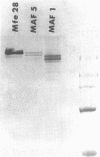
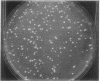
Abstract
The complete nucleotide sequence was determined for the Streptococcus sobrinus MFe28 gtfI gene, which encodes a glucosyltransferase that produces an insoluble glucan product. A single open reading frame encodes a mature glucosyltransferase protein of 1,559 amino acids (Mr, 172,983) and a signal peptide of 38 amino acids. In the C-terminal one-third of the protein there are six repeating units containing 35 amino acids of partial homology and two repeating units containing 48 amino acids of complete homology. The functional role of these repeating units remains to be determined, although truncated forms of glucosyltransferase containing only the first two repeating units of partial homology maintained glucosyltransferase activity and the ability to bind glucan. Regions of homology with alpha-amylase and glycogen phosphorylase were identified in the glucosyltransferase protein and may represent regions involved in functionally similar domains.
Full text
PDF




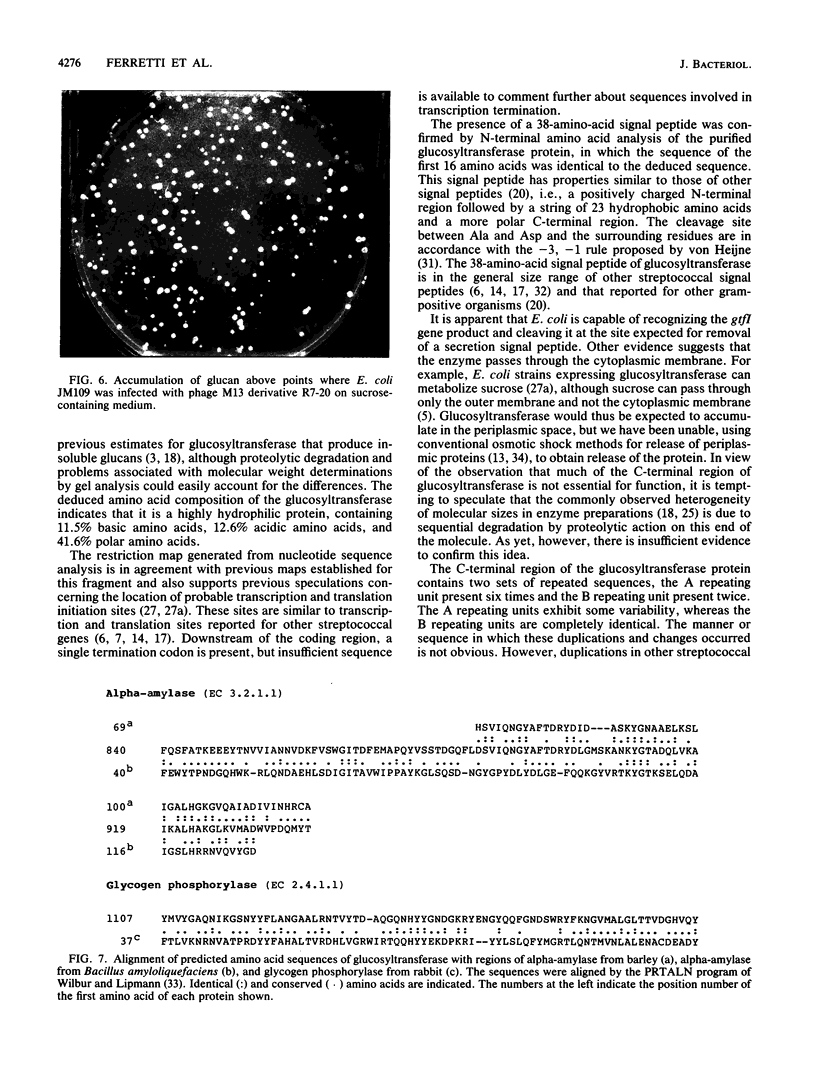


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki H., Shiroza T., Hayakawa M., Sato S., Kuramitsu H. K. Cloning of a Streptococcus mutans glucosyltransferase gene coding for insoluble glucan synthesis. Infect Immun. 1986 Sep;53(3):587–594. doi: 10.1128/iai.53.3.587-594.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burne R. A., Rubinfeld B., Bowen W. H., Yasbin R. E. Cloning and expression of a Streptococcus mutans glucosyltransferase gene in Bacillus subtilis. Gene. 1986;47(2-3):201–209. doi: 10.1016/0378-1119(86)90064-8. [DOI] [PubMed] [Google Scholar]
- Decad G. M., Nikaido H. Outer membrane of gram-negative bacteria. XII. Molecular-sieving function of cell wall. J Bacteriol. 1976 Oct;128(1):325–336. doi: 10.1128/jb.128.1.325-336.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahnestock S. R., Alexander P., Nagle J., Filpula D. Gene for an immunoglobulin-binding protein from a group G streptococcus. J Bacteriol. 1986 Sep;167(3):870–880. doi: 10.1128/jb.167.3.870-880.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti J. J., Gilmore K. S., Courvalin P. Nucleotide sequence analysis of the gene specifying the bifunctional 6'-aminoglycoside acetyltransferase 2"-aminoglycoside phosphotransferase enzyme in Streptococcus faecalis and identification and cloning of gene regions specifying the two activities. J Bacteriol. 1986 Aug;167(2):631–638. doi: 10.1128/jb.167.2.631-638.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin M. L., Russell R. R., Morrissey P. Cloning and expression of two Streptococcus mutans glucosyltransferases in Escherichia coli K-12. Infect Immun. 1985 Aug;49(2):414–416. doi: 10.1128/iai.49.2.414-416.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guss B., Eliasson M., Olsson A., Uhlén M., Frej A. K., Jörnvall H., Flock J. I., Lindberg M. Structure of the IgG-binding regions of streptococcal protein G. EMBO J. 1986 Jul;5(7):1567–1575. doi: 10.1002/j.1460-2075.1986.tb04398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelbauer G. L., Harayama S. Mutants in transmission of chemotactic signals from two independent receptors of E. coli. Cell. 1979 Mar;16(3):617–625. doi: 10.1016/0092-8674(79)90035-7. [DOI] [PubMed] [Google Scholar]
- Hollingshead S. K., Fischetti V. A., Scott J. R. Complete nucleotide sequence of type 6 M protein of the group A Streptococcus. Repetitive structure and membrane anchor. J Biol Chem. 1986 Feb 5;261(4):1677–1686. [PubMed] [Google Scholar]
- Kühn S., Fritz H. J., Starlinger P. Close vicinity of IS1 integration sites in the leader sequence of the gal operon of E. coli. Mol Gen Genet. 1979 Jan 2;167(3):235–241. doi: 10.1007/BF00267414. [DOI] [PubMed] [Google Scholar]
- Malke H., Roe B., Ferretti J. J. Nucleotide sequence of the streptokinase gene from Streptococcus equisimilis H46A. Gene. 1985;34(2-3):357–362. doi: 10.1016/0378-1119(85)90145-3. [DOI] [PubMed] [Google Scholar]
- Müller-Hill B., Crapo L., Gilbert W. Mutants that make more lac repressor. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1259–1264. doi: 10.1073/pnas.59.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. Protein secretion in Escherichia coli. Annu Rev Microbiol. 1985;39:615–648. doi: 10.1146/annurev.mi.39.100185.003151. [DOI] [PubMed] [Google Scholar]
- Palm D., Goerl R., Burger K. J. Evolution of catalytic and regulatory sites in phosphorylases. Nature. 1985 Feb 7;313(6002):500–502. doi: 10.1038/313500a0. [DOI] [PubMed] [Google Scholar]
- Pucci M. J., Macrina F. L. Molecular organization and expression of the gtfA gene of Streptococcus mutans LM7. Infect Immun. 1986 Oct;54(1):77–84. doi: 10.1128/iai.54.1.77-84.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robeson J. P., Barletta R. G., Curtiss R., 3rd Expression of a Streptococcus mutans glucosyltransferase gene in Escherichia coli. J Bacteriol. 1983 Jan;153(1):211–221. doi: 10.1128/jb.153.1.211-221.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. R., Coleman D., Dougan G. Expression of a gene for glucan-binding protein from Streptococcus mutans in Escherichia coli. J Gen Microbiol. 1985 Feb;131(2):295–299. doi: 10.1099/00221287-131-2-295. [DOI] [PubMed] [Google Scholar]
- Russell R. R., Gilpin M. L., Mukasa H., Dougan G. Characterization of glucosyltransferase expressed from a Streptococcus sobrinus gene cloned in Escherichia coli. J Gen Microbiol. 1987 Apr;133(4):935–944. doi: 10.1099/00221287-133-4-935. [DOI] [PubMed] [Google Scholar]
- Shiroza T., Ueda S., Kuramitsu H. K. Sequence analysis of the gtfB gene from Streptococcus mutans. J Bacteriol. 1987 Sep;169(9):4263–4270. doi: 10.1128/jb.169.9.4263-4270.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks C. R., Ferretti J. J. Nucleotide sequence of the type A streptococcal exotoxin (erythrogenic toxin) gene from Streptococcus pyogenes bacteriophage T12. Infect Immun. 1986 Apr;52(1):144–150. doi: 10.1128/iai.52.1.144-150.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witholt B., Boekhout M., Brock M., Kingma J., Heerikhuizen H. V., Leij L. D. An efficient and reproducible procedure for the formation of spheroplasts from variously grown Escherichia coli. Anal Biochem. 1976 Jul;74(1):160–170. doi: 10.1016/0003-2697(76)90320-1. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]