Abstract
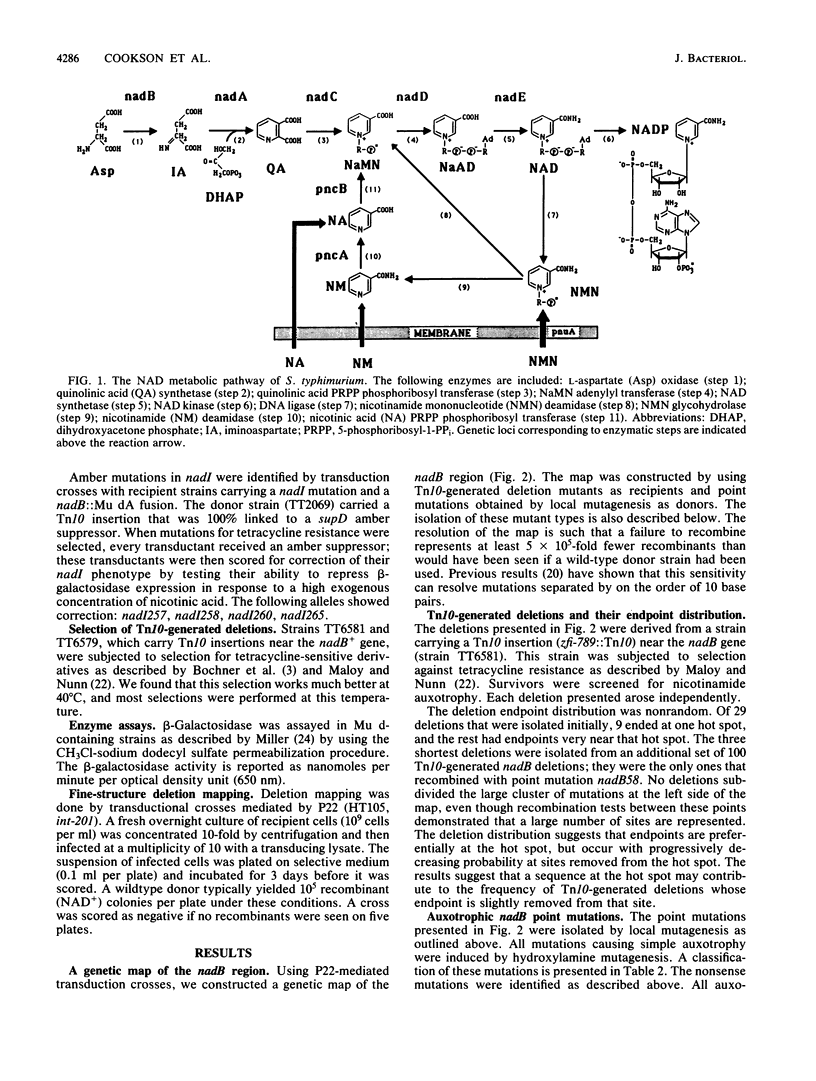
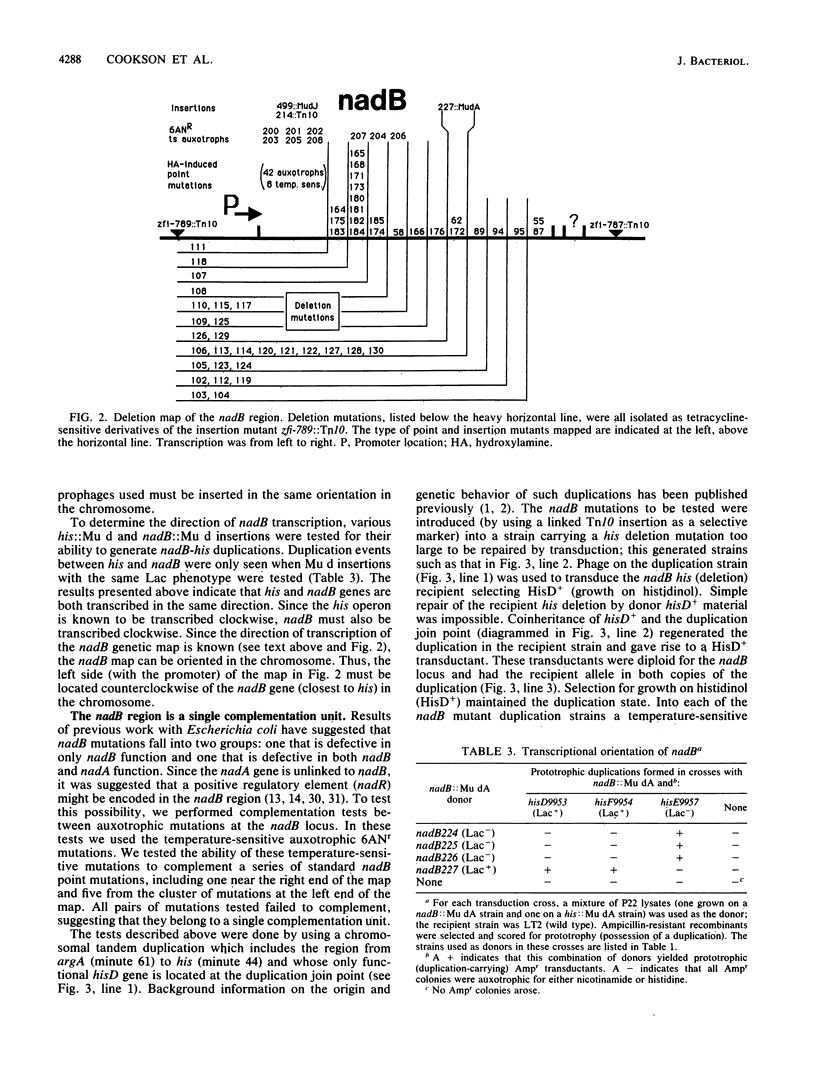
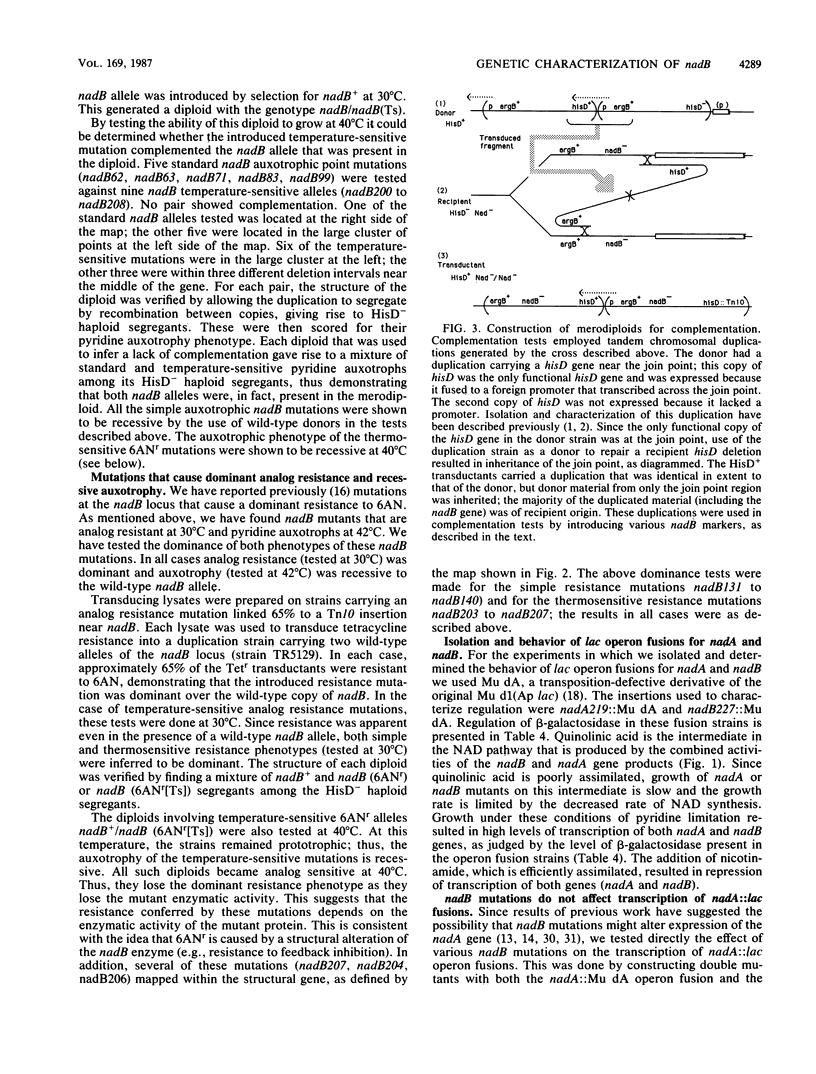
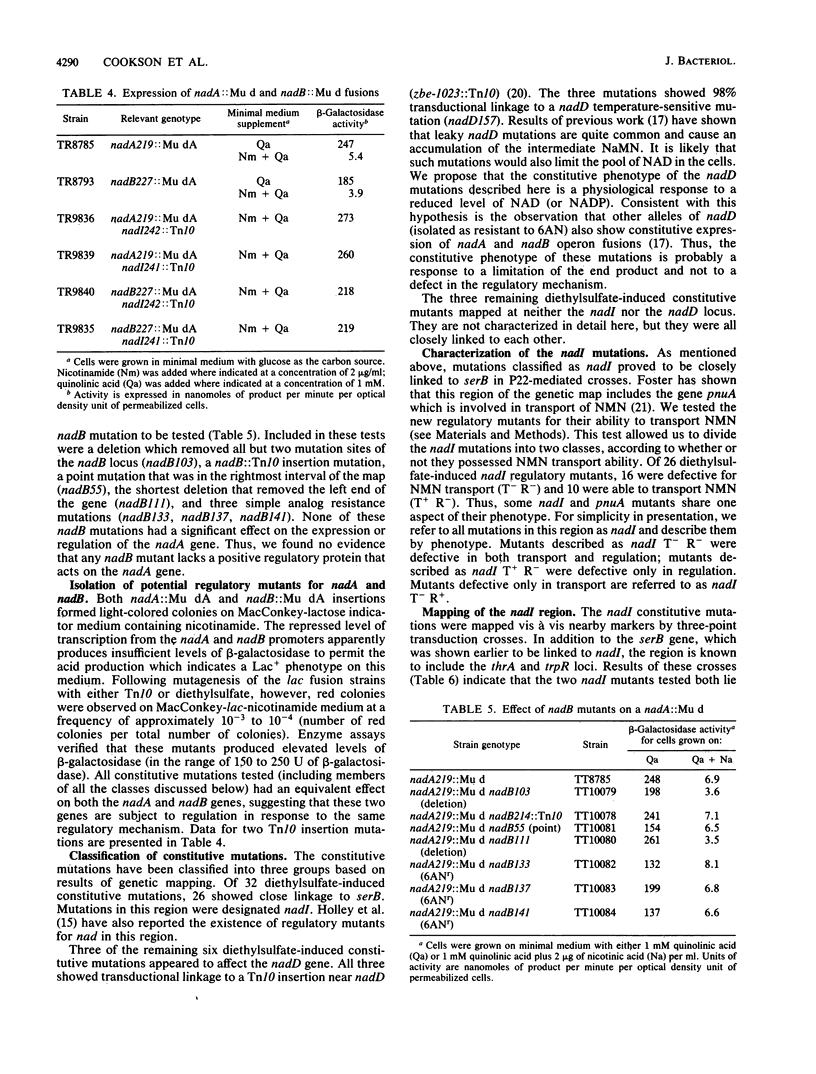
The nadB locus encodes the first enzyme of NAD synthesis. It has been reported that this gene and nadA are regulated by a positive regulatory protein encoded in the nadB region. In pursuing this regulatory mechanism, we constructed a fine-structure genetic map of the nadB gene. The region appears to include a single complementation group; no evidence for a positive regulatory element was found. Several mutations causing resistance to the analog 6-aminonicotinamide mapped within the structural gene and probably cause resistance to feedback inhibition. Regulatory mutations for nadB were isolated. These mutants mapped far from nadB near the pnuA gene, which encodes a function required for nicotinamide mononucleotide transport. The regulatory mutations appear to affect a distinct function encoded in the same operon as pnuA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. P., Roth J. R. Tandem chromosomal duplications in Salmonella typhimurium: fusion of histidine genes to novel promoters. J Mol Biol. 1978 Feb 15;119(1):147–166. doi: 10.1016/0022-2836(78)90274-7. [DOI] [PubMed] [Google Scholar]
- Anderson R. P., Roth J. R. Tandem genetic duplications in phage and bacteria. Annu Rev Microbiol. 1977;31:473–505. doi: 10.1146/annurev.mi.31.100177.002353. [DOI] [PubMed] [Google Scholar]
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho B. A., Olfson P., Casadaban M. J. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol. 1984 May;158(2):488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler J. L., Gholson R. K. De novo biosynthesis of nicotinamide adenine dinucleotide in Escherichia coli: excretion of quinolinic acid by mutants lacking quinolinate phosphoribosyl transferase. J Bacteriol. 1972 Jul;111(1):98–102. doi: 10.1128/jb.111.1.98-102.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler J. L., Gholson R. K., Scott T. A. Studies on the de novo biosynthesis of NAD in Escherichia coli. I. Labelling patterns from precursors. Biochim Biophys Acta. 1970 Nov 24;222(2):523–526. doi: 10.1016/0304-4165(70)90145-5. [DOI] [PubMed] [Google Scholar]
- Chandler J. L., Gholson R. K. Studies on the biosynthesis of NAD in Escherichia coli. 3. Precursors of quinolinic acid in vitro. Biochim Biophys Acta. 1972 Apr 21;264(2):311–318. doi: 10.1016/0304-4165(72)90295-4. [DOI] [PubMed] [Google Scholar]
- Chumley F. G., Menzel R., Roth J. R. Hfr formation directed by tn10. Genetics. 1979 Apr;91(4):639–655. doi: 10.1093/genetics/91.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Moat A. G. Mapping and characterization of the nad genes in Salmonella typhimurium LT-2. J Bacteriol. 1978 Feb;133(2):775–779. doi: 10.1128/jb.133.2.775-779.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Moat A. G. Nicotinamide adenine dinucleotide biosynthesis and pyridine nucleotide cycle metabolism in microbial systems. Microbiol Rev. 1980 Mar;44(1):83–105. doi: 10.1128/mr.44.1.83-105.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith G. R., Chandler J. L., Gholson R. K. Studies on the de novo biosynthesis of NAD in Escherichia coli. The separation of the nadB gene product from the nadA gene product and its purification. Eur J Biochem. 1975 May;54(1):239–245. doi: 10.1111/j.1432-1033.1975.tb04133.x. [DOI] [PubMed] [Google Scholar]
- Holley E. A., Spector M. P., Foster J. W. Regulation of NAD biosynthesis in Salmonella typhimurium: expression of nad-lac gene fusions and identification of a nad regulatory locus. J Gen Microbiol. 1985 Oct;131(10):2759–2770. doi: 10.1099/00221287-131-10-2759. [DOI] [PubMed] [Google Scholar]
- Hughes K. T., Cookson B. T., Ladika D., Olivera B. M., Roth J. R. 6-Aminonicotinamide-resistant mutants of Salmonella typhimurium. J Bacteriol. 1983 Jun;154(3):1126–1136. doi: 10.1128/jb.154.3.1126-1136.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. T., Ladika D., Roth J. R., Olivera B. M. An indispensable gene for NAD biosynthesis in Salmonella typhimurium. J Bacteriol. 1983 Jul;155(1):213–221. doi: 10.1128/jb.155.1.213-221.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. T., Roth J. R. Conditionally transposition-defective derivative of Mu d1(Amp Lac). J Bacteriol. 1984 Jul;159(1):130–137. doi: 10.1128/jb.159.1.130-137.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. T., Roth J. R. Directed formation of deletions and duplications using Mud(Ap, lac). Genetics. 1985 Feb;109(2):263–282. doi: 10.1093/genetics/109.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston H. M., Roth J. R. Genetic analysis of the histidine operon control region of Salmonella typhimurium. J Mol Biol. 1981 Feb 5;145(4):713–734. doi: 10.1016/0022-2836(81)90311-9. [DOI] [PubMed] [Google Scholar]
- Kinney D. M., Foster J. W., Moat A. G. Pyridine nucleotide cycle of Salmonella typhimurium: in vitro demonstration of nicotinamide mononucleotide deamidase and characterization of pnuA mutants defective in nicotinamide mononucleotide transport. J Bacteriol. 1979 Nov;140(2):607–611. doi: 10.1128/jb.140.2.607-611.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981 Feb;145(2):1110–1111. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren J., Ngo D. T., Olivera B. M. Pyridine nucleotide metabolism in Escherichia coli. 3. Biosynthesis from alternative precursors in vivo. J Biol Chem. 1973 Jul 25;248(14):5144–5149. [PubMed] [Google Scholar]
- Nasu S., Wicks F. D., Gholson R. K. L-Aspartate oxidase, a newly discovered enzyme of Escherichia coli, is the B protein of quinolinate synthetase. J Biol Chem. 1982 Jan 25;257(2):626–632. [PubMed] [Google Scholar]
- Saxton R. E., Rocha V., Rosser R. J., Andreoli A. J., Shimoyama M., Kosaka A., Chandler J. L., Gholson R. K. A comparative study of the regulation of nicotinamide-adenine dinucleotide biosynthesis. Biochim Biophys Acta. 1968 Feb 1;156(1):77–84. doi: 10.1016/0304-4165(68)90106-2. [DOI] [PubMed] [Google Scholar]
- Schmid M. B., Roth J. R. Genetic methods for analysis and manipulation of inversion mutations in bacteria. Genetics. 1983 Nov;105(3):517–537. doi: 10.1093/genetics/105.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector M. P., Hill J. M., Holley E. A., Foster J. W. Genetic characterization of pyridine nucleotide uptake mutants of Salmonella typhimurium. J Gen Microbiol. 1985 Jun;131(6):1313–1322. doi: 10.1099/00221287-131-6-1313. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Carlson J., Griffith G., Gholson R. K. Studies on the de novo biosynthesis of NAD in Escherichia coli. V. Properties of the quinolinic acid synthetase system. Biochim Biophys Acta. 1973 Apr 28;304(2):309–315. doi: 10.1016/0304-4165(73)90249-3. [DOI] [PubMed] [Google Scholar]
- Tritz G. J., Chandler J. L. Recognition of a gene involved in the regulation of nicotinamide adenine dinucleotide biosynthesis. J Bacteriol. 1973 Apr;114(1):128–136. doi: 10.1128/jb.114.1.128-136.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritz G. J. Characterization of the nadR locus in Escherichia coli. Can J Microbiol. 1974 Feb;20(2):205–209. doi: 10.1139/m74-031. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]



