane (Boehringer Mannheim). The Southern blots were hybridized with a 32P-dCTP random primer-labeled LL-37/hCAP-18 cDNA probe (Rediprime DNA labeling system, Amersham) and washed at high stringency. Results were recorded by autoradiography.
Dot Blot Analysis.
A nylon filter with dotted mRNAs from 50 different human organs was purchased (Human RNA Master Blot, CLONTECH), hybridized separately with a 32P-dCTP random primer-labeled probe of LL-37/hCAP-18 and ubiquitin cDNA, and washed under high stringency conditions. The signal of each mRNA dot was quantified by using a PhosphorImager 445 SI (Molecular Dynamics). The expression data of LL-37/hCAP-18 were normalized by using the signal quantities for the housekeeping gene ubiquitin. Hybriziation of the blot with a probe for this housekeeping gene resulted in almost identical signals for all of the samples from human organs.
In Situ Hybridization.
Various human tissues were embedded in OCT (Tissue–Tek, Miles), cryosectioned (6 μm), mounted on slides, and fixed in 4% paraformaldehyde in PBS (pH 7.4) for 4 hr at 4°C. After dehydration through graded concentrations of ethanol, sections were desiccated overnight under vacuum and stored at −20°C. The next day, sections were treated with 10 μg/ml of proteinase K for 30 min at 30°C, rinsed twice in 0.2× standard saline citrate (SSC) for 30 sec each time, fixed in 4% paraformaldehyde in PBS, rinsed twice in 0.1 M triethanolamine (pH 8.0) for 4 min each time, incubated with 0.25% acetic anhydride in 0.1 M triethanolamine for 10 min at room temperature, and dehydrated through ethanol. After drying under vacuum, prehybridization was performed for 4 hr at 54°C in 10 mM Tris (pH 8.0), 50% formamide, 2.5× Denhardt’s solution, 0.6 M NaCl, 1 mM EDTA, 0.1% SDS, 500 μg/ml of tRNA, and 10 mM DTT. RNase control sections were treated with 200 μg/ml of RNase A for 1 hr at 37°C before the prehybridization step. Sections then were hybridized with digoxigenin-labeled antisense or sense probes for 16 hr at 54°C in the prehybridization solution. Probes were synthesized by in vitro transcription by the SP6 or T7 RNA polymerase of full-length LL-37/hCAP-18 cDNA cloned in pBluescript II KS−. For immunological detection of the probe, sections were incubated with antibodies against digoxigenin conjugated with alkaline phosphatase (Boehringer Mannheim) followed by a solution of nitro-blue tetrazolium (NBT)/5-bromo-4-chloro-3-indolyl-phosphate (BCIP).
In situ hybridization of human proximal airway using LL-37/hCAP-18-specific sense and antisense riboprobes was performed as described above. For immunodetection, slides were incubated with antibodies to digoxigenin conjugated with Texas Red (Boehringer Mannheim). After in situ hybridization, the sections were treated with PBS containing 20% goat serum for 30 min at room temperature and incubated for 90 min in 1.5% goat serum/PBS containing 28 μg/ml of rabbit anti-human lysozyme (Dako). After washes, the sections were incubated with fluorescein isothiocyanate-conjugated anti-rabbit IgG-antibody (goat) and covered with mounting medium containing 1.5 μg/ml of 4′, 6-diamidine-2′-phenylindole dihydrochloride (DAPI) (Vector Laboratories).
Western Blot Analysis of Airway Surface Fluid from Human Lung and Human Bronchial Xenografts.
The airways of explanted lungs were lavaged by using 150 ml of PBS. Further, airway surface fluid was expelled with air from a human bronchial xenograft model as previously described (8). In short, respiratory primary cells removed from human bronchus by digestion with protease 14 (Sigma) and maintained in culture for 5–7 days were seeded (2 × 106 cells per graft, in 30 μl of hormonally defined growth medium; Clonetics) in tracheas obtained from CO2-asphyxiated Fisher 344 rats, from which the epithelium was denuded by three rounds of freeze thawing. These tracheas were ligated to plastic tubing, implanted s.c. in the flanks of nu/nu BALB/c mice and maintained for 3 weeks to allow for maturation of a fully differentiated epithelium. The collected liquids from lung and xenografts were extracted in acetonitrile (final concentration 60%) and trifluoroacetic acid (TFA, final concentration 1%) overnight at room temperature. After lyophilization, the substance was resuspended in water, cleared by centrifugation at 10,000 g for 20 min, and further purified on Beckman Ultrasphere C-18 RP-HPLC columns (4.6 mm × 25 cm or 10 mm × 25 cm) using a linear gradient of acetonitrile with 0.1% TFA. Fractions were dried by vacuum centrifugation, resuspended in 50 μl of distilled water, and tested for antimicrobial activity in agarose diffusion assays (see below). From each fraction, 3 μl was dotted onto a nitrocellulose membrane, and immunolabeling was performed by using the polyclonal rabbit antibody to LL-37/hCAP-18 (1:800) followed by a monoclonal peroxidase-conjugated anti-rabbit antibody from goat (Sigma). After washes, bound antibodies were visualized by using chemoluminescent substrate (ECL Western blotting detection kit, Amersham) and exposure to x-ray films. Positive fractions were used in a SDS/PAGE using a 15% tris-tricine running gel. After electrophoretic separation, the proteins were blotted onto nitrocellulose, and LL-37/hCAP-18 immunoreactive bands were visualized as described above. Further, samples of immunopositive fractions were subjected to mass spectrometry (Voyager BioSpectrometry Workstation, PerSeptive Biosystems).
Peptide Synthesis and Antimicrobial Assays.
To screen chromatography fractions for antibacterial activity the following agarose diffusion assay system was used. Ten milliliters of quarter-strength Luria–Bertani medium (2.5 g/liter tryptone/1.25 g/liter yeast extract/1.25 g/liter NaCl) containing 0.7% agarose were poured into a 150-mm plastic dish. After solidification, a second layer of 10 ml medium containing Escherichia coli D31 (23) was poured onto the first layer. The bacteria (5 × 108 colony-forming units) were added to the 42°C warm medium shortly before application, followed by short vortexing. Finally, 2 μl of each chromatography fraction was applied onto the solid surface of the agarose and air-dried. After incubation for 12 hr at 37°C, fractions with antimicrobial activity were determined visually.
Chemical synthesis of LL-37 was performed by using an automatic peptide synthesizer, and quality of the procedure was monitored by mass spectrometry and capillary electrophoresis (Louisiana State University Medical Center, Core Laboratories). Serial dilutions of the peptide (stock solution of 500 μg/ml) were made in water using polypropylene tubes and used in various antibacterial assays against the following bacteria: Escherichia coli D31, E. coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Enterococcus faecalis ATCC 29212, and Staphylococcus aureus ATCC 29213. Bacteria were grown in the recommended growth media. The minimal inhibitory concentration (MIC) of LL-37 alone (with or without addition of medium E (50× medium E: 10 g/liter of MgSO4 × 7 H2O, 100 g/liter of citric acid × H2O, 500 g/liter of K2HPO4, 175 g/liter of NaNH4HPO4 × H2O; ref. 24), LL-37 in various concentrations of NaCl and LL-37 in mixtures with lysozyme from human milk (Sigma), lactoferrin from human milk (Sigma), and as negative control phospholipase A2 from bovine pancreas (Sigma) were determined by a microdilution method using polypropylene 96-well tissue culture plates and reading the OD595 after 12 hr. The MIC was the lowest antimicrobial concentration at which there was no visible turbidity or increase of the OD595 after 12 hr. All experiments were repeated four times. For MIC testing of LL-37 alone, 2-fold serial dilutions were prepared in half-strength Mueller-Hinton broth. Bacteria were grown overnight in the appropriate media and inocula of approximately 1 × 105 colony-forming units were added to each well. The total volume in each well was 50 μl, and the Mueller-Hinton broth was finally diluted 1:2.5 (containing 60 mM NaCl as measured by ion-sensitive electrodes). After 12 hr of shaking at 200 rpm at 37°C, bacterial growth was determined by visual analysis and determining the OD595 of each well. To determine whether medium E increases the antimicrobial activity, the experiments were repeated in half-strength Mueller-Hinton broth and 1 × medium E (16 mM Na+/30 mM K+/0.8 mM Mg2+). For testing the dependency of antimicrobial activity of LL-37 on the concentration of NaCl, E. coli D31 was used in microdilution assays combining serial dilutions of LL-37 and NaCl in quarter-strength Luria–Bertani medium (2.5 g/liter of tryptone, 1.25 g/liter of yeast extract, NaCl to obtain final concentration between 20 and 300 mM as determined by ion-sensitive electrodes). To test whether LL-37 acts synergistically with other antimicrobial substances found in mucosal secretions, we performed antibacterial assays combining serial dilutions of LL-37 with lysozyme (500 μg/ml) and lactoferrin (1 mg/ml, 250 μg/ml for Staphylococcus aureus ATCC 29213), applying concentrations that were at least one-quarter of the MIC of the substance or less. Pancreatic phospholipase A2 (1.25 mg/ml) was used as negative control. Inocula and growth conditions were the same as described for the MIC determination.
RESULTS
LL-37/hCAP-18 Is Expressed in Epithelia of a Number of Human Organs.
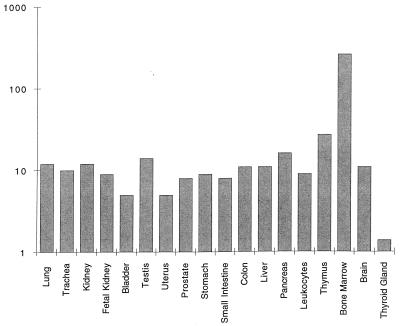
A previous RNA hybridization study demonstrated expression of LL-37/hCAP-18 primarily in testis and bone marrow (16). Techniques of in situ hybridization and immunocytochemistry also detected LL-37/hCAP-18 in inflamed human epidermal cells (22). We screened mRNAs isolated from more than 50 human organs using dot blot hybridization technique. A commercially available nylon filter dotted with the tissue-derived mRNAs was hybridized to a LL-37/hCAP-18-specific probe. The resulting autoradiograph was quantified on a PhosphorImager, and the expression levels were normalized by using the signal intensities of the cDNA probe of a housekeeping gene hybridized to the same filter. Fig. 1 presents results from mRNAs of all organs, which demonstrated a hybridization signal in addition to one negative sample (i.e., thyroid gland). The highest level of LL-37/hCAP-18 RNA was found in bone marrow consistent with the previous report (16). Ten- to 20-fold lower RNA levels were found in a number of other organ systems, including the lung, urogenital system, and gastrointestinal tract.
Figure 1.
Tissue distribution of LL-37/hCAP-18 expression in human tissues. A filter dotted with mRNA from a number of human tissues was hybridized to a LL-37/hCAP-18 probe. Signals were quantified by using a PhosphorImager system and normalized to the expression of the housekeeping gene ubiquitin. Data are expressed as relative hybridization signals and are plotted on a log scale. Only those tissues that demonstrated a significant signal over background are presented, except for thyroid gland, which is an example of a negative organ. The experiment was repeated on three occasions with virtually identical results.
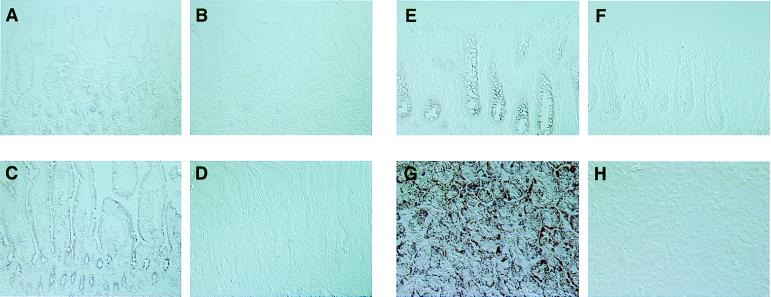
Human tissues from selected organs were obtained and analyzed for expression of LL-37/hCAP-18 by in situ hybridization using alkaline phosphatase as a reporter. LL-37hCAP-18 RNA was detected diffusely throughout the epithelium of the proximal bowel including the stomach (Fig. 2A) and duodenum (Fig. 2C) as well as the large bowel (Fig. 2E). Hybridization to the antisense probe was seen throughout the acinar cells of the pancreas (Fig. 2G) and the collecting system of the kidney (data not shown). Specificity of the assay was demonstrated in sections hybridized to a sense probe (Fig. 2 B, D, F, and H) or with an antisense probe after treatment with RNase (data not shown).
Figure 2.
In situ hybridization of LL-37/hCAP-18 in epithelia of nonpulmonary tissues. A subset of tissues positive by dot blot analysis was analyzed by in situ hybridization using a probe specific for LL-37/hCAP-18. In each case, tissue sections were hybridized to both an antisense and a sense probe. The following tissues were analyzed: stomach (antisense, A and sense, B), small bowel (antisense, C and sense, D), colon (antisense, E and sense, F), and pancreas (antisense, G and sense, H). In each case the magnification is ×100.
Expression of LL-37/hCAP-18 in Human Lung and Its Secretion onto the Airway Surface.
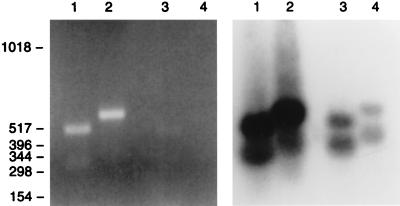
RNA hybridization experiments, shown in Fig. 1, indicated expression of LL-37/hCAP-18 in trachea and lung. The expression of LL-37/hCAP-18 in respiratory epithelial cells was confirmed by RT-PCR in experiments presented in Fig. 3. RNA isolated from whole lung and primary airway epithelial cells was analyzed by using two different sets of primers specific for LL-37/hCAP-18. The predicted bands for LL-37/hCAP-18 transcripts were amplified from RNA of total human lung (Fig. 3, lanes 1 and 2) and to a lesser extent from the primary airway epithelial cells (Fig. 3, lanes 3 and 4). Hybridization to a LL-37/hCAP-18 probe confirmed the identification of amplified bands (Fig. 3).
Figure 3.
Detection of LL-37/hCAP-18 RNA in airway tissue and cultivated respiratory epithelial cells by RT-PCR. Poly(A)+ RNA was isolated from human lung (lanes 1 and 2) and cultured airway epithelial cells (lanes 3 and 4) and subjected to RT-PCR by using two different sets of primers specific for LL-37/hCAP-18. The PCR mixtures were fractionated on an agarose gel that was stained with ethidium bromide (Left). DNA from the gel was transferred to a filter, which was hybridized to a LL-37/hCAP-18 probe (Right). An autoradiograph of this experiment is shown. Lanes 1 and 3 represent reactions using the primer combination fall 1 and fall 2, whereas lanes 2 and 4 are reactions using combination fall 1 and fall 8. Molecular weight markers measured as base pairs are shown along the left border.
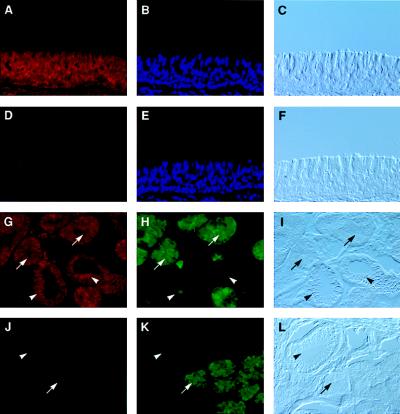
Tissues from a number of human lung samples were analyzed by fluorescent in situ hybridization. These included surgical specimens as well as organs harvested but not used for lung transplantation. Representative micrographs are shown in Fig. 4. Hybridization of the antisense LL-37/hCAP-18 probe to sections from proximal airway demonstrated uniform staining throughout the surface epithelia (Fig. 4A) as well as components of the submucosal glands (Fig. 4G). Further experiments were performed to determine the cell types within the submucosal glands that express LL-37/hCAP-18. The serous tubules are the most distal part of the glands, which contain cells that secrete a number of proteins and peptides, including lysozyme and lactoferrin (25). Serous cells are also the predominant site of cystic fibrosis transmembrane conductance regulator (CFTR) expression in human lung (26). The more proximal mucous tubules have large secretory granules primarily comprised of mucins. The collecting duct of the gland empties into the lumen of the airway surface. Serous and mucous tubules are indicated in Fig. 4 G-L by arrows and arrowheads, respectively. In situ hybridization experiments were performed together with immunocytochemistry for lysozyme to specifically mark the serous tubules (Fig. 4 H and K). Expression of LL-37/hCAP-18 in submucosal glands was found in both serous and mucous cells of the submucousal glands (Fig. 4 G–I). Morphology of the submucosal glands was demonstrated in unstained sections by Nomarski optics in Fig. 4 I and L. Specificity of the in situ hybridization was confirmed with a sense probe (Fig. 4 D–F and J–L) as well as in sections treated with RNase before hybridization to the antisense probe (data not shown). Expression of LL-37/hCAP-18 in surface epithelia of the distal airway was markedly reduced (data not shown). A similar distribution of LL-37/hCAP-18 mRNA expression was found in lung tissues harvested from a number of CF patients and the surface epithelium of human bronchial xenografts (data not shown).
Figure 4.
Fluorescent in situ hybridization of LL-37/hCAP-18 in proximal human lung. Sections of human lung were hybridized to a LL-37/hCAP-18 RNA probe and immunolabeled by using an antibody to lysozyme as described in Materials and Methods. (A–F) The bronchial surface epithelium, hybridized to the LL-37/hCAP-18 antisense (A) or sense probe (D), stained with 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI) (B and E) and visualized by Nomarski optics (C and F). (G–L) Specific evaluation of the submucosal glands, showing the hybridization to the LL-37/hCAP-18 antisense (G) or sense probe (J), the colocalization with lysozyme by immunohistochemistry (H and K), and the visualization by Nomarski optics (I and L). Arrows indicate serous cells and arrowheads indicate mucous cells. In each case the magnification is ×100.
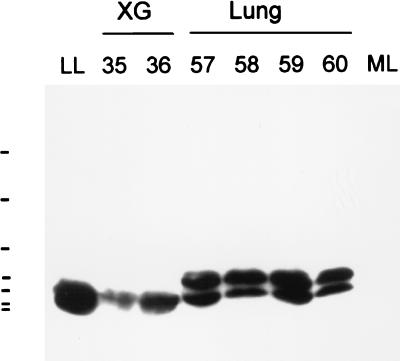
Additional experiments were performed to determine whether LL-37/hCAP-18 is indeed secreted into the airway surface fluid. Lungs obtained from nondiseased humans that were harvested for transplantation but not used were lavaged with PBS. Further, airway surface fluid from a human bronchial xenograft model was used for this experiment to avoid a possible contamination with human inflammatory cells. The lavage fluids were extracted in acetonitrile, concentrated by lyophilization, and fractionated over reverse-phase HPLC. Peptides were eluted by using a linear gradient of acetonitrile plus 0.1% TFA. The resulting fractions were recovered and analyzed for antimicrobial activity. Several peaks of bacterial killing were observed; an aliquot of each fraction was subjected to dot blot analysis using an antibody specific for LL-37/hCAP-18. Fractions that showed antimicrobial activity and were immunopositive were analyzed by Western blot analysis (Fig. 5). The peaks eluting at approximately 48% acetonitrile did contain detectable quantities of LL-37 based on Western blot analysis. In airway surface fluid from xenografts, one band of approximately 4.5-kDa mass was detected, whereas two forms of the peptide were found in fluid obtained from human lung, including the mature form of approximately 4.5-kDa mass and a substantially larger version, which may represent the unprocessed precursor. Using mass spectrometry, in two of the fractions from airway surface fluid obtained from human lung, a substance of 4496.8 Da was detected (data not shown).
Figure 5.
Western blot analysis of HPLC purified airway surface fluid. Airway surface fluid from human lung and a human bronchial xenograft model was extracted, lyophylized, resuspended in water, and fractionated over an HPLC column. Fractions that showed antimicrobial activity and positive immunoreactivity with the anti-LL-37/hCAP-18 antibody in dot blot analysis were analyzed by Western blot. Shown are the results of the Western blot for fractions 35 and 36 from the xenograft (XG) and 57 to 60 from human lung (Lung). The control is 40 ng of synthetic LL-37 (lane LL); 20 μg of a low molecular weight fraction from total mouse lung was loaded into lane ML. Bars along the left border indicate molecular mass markers of 2.3, 3.4, 6.5, 14.3, 21.5, 30.0, and 46.0 kDa.
LL-37/hCAP-18 Has Broad Antimicrobial Activity That Is Synergistic with Other Proteins.
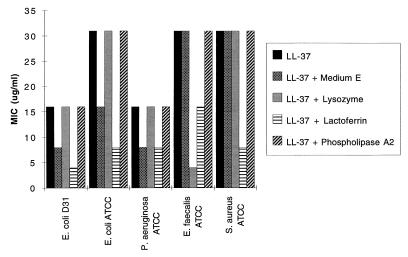
The mature peptide of LL-37/hCAP-18 consisting of the 37 C-terminal amino acids (LL-37) was chemically synthesized and its structure confirmed by mass spectrometry and capillary electrophoresis. The MIC of the peptide was measured against a number of bacteria, including E. coli, P. aeruginosa, E. faecalis, and S. aureus. Initial OD595 values were 0.05 (±0.01); final values of wells with bacterial growth after 12 h were 0.2 (±0.07). The MICs of LL-37 for these organisms ranged from 16 to 31 μg/ml (Fig. 6). A 2-fold decrease in MICs was noted against Gram-negative organisms when LL-37/hCAP-18 was assayed in medium containing medium E (16). Wells without turbicity after the incubation were subcultured on several occasions and results indicated that the minimal bactericidal concentration is about double the MIC for most conditions and bacteria (data not shown).
Figure 6.
Synergism of LL-37 with other antimicrobial proteins in killing of various bacteria. The MIC for LL-37 was determined against a number of bacteria (two strains of E. coli, P. aeruginosa, E. faecalis, and S. aureus). The MICs were determined with or without the addition of medium E or the presence of lysozyme (500 μg/ml) and lactoferrin (1 mg/ml for all except S. aureus, which was 250 μg/ml). Pancreatic phospholipase A2 (1.25 mg/ml) was used as negative control. This experiment was repeated on three occasions with essentially identical results.
Additional experiments were performed to determine whether LL-37/hCAP-18 is synergistic with other known antimicrobial proteins found in the airway surface fluid, including lysozyme (27) and lactoferrin (28, 29) (Fig. 6). These purified proteins were titrated against the various bacteria to determine a concentration at which they did not have an effect on bacterial growth alone. The MICs for LL-37/hCAP-18 were repeated in the presence of noninhibitory concentrations of these individual proteins. Synergistic effects were not detected with the control phospholipase A2 whereas lysozyme decreased the MIC of LL-37/hCAP-18 4-fold for E. faecalis but did not affect the activity of LL-37/hCAP-18 against the other organisms. The most substantial synergistic effect was documented with lactoferrin where the MICs were reduced 2- to 4-fold against all bacterial studied. Of relevance to the study of CF pathogenesis is the effect of NaCl on LL-37/hCAP-18 activity (Table 1). Note that the activity of LL-37/hCAP-18 diminishes 4- to 5-fold as the total concentration of NaCl is elevated from 60 to 155 mM.
Table 1.
Dependency of the MIC of LL-37 against E. coli D31 on the salt concentration of the medium
| NaCl, mM | MIC of LL-37 against E. coli D31, μg/ml |
|---|---|
| 20 | 16 |
| 60 | 62 |
| 100 | 125 |
| 155 | 250 |
| 300 | >500 |
Serial dilutions of the peptide were used in microbroth assays with increasing total concentrations of NaCl.
DISCUSSION
The impetus for this study was to learn about innate immunity in the lung and its relevance to pathogenesis of CF. Our hypothesis is that CF is caused by a breach in host defense secondary to a defect in CFTR. Children with this disease are born with normal pulmonary function. However, within the first few years of life their airways become colonized with pathogenic bacteria such as P. aeruginosa that is associated with chronic respiratory inflammation (30, 31). Smith et al. (32) were the first to demonstrate that airway surface fluid from CF is defective in its ability to kill certain bacteria.
One component of innate immunity potentially relevant to CF pathogenesis is the epithelial cell-derived human defensin called hBD-1. We previously showed that this peptide is expressed in the surface epithelia and submucosal glands in human lung and is inactivated in the high-salt milieu of the CF airway surface fluid (8). Antisense inhibition of its expression from a normal epithelium partially ablated killing of P. aeruginosa.
The cathelicidins represent another family of antimicrobial peptides that we considered in the context of pulmonary host defense (10–12). Members of this family have been isolated from a number of mammalian species and are characterized by a highly conserved N terminus with a more divergent C terminus representing the mature peptide (13–15). Cells of the myeloid lineage, specifically neutrophils, are the primary site of expression of most cathelicidins where they reside in cytoplasmic granules. A human cathelicidin called LL-37/hCAP-18 recently was cloned from a human bone marrow library (16–18). Like most other cathelicidins, this peptide is primarily found in inflammatory cells (21), although some expression also has been detected in testis (16) and inflamed epidermis (22).
We evaluated the tissue distribution of LL-37/hCAP-18 expression and were surprised to find it in a large number of organs in addition to bone marrow and testis. In situ hybridization demonstrated a high level of LL-37/hCAP-18 mRNA in epithelia of these organs. An example is in the gastrointestinal tract. LL-37/hCAP-18 mRNA is found diffusely throughout most epithelial cells of the proximal and distal gut, such as the stomach, small bowel, and colon. The distribution of LL-37/hCAP-18 expression in human bowel resembles that of the cryptin family of defensin peptides that are expressed in the murine intestine (33). The broad expression of LL-37/hCAP-18 within granules of phagocytes and diffusely through the epithelia is unusual and suggests it may have functions distinct from direct bacterial killing, such as its previously described binding to endotoxin (18). Other functions for this broadly expressed peptide should be evaluated.
Expression of LL-37/hCAP-18 in lung mirrors that of hBD-1 in that it was found in both surface epithelia of the proximal airway as well as cells of the submucosal glands; expression in distal airway was diminished as compared with that observed with hBD-1. We were able to isolate detectable quantities of the active, mature peptide from airway surface fluid of human lung and a human bronchial xenograft model. This finding supports the role of the secreted version of this peptide in host defense and indicates that the source of the peptide in the airway surface fluid is indeed the epithelial cells. It is interesting that in the airway surface fluid from lung a larger form of the peptide, presumably representing the unprocessed form, was detected. This finding possibly indicates a contamination by lysed leukocytes. It generally is believed that the prepro-sequence of cathelicidins must be removed for the C terminus to have antimicrobial activity (34).
Our data suggest that LL-37/hCAP-18 may contribute to the normal host defense in the lung. An important question is the relative biological activity of various antibacterial proteins/peptides contained within the airway surface fluid. We showed that the MIC for LL-37/hCAP-18 is decreased in the presence of lactoferrin and lysozyme, suggesting synergism between peptides and proteins found within the airway surface fluid. These functional interactions are interesting in light of the fact that each is expressed within the serous cells of the submucosal glands. Synergism described in this study also illustrates the difficulty in extrapolating from biological assays of purified peptides/proteins to their relative function in vivo.
The relevance of LL-37/hCAP-18 to CF still remains speculative. Similar to hBD-1, LL-37/hCAP-18 is inactivated by the high-salt milieu that has been described in the CF airway surface fluid (8). The activity is diminished 4- to 5-fold over a range of NaCl that distinguishes the non-CF from the CF airway surface fluid (i.e., 80 mM for non-CF to 140 mM in CF) (35, 36). Whether the high salt serves as a trigger to inactivate LL-37/hCAP-18 and hBD-1 remains controversial, caused in part by technical problems in measuring the actual salt concentration of the airway surface fluid. Compromised activity of LL-37/hCAP-18 in the CF airway also could be explained by inactivation through other components of the airway surface fluid or a defect in its biogenesis secondary to cystic fibrosis transmembrane conductance regulator (CFTR) expression. The latter is a particularly intriguing hypothesis in light of the fact that CFTR and LL-37/hCAP-18 are expressed in essentially the same cells of the human airway.
In summary, our demonstration of diffuse expression of this human cathelicidin throughout epithelia of many organs expands its potential role in human health and disease.
Acknowledgments
We thank the contributions of the Cell Morphology and Vector Cores at the Institute for Human Gene Therapy. Discussions with Dr. Mitchell Goldman also were appreciated. R.B. is a recipient of a fellowship of the Deutsche Forschungsgemeinschaft. This work was supported by the Cystic Fibrosis Foundation, the National Institutes of Health (P30 DK 47757-04 and RO1 HL4 9040-06), and Genovo Inc., a company that J.M.W. is a founder of and holds equity in.
ABBREVIATIONS
- hBD-1
human β-defensin 1
- CF
cystic fibrosis
- RT-PCR
reverse transcription–PCR
- MIC
minimal inhibitory concentration
References
- 1.Sherman M P, Ganz T. Annu Rev Physiol. 1992;54:331–350. doi: 10.1146/annurev.ph.54.030192.001555. [DOI] [PubMed] [Google Scholar]
- 2.Toews G B. Semin Respir Infect. 1993;8:160–167. [PubMed] [Google Scholar]
- 3.Ganz T, Lehrer R-I. Pharmacol Ther. 1995;66:191–205. doi: 10.1016/0163-7258(94)00076-f. [DOI] [PubMed] [Google Scholar]
- 4.Ganz T, Lehrer R-I. Immunology. 1994;6:584–589. doi: 10.1016/0952-7915(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 5.Diamond G, Zasloff M, Eck H, Brasseur M, Maloy W L, Bevins C L. Proc Natl Acad Sci USA. 1994;88:3952–3956. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schonwetter B-S, Stolzenber E-D, Zasloff M-A. Science. 1995;267:1645–1648. doi: 10.1126/science.7886453. [DOI] [PubMed] [Google Scholar]
- 7.Bensch K-W, Raida M, Magert H-J, Schultz-Knappe P, Forssmann W-G. FEBS Lett. 1995;368:331–335. doi: 10.1016/0014-5793(95)00687-5. [DOI] [PubMed] [Google Scholar]
- 8.Goldman M-J, Anderson G-M, Stolzenberg E-D, Kari U-P, Zasloff M, Wilson J-M. Cell. 1997;88:553–560. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 9.McCray P B, Bentley L. Am J Respir Cell Mol Biol. 1997;16:343–349. doi: 10.1165/ajrcmb.16.3.9070620. [DOI] [PubMed] [Google Scholar]
- 10.Zanetti M, Gennaro R, Romeo D. FEBS Lett. 1995;374:1–5. doi: 10.1016/0014-5793(95)01050-o. [DOI] [PubMed] [Google Scholar]
- 11.Agerberth B, Gunne H, Odeberg J, Kogner P, Boman H-G, Gudmundsson G-H. Proc Natl Acad Sci USA. 1995;92:195–199. doi: 10.1073/pnas.92.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ritonja A, Kopitar M, Jerala R, Turk V. FEBS Lett. 1989;255:211–214. doi: 10.1016/0014-5793(89)81093-2. [DOI] [PubMed] [Google Scholar]
- 13.Storici P, Del Sal G, Schneider C, Zanetti M. FEBS Lett. 1992;314:187–190. doi: 10.1016/0014-5793(92)80971-i. [DOI] [PubMed] [Google Scholar]
- 14.Del Sal G, Storici P, Schneider C, Romeo D, Zanetti M. Biochem Biophys Res Commun. 1992;187:467–472. doi: 10.1016/s0006-291x(05)81517-7. [DOI] [PubMed] [Google Scholar]
- 15.Tossi A, Scocchi M, Zanetti M, Storici P, Gennaro R. Eur J Biochem. 1995;228:941–946. doi: 10.1111/j.1432-1033.1995.tb20344.x. [DOI] [PubMed] [Google Scholar]
- 16.Agerberth B, Gunne H, Odeberg J, Kogner P, Boman H-G, Gudmundsson G-H. Proc Natl Acad Sci USA. 1995;92:195–199. doi: 10.1073/pnas.92.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cowland J-B, Johnsen A-H, Borregarrd N. FEBS Lett. 1995;368:173–176. doi: 10.1016/0014-5793(95)00634-l. [DOI] [PubMed] [Google Scholar]
- 18.Larrick J-W, Hirata M, Balint R-F, Lee J, Zhong J, Wright S-C. Infect Immun. 1995;63:1291–1297. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gudmundsson G-H, Magnusson K-P, Chowdhary B-P, Johansson M, Andersson L, Boman H-G. Proc Natl Acad Sci USA. 1995;92:7085–7089. doi: 10.1073/pnas.92.15.7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gudmundsson G-H, Agerberth B, Odeberg J, Bergman T, Olsson B, Salcedo R. Eur J Biochem. 1996;238:325–332. doi: 10.1111/j.1432-1033.1996.0325z.x. [DOI] [PubMed] [Google Scholar]
- 21.Sørensen O, Arnljots K, Cowland J-B, Bainton D-F, Borregaard N. Blood. 1997;90:2796–2803. [PubMed] [Google Scholar]
- 22.Frohm M, Agerberth B, Ahangari G, Ståhle-Bäckdahl M, Lidén S, Wigzell H, Gudmundsson G-H. J Biol Chem. 1997;272:15258–15263. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 23.Steiner H, Hultmark D, Engstroem A, Bennich H, Boman H-G. Nature (London) 1981;292:246–248. doi: 10.1038/292246a0. [DOI] [PubMed] [Google Scholar]
- 24.Vogel H-J, Bonner D-M. J Biol Chem. 1955;218:97–106. [PubMed] [Google Scholar]
- 25.Basbaum C B, Jany B, Finkbeiner W E. Annu Rev Physiol. 1990;52:97–113. doi: 10.1146/annurev.ph.52.030190.000525. [DOI] [PubMed] [Google Scholar]
- 26.Engelhardt J F, Yankaskas J R, Ernst S A, Yang Y, Marino C, Boucher R C, Cohn J A, Wilson J M. Nat Genet. 1992;2:240–248. doi: 10.1038/ng1192-240. [DOI] [PubMed] [Google Scholar]
- 27.Ellison-III R-T, Giehl T-J. J Clin Invest. 1991;88:1080–1091. doi: 10.1172/JCI115407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomita, M., Takase, M., Wakabayashi, H. & Bellamy, W. (1994) Struct. Funct. 208–218.
- 29.Sherman M P, Ganz T. Annu Rev Physiol. 1992;54:331–350. doi: 10.1146/annurev.ph.54.030192.001555. [DOI] [PubMed] [Google Scholar]
- 30.Armstrong D-S, Grimwood K, Carzino R, Carlin J-B, Olinsky A, Phelan P-D. Br Med J. 1995;310:1571–1572. doi: 10.1136/bmj.310.6994.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armstrong D-S, Grimwood K, Carlin J-H-B. Pediatr Pulmonol. 1996;21:267–275. doi: 10.1002/(SICI)1099-0496(199605)21:5<267::AID-PPUL1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 32.Smith J-J, Travis S-M, Greenberg E-P, Welsh M-J. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 33.Huttner K-M, Selsted M-E, Ouellette A-J. Genomics. 1994;19:448–453. doi: 10.1006/geno.1994.1093. [DOI] [PubMed] [Google Scholar]
- 34.Zanetti M, Litteri L, Griffiths G, Gennaro R, Romeo D. J Immunol. 1991;146:4295–4300. [PubMed] [Google Scholar]
- 35.Gilljam H, Ellin A, Strandvik B. Scand J Clin Lab Invest. 1989;49:121–124. doi: 10.3109/00365518909105409. [DOI] [PubMed] [Google Scholar]
- 36.Joris L, Dab I, Quinton P M. Am J Respir Dis. 1993;148:1633–1637. doi: 10.1164/ajrccm/148.6_Pt_1.1633. [DOI] [PubMed] [Google Scholar]